Abstract
Purpose
The study aims to test the hypothesis that platelet-rich plasma (PRP) stimulates cellular processes involved in endometrial regeneration relevant to clinical management of poor endometrial growth or intrauterine scarring.
Methods
Human endometrial stromal fibroblasts (eSF), endometrial mesenchymal stem cells (eMSC), bone marrow-derived mesenchymal stem cells (BM-MSC), and Ishikawa endometrial adenocarcinoma cells (IC) were cultured with/without 5% activated (a) PRP, non-activated (na) PRP, aPPP (platelet-poor-plasma), and naPPP. Treatment effects were evaluated with cell proliferation (WST-1), wound healing, and chemotaxis Transwell migration assays. Mesenchymal-to-epithelial transition (MET) was evaluated by cytokeratin and vimentin expression. Differential gene expression of various markers was analyzed by multiplex Q-PCR.
Results
Activated PRP enhanced migration of all cell types, compared to naPRP, aPPP, naPPP, and vehicle controls, in a time-dependent manner (p < 0.05). The WST-1 assay showed increased stromal and mesenchymal cell proliferation by aPRP vs. naPRP, aPPP, and naPPP (p < 0.05), while IC proliferation was enhanced by aPRP and aPPP (p < 0.05). There was no evidence of MET. Expressions of MMP1, MMP3, MMP7, and MMP26 were increased by aPRP (p < 0.05) in eMSC and eSF. Transcripts for inflammation markers/chemokines were upregulated by aPRP vs. aPPP (p < 0.05) in eMSC and eSF. No difference in estrogen or progesterone receptor mRNAs was observed.
Conclusions
This is the first study evaluating the effect of PRP on different human endometrial cells involved in tissue regeneration. These data provide an initial ex vivo proof of principle for autologous PRP to promote endometrial regeneration in clinical situations with compromised endometrial growth and scarring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 13% of couples worldwide have infertility [1] due to several factors, including impaired embryo quality and endometrial receptivity. The latter can be affected by altered programmed responsiveness to steroid hormones [2, 3], a non-lactobacillus dominant endometrial microbiome [4], or structurally due to uterine anomalies, scarring and intrauterine adhesions (Asherman’s syndrome (AS)), or an unexplained thin lining. Endometrial thickness is commonly used as a clinical marker of endometrial receptivity and a prognostic factor for pregnancy outcome after embryo transfer [5]. An endometrial thickness <7–8 mm at the end of the follicular phase is associated with reduced pregnancy rates [6, 7], poor pregnancy outcomes [5], and may result in a cycle cancelation [8]. Unexplained thin endometrium and AS are among the most challenging obstacles in fertility care, often resulting in patients pursuing gestational surrogacy [9].
The main complications of Asherman’s syndrome are infertility (43%), impairment of menstrual flow (62%), and abnormal placentation if pregnancy is achieved [10], with surgery (hysteroscopy) with or without oral estrogens being the standard treatment [11].
Recently, the use of biologics has been pursued to boost the regeneration process and/or minimize post-operative adhesions, including intrauterine infusion of autologous bone marrow-derived mesenchymal stem cells (BM-MSCs) [12], human amnion graft placement [13], or autologous peripheral blood CD133+ BM-MSC delivered into the uterine spiral arterioles by catheterization [14]. These have not been widely adopted likely due to the complexity of the techniques and conflicting outcomes [12,13,14].
In the setting of persistent thin lining and normal uterine cavity (without scarring), several strategies to improve thickness have been investigated, including use of exogenous estrogens, vaginal sildenafil, low-dose aspirin, and granulocyte colony stimulation factor [15,16,17,18]; however, a large proportion of women with thin lining remain refractory to such therapies. Two studies reported on successful use of an intrauterine infusion of platelet-rich plasma (PRP) in infertile women with thin endometrium [19, 20].
PRP is an autologous concentration of platelets in plasma that has been increasingly used to support tissue growth and repair in orthopedics, dental and plastic surgery, diabetic wound healing, and dermatology [21,22,23,24], but has been minimally investigated to date in gynecology. Platelets contain granules that store growth factors and cytokines (e.g., VEGF, TGFβ, PDGF, IGF1, FGF, EFG, HGF, CXCL12, CCL5) released upon platelet activation at the site of injury or inflammation. These factors are critical in activation of fibroblasts and recruitment of leukocytes to the injury site, inducing and regulating proliferation and migration of other cell types involved in tissue repair such as smooth muscle cells and mesenchymal stem cells, and promoting angiogenesis [25, 26]. Importantly, platelet-derived factors are essential for endometrial progenitor cell activity [27], and PDGF isoforms significantly promote endometrial stromal cell proliferation, migration, and contractility [28]. Endometrium contains epithelial, mesenchymal, and endothelial stem/progenitor cells [29, 30]. BM-MSCs have been proposed as a potential source of endometrial regeneration [31,32,33,34]. In mice, platelets recruit circulating progenitors to exposed collagen in damaged blood vessels and induce differentiation into mature endothelial cells [25], which can be one of the regeneration mechanisms initiated by platelets.
Using an in vitro approach, our goal was to investigate the potential pharmacologic use of PRP in promoting biological processes involved in endometrial regeneration as relevant to the management of Asherman’s syndrome or thin endometrial lining in infertility patients. To this end, we studied the effects of PRP and platelet-poor plasma (PPP) on the proliferation and migration, as well as gene expression, of endometrial stromal fibroblasts (eSF), endometrial mesenchymal stem cells (eMSC), BM-MSCs, and Ishikawa cells (IC), as well as mesenchymal-to-epithelial transition (MET) of eSF and eMSC.
Materials and methods
Human subjects
Primary human eutopic eSF and eMSC were obtained through the UCSF NIH Human Endometrial Tissue and DNA Bank. Written informed consent was given by all participants under the active IRB protocol approved by the Institutional Committee on Human Research.
Study design
Human primary eSF, eMSC, BM-MSCs, and Ishikawa endometrial adenocarcinoma cells (IC) were cultured without (control group) and with 5% activated (a) PRP, non-activated (na)PRP, activated platelet-poor-plasma (aPPP), and naPPP (5 groups total). Effects of treatments were evaluated using in vitro assays for cell proliferation (WST-1), wound-healing migration, and chemotaxis Transwell migration. Mesenchymal-to-epithelial transition of eSF, eMSC, and BM-MSC was evaluated with cytokeratin and vimentin immunofluorescence. Differential gene expression of growth factor receptors, extracellular matrix markers, cell surface markers, inflammation markers, and chemokines were analyzed by multiplex q-RT-PCR (Fluidigm, South San Francisco, CA) (Fig. 1).
Endometrial stromal fibroblast isolation and culture
Isolated and cultured eSFs from women with either no uterine pathology or non-cavity-distorting fibroids and without endometriosis, (n = 3) before and after PRP/PPP treatment, were used for the WST-1 proliferation assay, migration scratch and Transwell assays, immunohistochemistry, and real-time RT-PCR. Fresh endometrial samples were digested with collagenase as described previously [35]. Human eSF were separated from epithelium based on size and plated with the use of Dulbecco modified Eagle medium (DMEM; Life Technologies, Foster City, CA) and 25% MCDB-105 (Sigma-Aldrich, St Louis, MO), containing 10% charcoal-stripped fetal bovine serum (FBS; HyClone, Thermo Scientific, Inc., Waltham, MA), 1 mM sodium pyruvate (Sigma-Aldrich), 1% antibiotic-antimycotic solution (Life Technologies), and 5 μg/ml insulin (Gemini, Sacramento, CA), as described previously [35, 36]. At passage 2, cells were cultured to near confluence in the same medium, followed by changing to low-serum medium (2% FBS) and cultured for 24 h before the onset of treatment. eSF culture purity (~99% stromal fibroblasts) was determined by cytokeratin, vimentin, and CD45 by immunostaining, as described previously [35]. All cell culture experiments were conducted with the use of second to fourth cell passages. Primary cells are archived in our Tissue Bank, and we have extensively studied their stability and response to steroid hormones after thawing, plating, and multiple passages in vitro [37]. Once cultured, these cells retain their viability and steroid hormone response uniformly over passages 1–4 [38].
Endometrial mesenchymal stem cell isolation and culture
CD146(+)/PDGFRB(+) eMSC that were isolated previously from eutopic endometrial samples (healthy oocyte donors, n = 3) using fluorescence-activated cell sorting (FACS) with demonstration of their clonogenicity [39] were used in this study. Primary cultures were established and used as described [40], with modifications to the culture medium which was composed of 75% high-glucose phenol red-free of DMEM/MCDB-105 medium, supplemented with 10% charcoal-stripped FBS, 1 mM sodium pyruvate, and 25 ng/ml basic FGF (Sigma-Aldrich). Cells were expanded by serial passage with subsequent cryopreservation of eMSC for further use. eMSC from [43] at second and third passages were used for current experiments. As it was previously shown, eMSC cultured in the presence of basic FGF will largely retain the eMSC phenotype over serial passages with ~70% of SUSD2+ cells and persistence of multi-lineage differentiation capacity at passage 6 [41].
Cell lines
BM-MSCs were purchased from Cambrex Biosciences, East Rutherford, NJ and expanded in high-glucose DMEM containing 10% FBS, 1% penicillin, and 1% streptomycin [32]. At confluency, BM-MSCs were trypsinized and plated at a specific density as required for different experiments. All treatments were performed in triplicate under identical culture conditions. All experiments were performed with the second and third passages.
IC
This cell line (Sigma-Aldrich) was used in our study as a model for primary endometrial epithelial cells because of the limitations in obtaining sufficient numbers for primary epithelial culture and expansion for all experiments planned. Ishikawa cells were expanded in minimal essential Eagle’s medium with 10% charcoal-stripped FBS, 2 mM L-glutamine, 1% penicillin/streptomycin, and 1% non-essential amino acids at 37 °C in a 95% air/5% CO2 humidified incubator [42]. The medium was changed every second day. When cells became confluent, they were trypsinized and plated at a specific density as required for different experiments. All treatments were performed in triplicate under identical culture conditions.
Preparation of PRP
Leukocyte reduced apheresis platelets were purchased from the Blood Centers of the Pacific (San Francisco, CA), from a white 59-year-old male donor, blood group A, Rh positive, with platelet concentration 2.2 × 1011 in 300 ml of apheresis platelet concentrate. We specifically used a male donor to eliminate any potential influences of hormonal variations in the growth factor/cytokine composition. The PRP and PPP were prepared as described [43, 44]. Briefly, the platelet-containing plasma was centrifuged for 15 min at 3200 rpm at room temperature (RT), and PPP was separated out. The plasma supernatant was used as PPP and the thrombocyte pellet in 1.0 ml of plasma was used as PRP. Platelets were activated with thrombin (1 U/ml) and 0.5 M of calcium chloride [45]. An activating 1:1 (v/v) mixture of calcium chloride and thrombin was prepared in advance. A 10:1 (v/v) mixture of PRP or PPP and activator was incubated for 10 min at RT. Activated samples are designated as activated (a) PRP or PPP and samples not activated are designated as non-activated (na)PRP or PPP. As platelets will not survive extended storage, aPRP and aPPP were centrifuged again at 3200 rpm for 15 min and the resulting supernatant was stored at − 20 °C until further use.
WST-1 proliferation assay
WST-1 assay was used to assess metabolic activity of all primary cells and cell lines investigated herein. Cells were seeded at a density of 5 × 104 cells/well in 96-well plates in triplicate and cultured overnight (n = 3 in each group and each cell type). After media change, the cells were incubated for total of three (eSF and eMSC) or seven (BM-MSC and Ishikawa) days, depending on the growth rate. Ten microliters of WST-1 reagent (Roche Diagnostics, Laval, Quebec, Canada) was added to each well and incubated for another 4 h at 37 °C (incubation time verified by preliminary experiments; data not shown). The absorbance was determined using a microplate reader at a test wavelength of 450 nm and reference wavelength of 650 nm (Bio-Rad Laboratories, Hercules, CA).
PRP dose-finding experiments in eSF proliferation assay
eSF were seeded at a density of 5 × 104 cells/well in 96-well plates. After overnight serum starvation, the cells were cultured in serum-free DMEM supplemented with 0 (control), 1, 5, 10, and 20% aPRP and aPPP for 3 and 5 days. WST-1 proliferation assay was performed as described above, with results demonstrating no significant differences between proliferation potential in the 1, 5, and 10% groups, and decrease in proliferation at 20% PRP concentration (data not shown). Since 5% PRP use was also reported previously in studies on dermal fibroblasts [34, 44], we used that concentration in our experiments.
Scratch wound-healing assay
Cells were seeded in 6- or 12-well plates with 1 × 105 cells per well and cultured to confluency (n = 3 for eSF, eMSC, and IC and n = 2 for BM-MSC, in triplicate or duplicate). Monolayers of confluent cells were scratched with a 100-μl pipette tip without damaging the plastic and then washed with PBS to remove non-adherent cells, as described [46]. The wells were divided according to the study protocol into control, aPRP, naPRP, aPPP, and naPPP groups and treated accordingly, with incubation times of 24 h for eSF and eMSC, 48 h for BM-MSC, and 72 h for Ishikawa cells. The total incubation time was identified by preliminary experiments to evaluate the time needed for complete closure of the wound line. Migration was monitored by time-lapsed microscopy using a Leica DFC360FX 1.4-megapixel monochrome digital camera mounted to a Leica DMI6000B inverted microscope fitted with a motorized stage and digital camera (DFC360FX) powered by a Leica CTR6500 HS electronics box. Sequential images for three randomly selected areas were acquired per well per experimental group every 6–24 h as programmed for different cell types, at ×100 magnification, and the coordinates were saved for time-lapse imaging.
The wound repair was assessed by calculating the area in square micrometers between the lesion edges (the wound area) using the public domain ImageJ program developed at the National Institutes of Health (Bethesda, MD), and the average of data from duplicate/triplicate cultures were used for statistical analysis.
Transwell chemotaxis migration assay
For the Transwell migration assay, 3 × 103 cells were seeded on the upper surface of the polycarbonate Transwell filter (8-μm pore size, Corning, New York, NY) with serum-free DMEM (n = 3 for eSF, eMSC, and IC, and n = 2 for BM-MSC, in duplicate or triplicate) [47]. DMEM with vehicle and 5% aPRP, naPRP, aPPP, and naPPP were added to the lower chambers. The incubation time was 14 and 24 h for eSF and eMSC, 16 and 24 h for BM-MSC, and 24–96 h for Ishikawa cells. At the termination of the experiments, the upper membrane was carefully cleaned with a q-tip to remove all adherent cells, so that the cells on the upper surface of the membrane were not mistaken for the migrated cells on the bottom membrane of the insert and counted as such. Thereafter, membranes were washed twice in phosphate-buffered saline (PBS) and fixed with methanol for 5 min at − 20 °C. Then, the chambers were stained with 0.5% crystal violet solution for 15 min, air-dried, and photographed at ×200 magnification. A total of three fields were counted for each Transwell filter, and the average number of cells was used for statistical analysis.
Immunofluorescence
Immunocytochemistry analyses with cytokeratin and vimentin antibodies were performed to analyze possible mesenchymal-to-epithelial transition under the experimental conditions. Indirect immunofluorescence was conducted following previously reported methods [48]. Briefly, cells were cultured in 96-well plates until confluency and were divided into five groups and treated for 72 h according to the study protocol as above with vehicle, 5% aPRP, naPRP, aPPP, and naPPP. Cells were then fixed in 2% paraformaldehyde and 100% methanol and stored until use. Cells were permeabilized with 0.1% Triton X-100, blocked with 10% normal goat serum, and incubated overnight at 4 °C with the following primary antibodies: rabbit anti-human cytokeratin 18 (1:100; ab32118, Abcam, Cambridge, MA), rabbit anti-human cytokeratin 5 (1:100; ab24647, Abcam), mouse anti-human vimentin (1:100; 180,052, Life Technologies), and mouse anti-human E cadherin (1:100; ab1416, Abcam). Cells were then washed three times with PBS/0.1% Tween 20 buffer and incubated for 1 h at room temperature with the corresponding Alexa Fluor 488 conjugated goat anti-mouse, or 488 or 594 conjugated goat anti-rabbit secondary antibodies (1:250; A-11001 and A-11008 or A-11012, respectively, Invitrogen) and washed thereafter. Negative control wells were treated with the corresponding mouse or rabbit non-immune IgG. Results were viewed on a Leica DM 5000 microscope equipped with epifluorescence optics (Leica Microsystems, Inc.).
RNA isolation
Total RNA was purified using Qiagen RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions, and quantified by spectroscopy as previously described [36, 40]. Purity was analyzed by the 260/280 absorbance ratio and RNA integrity was assessed using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). For quantitative RT-PCR analysis, 1 μg of RNA was converted to complementary DNA (cDNA) using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, USA).
Quantitative RT-PCR with multiplex Fluidigm array
All cDNA samples from cultured eSF, eMSC, BM-MSC, and Ishikawa cells in control, aPRP, naPRP, aPPP, and naPPP groups (n = 3 in each group, in duplicate) were assayed by q-RT-PCR using the Fluidigm Dynamic Array Integrated Fluidic Circuits and the BioMark HD system (www.fluidigm.com/biomark-system.html) as previously described [40, 49]. Briefly, cDNA was pre-amplified to generate a pool of target genes using Taq-Man Pre-Amp master mix (Applied Biosystems), 100 ng cDNA, and 500 nM for each primer pair. Samples were then treated with exonuclease (Exonuclease I; New England BioLabs) per protocol and diluted 1:5 in a Tris-ethylenediaminetetraacetic acid dilution buffer (TEKnova) using previously generated optimal dilution curves. q-RT-PCR was performed using SsoFast Evagreen supermix with low ROX binding dye (Biotium Inc.) at a primer concentration of 5 μM (primers were designed by Fluidigm). Data were processed by user-detected threshold settings and linear baseline correction using Biomark real-time PCR Analysis software (version 3.0.4). The YWHAZ housekeeping gene was used as a normalizer. The comparative (ΔΔ) Ct method was used to calculate relative fold changes (docs.appliedbiosystems.com/pebiodocs/04303859.pdf).
Statistical analysis
Statistical analysis for the WST-1 proliferation assay, scratch migration, and Transwell migration assays and for the quantitative RT-PCR was performed using two-way repeated measures ANOVA with post hoc Tukey-Kramer test for multiple pairwise comparison corrections. Statistical significance was determined at p ≤ 0.05.
Results
Activated platelet-rich plasma promotes cell proliferation
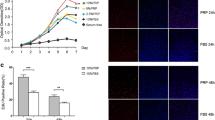
In the WST-1 assay, the absorbance values indicative of relative cell proliferation showed an increase in stromal and mesenchymal cell proliferation by activated PRP versus non-activated PRP and PPP (p < 0.05), while Ishikawa epithelial cell proliferation was significantly affected by both activated, but not non-activated, PRP and PPP (p < 0.05) (Fig. 2). It is important to note that while aPRP had the most significant effect on proliferation of all cell types studied, naPRP as well as aPPP and naPPP also showed various degrees of increased proliferation when compared to control cells after 3 days (Fig. 2). eSF and BM-MSC demonstrated significantly higher proliferation in all groups compared to control after 3 (eSF) and 7 (BM-MSC) days, respectively, while such significant effect was seen in eMSC in aPPP and naPRP groups, and in aPPP and naPPP in Ishikawa cells after 3 days. The decreased proliferation of Ishikawa cells on the seventh day may be explained by confluent culture.
Time-dependent proliferative effect of PRP and PPP on endometrial stromal fibroblasts (eSF), endometrial mesenchymal stem cells (eMSC), bone marrow-derived mesenchymal stem cells (BM-MSCs), and Ishikawa cells assayed with the WST-1 assay. Absorbance values indicate relative cell proliferation. Data represent the mean ± SD. Asterisk indicates the significance at p < 0.05 compared with the control group. aPRP activated platelet-rich plasma, naPRP non-activated platelet-rich plasma, aPPP activated platelet-poor plasma, naPPP non-activated platelet-poor plasma
Activated platelet-rich plasma promote cell migration
Activated PRP promoted the migration of human eSF, eMSC, BM-MSC, and IC compared to non-activated PRP, PPP, and vehicle controls, in both wound healing (Fig. 3 and images in Supplemental Fig. 1) and chemotaxis assays (Fig. 4 and images in Supplemental Fig. 2), at various time points studied (p < 0.05). As shown in both figures, Ishikawa cells required substantially longer time to close the wound or to migrate through the pores of a Transwell membrane. The difference in migration was largely not significant after short-time exposure, but reached significance upon longer exposure in all cell types in both migration assays. It is worth noting that there were significant differences in the migration potential of different cell types studied herein between the control (untreated) group and naPRP, aPPP, and naPPP groups for the most part as well (p < 0.01; Figs. 3 and 4), signifying the overall stimulatory effect of human platelet-rich or platelet-poor plasma on endometrial cell migration.
a Wound-healing assays for endometrial stromal fibroblasts (eSF), endometrial mesenchymal stem cells (eMSC), bone marrow-derived mesenchymal stem cells (BM-MSCs), and Ishikawa cells. Graphs show the percentage of covered surface (n = 3 images per well, in duplicate or triplicate, n = 3 for each cell type) for the control and treatment groups. Data represent the mean ± SD. Statistical significance accepted at p ≤ 0.05. Significant difference between different time points within the same treatment groups indicated by the same letter. Asterisk indicates significant difference compared to the respective time point in the aPRP group. aPRP activated platelet-rich plasma, naPRP non-activated platelet-rich plasma, aPPP activated platelet-poor plasma, naPPP non-activated platelet-poor plasma. b Transwell migration assays for endometrial stromal fibroblasts (eSF), endometrial mesenchymal stem cells (eMSC), bone marrow-derived mesenchymal stem cells (BM-MSCs), and Ishikawa cells at different time points. Graphs show the average number of migrated cells (n = 3 images per insert, in duplicate or triplicate, n = 3 for each cell type) for the control and treatment groups. Data represent the mean ± SD. Statistical significance accepted at p ≤ 0.05. Significant difference between different time points within the same treatment group is indicated by the same letter. Asterisk indicates significant difference compared to the respective time point in the aPRP group. aPRP activated platelet-rich plasma, naPRP non-activated platelet-rich plasma, aPPP activated platelet-poor plasma, naPPP non-activated platelet-poor plasma. The y axis range is not unified so that the smaller-scale changes remain apparent to the reader
mRNA expression of a MMPs (MMP 1, 3, 7, 26); b transgelin; c interleukins (IL1A, IL1B) and receptor IL1R2, and chemokines (CCL5, CCL7, and CXCL13); and d EGFR, FGFR2, PDGFRB, in endometrial stromal fibroblasts (eSF), endometrial mesenchymal stem cells (eMSC), bone marrow-derived mesenchymal stem cells (BM-MSCs) upon treatment with PRP or PPP, normalized to vehicle controls. Statistical significance accepted at p ≤ 0.05. Data represent the mean ± SD. Asterisk indicates significant difference compared to the aPRP group. aPRP activated platelet-rich plasma, naPRP non-activated platelet-rich plasma, aPPP activated platelet-poor plasma, naPPP non-activated platelet-poor plasma. The y axis range is not unified so that the smaller-scale changes remain apparent to the reader
Platelet involvement in endometrial regeneration may be mediated by inflammation and chemoattraction
We analyzed gene expression differences in all cell types and groups studies herein. Interestingly, there was no significant difference in estrogen or progesterone receptor gene expression between cells treated with or without PRP or PPP in all cell types studied (data not shown). In contrast, expression of matrix metalloproteinases MMP3, MMP7, and MMP26 were increased in the PRP and PPP groups in eSF with significant differences with control and between aPRP and other groups (p < 0.05), while in eMSC MMP1, MMP3 and MMP26 were significantly upregulated compared to controls and between aPRP and other groups (Fig. 4a). There was no difference in MMP2 and MMP7 transcript expression in any cell type studied, and no difference in messenger RNA (mRNA) expression of MMPs in BM-MSC or Ishikawa cells (data not shown). The cell transformation marker transgelin (TAGL) mRNA was upregulated in eSF in aPRP versus naPRP or aPPP (p < 0.05) (Fig. 4b), with no difference in E-cadherin (CDH1) expression (data not shown). Inflammation markers IL1A, IL1B, and IL1R2 were significantly upregulated in the aPRP group in eSF compared to all other groups; while in eMSC IL1A, IL15, and IL1R2 followed the same pattern of expression (Fig. 4c; non-significant data for BM-MSC and Ishikawa cells (latter not shown)). Several chemokines (CCL5, CCL7, and CXCL13) but not CCL2, CCL3, CCL4, or IL8 (data not shown) were upregulated in aPRP versus control, aPPP, naPRP, or naPPP-treated eSF, while eMSC demonstrated significant upregulation of CCL5 and CCL7 in aPRP compared to other groups (p < 0.05; Fig. 4c; non-significant data for BM-MSC and Ishikawa cells not shown). MMP7, TAGL, IL1B, and CXCL13 transcripts were not significantly regulated in eMSC (data not shown).
Transcripts for some growth factor receptors such as EGFR, PDGFRB, and FGFR2 were analyzed in eSF, eMSC, BM-MSC, and IC, and the results demonstrated various degrees of upregulation (Fig. 4d), with EGFR showing most consistent upregulation in response to treatment with aPRP in all cell types (Fig. 4d).
Mesenchymal-to-epithelial transition in eMSC and BM-MSC at mRNA or protein level
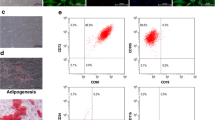
Under the experimental conditions tested, PRP did not induce changes in major terminal MET endpoints in eSF or eMSC, as shown by sustained expression of vimentin mRNA and protein, with no induction of KRT7 mRNA expression and absent cytokeratin 18 immunoreactivity (Fig. 5).
a Immunofluorescent analysis of vimentin (Vim) and cytokeratin 18 (KRT18) protein in human endometrial mesenchymal stem cells (eMSC) in control cultures and cultures exposed to 5% PRP or PPP. Magnification, ×200. Negative controls are presented as inserts for vimentin and cytokeratin 18, respectively. No difference was observed between the groups. Data on endometrial stromal fibroblasts (eSF) or bone marrow-derived mesenchymal stem cells (BM-MSCs) not shown. b mRNA expression of cytokeratin 7 (KRT7) in eMSC, eSF, and BM-MSC and vimentin (VIM) in eSF. Significance accepted at p ≤ 0.05. Data represent the mean ± SD. Vimentin mRNA data for eMSC or BM-MSC not shown (no significant difference). aPRP activated platelet-rich plasma, naPRP non-activated platelet-rich plasma, aPPP activated platelet-poor plasma, naPPP non-activated platelet-poor plasma
Discussion
General comments
The uterine endometrium is unique among adult human tissues in that it undergoes physiologic cyclic shedding and subsequent regeneration without scarring at roughly monthly intervals throughout women’s reproductive years. The successful execution of this remarkable recurrent tissue repair process requires the coordinated involvement of fibroblasts, epithelial, endothelial, and adult stem/progenitor cells, and their responses to local cues from the tissue microenvironment, including cell proliferation, migration, lineage differentiation, and transdifferentiation through MET [50, 51]. Disturbances of this tightly regulated homeostatic balance have been implicated in endometrial pathologies, infertility, and poor pregnancy outcomes [52].
In this context, the current study addressed the fundamental questions of whether and how a therapeutic intervention may affect these specific cellular processes towards alleviating related endometrial pathologies of inadequate growth or intracavitary scarring. Accordingly, our study evaluated for the first time the effects of PRP on biological responses of different human endometrial cells, and confirmed previously published data on BM-MSC. The main finding of the study is the demonstrated stimulatory effect of PRP on endometrial cell proliferation and migration, as well as expression of several factors potentially involved in endometrial regeneration and repair. Because stimulation of proliferation and migration is more robust when using a combination of growth factors rather than single agents [53], our approach in this in vitro study was to evaluate the effect of PRP, a complex mixture of key agents involved in these processes, on different cell types involved in endometrial regeneration. Another interesting finding, not entirely unexpected, was that not only aPRP but also naPRP and PPP did stimulate endometrial cell proliferation and migration. This demonstrated that even plasma that was relatively poor in its growth factor and cytokine content was able to stimulate key cellular processes, which could be an important asset in clinical situation.
PRP stimulates endometrial cell proliferation and migration
PRP has been shown to promote proliferation of human adipose-derived stem cells, human dermal fibroblasts, human synovial cells, human gingival fibroblasts, osteoblast-like cells, and stromal cells [34, 45, 54,55,56] among others. We were interested in evaluating if PRP (activated, non-activated, or both) exerts a similar effect on human endometrial stromal fibroblasts and other cell types in the endometrium. The WST-1 assay demonstrated that PRP stimulates the growth of all endometrial cell types studied (eSF, eMSC, and Ishikawa cells), as well as BM-MSC. Moreover, the scratch assay, which reflects the ability of cells to engage in wound healing, demonstrated the stimulating effect of aPRP (and even PPP) in this regard in all cell types studied. The Transwell assay demonstrated that particularly aPRP can mobilize endometrial cells by chemoattraction. Thus, our data demonstrate that PRP promotes endometrial cell proliferation and migration—characteristics that are fundamental for endometrial regeneration. Of note is that while all treatment groups (aPRP, naPRP, aPPP, and naPPP) affected proliferation and migration processes to various degrees, aPRP had the greatest and most consistent effect. Similar to data on other cell types, these effects are likely mediated via growth factor receptors, confirmed herein by upregulation of some of them in treated cells by aPRP.
Interestingly, different cells types tested here demonstrated more or less similar proliferative response to PRP, while migratory responses were more pronounced in eSFs, followed very closely by eMSC and then BM-MSC, whereas IC had longer doubling time and required much longer time for migration in our experimental settings. Whether this represents the order of first responders in the process of tissue repair is unclear and warrants further investigation, as well as potential effect that unique PRP contents from different patients could have on the rate of endometrial repair and regeneration. On the same note, further experiments can address if different doses of PRP may result in different proliferation rate of various endometrial residual and circulating cell types.
Transgelin is a cytoplasmic protein that is highly expressed in fibroblasts and participates in processes associated with a remodeling of the actin cytoskeleton, including cell migration, proliferation, differentiation, apoptosis, and matrix remodeling [57]. It is expressed in human endometrium and is overexpressed in ectopic endometriotic lesions [58]. Significant upregulation of its transcript in eSF upon aPRP and even naPRP treatment suggests its potential involvement in the fibroblast activation morphological transformation of cells with migration and proliferation under these conditions. In contrast to our findings with eSF from women without any gynecologic disorder, prolonged exposure of endometriotic and adenomyosis lesions to activated platelets results in fibrosis due to epithelial-to-mesenchymal transition, fibroblast-to-myofibroblast transdifferentiation, and repeated injury and healing [59, 60]. These processes are mediated by TGF-β1 and activation of TGF-β/SMAD signaling pathway, and anti-platelet therapy in a mouse model was shown to decrease the burden of endometriosis and adenomyosis [61, 62]. These data underscore baseline molecular differences between endometriotic/adenomyotic ectopic endometrial cells compared to eutopic endometrial cells from subjects without such pathology [36, 63], resulting in different molecular and functional responses when exposed to activated platelets. Whether eutopic endometrium from subjects with endometriosis/adenomyosis will respond similarly to ectopic cells or normal eutopic endometrial cells is currently unknown. Of note, the focus of the current study—i.e., processes resulting in endometrial regeneration in the setting of thin endometrium and endometrial scarring—is fundamentally different from endometriosis/adenomyosis, wherein ectopic endometrial cells are exposed repeatedly to bleeding and activated platelets. This clinical feature can explain the differences in the in vitro and anticipated in vivo results.
Inflammation, wound healing, and regeneration
PRP has been shown to attenuate fibronectin-induced expression of several pro-inflammatory chemokines and MMPs in human meniscocytes and articular chondrocytes, thus supporting use of PRP in the management of cartilage and meniscal injuries [64]. It was also proposed as a potential treatment for endometritis in cattle based on its abilities to downregulate the expression of pro-inflammatory genes in primary cultures of bovine endometrial cells after 48 h of incubation [65], and decreased intrauterine inflammatory response in mares [66]. In the present study, we observed a stimulatory effect of PRP on the expression of several pro-inflammatory cytokines, chemokines, and MMPs (Fig. 4), while others (IL8, IL1RL1; data not shown) did not significantly change. Inflammation is a hallmark of the first phase of wound healing, which is marked by platelet accumulation, coagulation, and leukocyte migration and mediated by inflammatory cytokines and growth factors [67]. Interestingly, post-menstrual repair is characterized by re-epithelialization and angiogenesis, processes that play a vital role in endometrial regeneration. While AS and thin lining patients do not have a wound within the uterine cavity per se, similar processes for wound healing and tissue regeneration prevail, and our results of activation of cytokine/chemokine gene expression support the effects of PRP in promoting these processes.
On the other hand, in the postpartum endometrial repair, which is characterized by massive endometrial regeneration, MET of endometrial progenitor cells has been described as an important mechanism [68]. However, in the present in vitro study on normal endometrial or progenitor cells, we did not observe any evidence of MET, which could probably be explained by a “smaller scale” or repair needed in our in vitro conditions.
Matrix metalloproteinases (MMPs) are involved in tissue regeneration and wound healing via degradation of extracellular matrix (ECM) and wound remodeling [69]. A wide range of cytokines and growth factors known to activate MMPs [70] are present in PRP. In our study, PRP and, to some extent, PPP increased expression of the matrix-degrading enzymes MMP1, MMP3, MMP7, and MMP26 (Fig. 4). This correlates well with previous data on human dermal fibroblasts and tenocytes [71, 72]. MMP7 in particular is required for re-epithelization of mucosal wounds [69], which adds to the significance of its upregulation in endometrial stromal cells upon PRP treatment in our study.
PRP effect of sex hormone receptor expression
In bovine endometrium, PRP upregulates progesterone receptor protein in glandular epithelial cells when administered to dairy cows compared to untreated controls [65]. In vitro, PRP upregulated the gene expression of ERalpha, ERbeta, and the progesterone receptor in cultured bovine endometrial cells [65], leading the authors to suggest that intrauterine infusion of PRP in cattle may improve their fertility [65, 73]. Activated platelets also induced ERbeta (the predominant ER in mediating estrogen action in endometriosis), mRNA, and protein expression in endometriotic stromal cells from ovarian endometrioma [74]. In our study, we did not observe differential expression of estrogen or progesterone receptor transcripts in response to PRP or PPP treatment likely due to species differences and different source of human cells used compared to above studies.
Clinical translation
While designing and performing this pre-clinical study, increased endometrial thickness and increased pregnancy rates were reported by others when PRP was infused intrauterine in patients with a thin endometrial lining in the setting of IVF [19, 20]. It is unclear from these studies, in the absence of investigating underlying mechanisms, whether the improved pregnancy rates were mainly due to improved endometrial regeneration or endometrial receptivity, or both. Thus, the current study provides the fundamental mechanistic, molecular, and cellular paradigm underlying the promise of PRP to improve pregnancy rates in women with scarred or thin endometrium.
Strengths and limitations
While providing major novel information, our study has both strengths and limitations. Strengths include the first comprehensive evaluation of the effect of PRP on different human endometrial cell types. The major limitation is the inability to obtain sufficient cultures of primary endometrial epithelial cells for the experiments, thus necessitating substitution of these with the Ishikawa endometrial adenocarcinoma cell line. While Ishikawa cells are used widely in endometrial research and do express estrogen and progesterone receptors, the data obtained should be interpreted with caution as this is an epithelial cancer cell line. Another limitation of the study is that we did not evaluate the concentrations of the reportedly main growth factors such as PDGF and TGFβ in PRP, which is going to be addressed in follow-up study. In addition, while we chose to analyze here PCR primers relevant to endometrial biology and previously validated in our and others studies evaluating endometrial gene expression in the mid-secretory phase and in endometrial stromal and mesenchymal stem cells, microarray analysis would be an important next step in the future to get a more comprehensive and unbiased assessment of genes and pathways affected.
An additional limitation of the study is that we isolated PRP from a single white 59-year-old male blood donor. While in clinical applications autologous PRP would be anticipated for testing in women with AS and/or thin lining, we chose to use PRP from a male donor in order to avoid possible effect of circulating hormones, which can fluctuate based on menstrual cycle phase (latter information is not provided to and by the blood bank). In addition, we did not expect that the age of the blood donor would have any significant effect on study results; however, this can be evaluated in follow-up studies using pooled blood from several donors. Despite these limitations, our study is the first, to our knowledge, to evaluate the effect of PRP on functional characteristics of endometrial cells, providing the fundamental mechanistic foundation and proof-of-principle for future controlled clinical studies.
In conclusion, we demonstrated herein that PRP enhances migration and proliferation of human endometrial epithelial (Ishikawa) cells, endometrial stromal fibroblasts, endometrial mesenchymal stem cells, and bone marrow-derived mesenchymal stem cells. These data provide an initial ex vivo proof of principle for the use of autologous PRP to promote endometrial regeneration and warrant pre-clinical studies in animal models and subsequently in the clinical setting. We believe that our results support the potential value of using PRP for endometrial regeneration in clinical settings with compromised endometrial growth, such as in Asherman’s syndrome and an atrophic or thin endometrial lining.
References
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. https://doi.org/10.1371/journal.pmed.1001356.
Horcajadas JA, Diaz-Gimeno P, Pellicer A, Simon C. Uterine receptivity and the ramifications of ovarian stimulation on endometrial function. Semin Reprod Med. 2007;25(6):454–60. https://doi.org/10.1055/s-2007-991043.
Labarta E, Martinez-Conejero JA, Alama P, Horcajadas JA, Pellicer A, Simon C, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26(7):1813–25. https://doi.org/10.1093/humrep/der126.
Moreno I, Codoner FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazan J, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215(6):684–703. https://doi.org/10.1016/j.ajog.2016.09.075.
Kumbak B, Erden HF, Tosun S, Akbas H, Ulug U, Bahceci M. Outcome of assisted reproduction treatment in patients with endometrial thickness less than 7 mm. Reprod BioMed Online. 2009;18(1):79–84.
Abdalla HI, Brooks AA, Johnson MR, Kirkland A, Thomas A, Studd JW. Endometrial thickness: a predictor of implantation in ovum recipients? Hum Reprod. 1994;9(2):363–5.
Dessolle L, Darai E, Cornet D, Rouzier R, Coutant C, Mandelbaum J, et al. Determinants of pregnancy rate in the donor oocyte model: a multivariate analysis of 450 frozen-thawed embryo transfers. Hum Reprod. 2009;24(12):3082–9. https://doi.org/10.1093/humrep/dep303.
Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(4):530–41. https://doi.org/10.1093/humupd/dmu011.
Lebovitz O, Orvieto R. Treating patients with “thin” endometrium—an ongoing challenge. Gynecol Endocrinol. 2014;30(6):409–14. https://doi.org/10.3109/09513590.2014.906571.
Schenker JG. Etiology of and therapeutic approach to synechia uteri. Eur J Obstet Gynecol Reprod Biol. 1996;65(1):109–13.
Fernandez H, Al-Najjar F, Chauveaud-Lambling A, Frydman R, Gervaise A. Fertility after treatment of Asherman's syndrome stage 3 and 4. J Minim Invasive Gynecol. 2006;13(5):398–402. https://doi.org/10.1016/j.jmig.2006.04.013.
Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J Hum Reprod Sci. 2011;4(1):43–8. https://doi.org/10.4103/0974-1208.82360.
Amer MI, Abd-El-Maeboud KH, Abdelfatah I, Salama FA, Abdallah AS. Human amnion as a temporary biologic barrier after hysteroscopic lysis of severe intrauterine adhesions: pilot study. J Minim Invasive Gynecol. 2010;17(5):605–11. https://doi.org/10.1016/j.jmig.2010.03.019.
Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–96. https://doi.org/10.1093/humrep/dew042.
Chen MJ, Yang JH, Peng FH, Chen SU, Ho HN, Yang YS. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. 2006;23(7–8):337–42. https://doi.org/10.1007/s10815-006-9053-1.
Weckstein LN, Jacobson A, Galen D, Hampton K, Hammel J. Low-dose aspirin for oocyte donation recipients with a thin endometrium: prospective, randomized study. Fertil Steril. 1997;68(5):927–30.
Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78(5):1073–6.
Gleicher N, Kim A, Michaeli T, Lee HJ, Shohat-Tal A, Lazzaroni E, et al. A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum Reprod. 2013;28(1):172–7. https://doi.org/10.1093/humrep/des370.
Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8(1):1286–90.
Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod. 2017;21(1):54–6. https://doi.org/10.5935/1518-0557.20170013.
Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42(3):610–8. https://doi.org/10.1177/0363546513518416.
Jo CH, Shin JS, Lee YG, Shin WH, Kim H, Lee SY, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blind, parallel-group trial. Am J Sports Med. 2013;41(10):2240–8. https://doi.org/10.1177/0363546513497925.
Cervelli V, Gentile P, Scioli MG, Grimaldi M, Casciani CU, Spagnoli LG, et al. Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15(4):625–34. https://doi.org/10.1089/ten.TEC.2008.0518.
Picard F, Hersant B, Bosc R, Meningaud JP. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: a review and a proposal for a new standard care. Wound Repair Regen. 2015;23(5):638–43. https://doi.org/10.1111/wrr.12317.
Langer HF, Stellos K, Steingen C, Froihofer A, Schonberger T, Kramer B, et al. Platelet derived bFGF mediates vascular integrative mechanisms of mesenchymal stem cells in vitro. J Mol Cell Cardiol. 2009;47(2):315–25. https://doi.org/10.1016/j.yjmcc.2009.03.011.
Stellos K, Seizer P, Bigalke B, Daub K, Geisler T, Gawaz M. Platelet aggregates-induced human CD34+ progenitor cell proliferation and differentiation to macrophages and foam cells is mediated by stromal cell derived factor 1 in vitro. Semin Thromb Hemost. 2010;36(2):139–45. https://doi.org/10.1055/s-0030-1251497.
Gargett CE, Chan RW, Schwab KE. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288(1–2):22–9. https://doi.org/10.1016/j.mce.2008.02.026.
Matsumoto H, Nasu K, Nishida M, Ito H, Bing S, Miyakawa I. Regulation of proliferation, motility, and contractility of human endometrial stromal cells by platelet-derived growth factor. J Clin Endocrinol Metab. 2005;90(6):3560–7. https://doi.org/10.1210/jc.2004-1918.
Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16(11):818–34. https://doi.org/10.1093/molehr/gaq061.
Kaitu'u-Lino TJ, Ye L, Salamonsen LA, Girling JE, Gargett CE. Identification of label-retaining perivascular cells in a mouse model of endometrial decidualization, breakdown, and repair. Biol Reprod. 2012;86(6):184. https://doi.org/10.1095/biolreprod.112.099309.
Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–6. https://doi.org/10.1634/stemcells.2006-0828.
Aghajanova L, Horcajadas JA, Esteban FJ, Giudice LC. The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol Reprod. 2010;82(6):1076–87. https://doi.org/10.1095/biolreprod.109.082867.
Ikoma T, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201(6):608 e1–8. https://doi.org/10.1016/j.ajog.2009.07.026.
Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122(5):1352–60. https://doi.org/10.1097/PRS.0b013e3181882046.
Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod. 2009;80(1):105–14.
Aghajanova L, Horcajadas JA, Weeks JL, Esteban FJ, Nezhat CN, Conti M, et al. The protein kinase a pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology. 2010;151(3):1341–55.
Chen JC, Hoffman JR, Arora R, Perrone LA, Gonzalez-Gomez CJ, Vo KC, et al. Cryopreservation and recovery of human endometrial epithelial cells with high viability, purity, and functional fidelity. Fertil Steril. 2016;105(2):501–10.e1. https://doi.org/10.1016/j.fertnstert.2015.10.011.
Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB. Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril. 1989;52(5):761–8.
Spitzer TL, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86(2):58. https://doi.org/10.1095/biolreprod.111.095885.
Barragan F, Irwin JC, Balayan S, Erikson DW, Chen JC, Houshdaran S, et al. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol Reprod. 2016;94(5):118. https://doi.org/10.1095/biolreprod.115.136010.
Gurung S, Werkmeister JA, Gargett CE. Inhibition of transforming growth factor-beta receptor signaling promotes culture expansion of undifferentiated human endometrial mesenchymal stem/stromal cells. Sci Rep. 2015;5:15042. https://doi.org/10.1038/srep15042.
Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, Hovatta O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril. 2011;95(1):230–7. 7 e1-2
Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212–8. https://doi.org/10.3343/kjlm.2011.31.3.212.
Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, et al. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. 2011;23(4):424–31. https://doi.org/10.5021/ad.2011.23.4.424.
Kushida S, Kakudo N, Suzuki K, Kusumoto K. Effects of platelet-rich plasma on proliferation and myofibroblastic differentiation in human dermal fibroblasts. Ann Plast Surg. 2013;71(2):219–24. https://doi.org/10.1097/SAP.0b013e31823cd7a4.
Hulkower KI, Herber RL. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics. 2011;3(1):107–24. https://doi.org/10.3390/pharmaceutics3010107.
Liao Y, He X, Qiu H, Che Q, Wang F, Lu W, et al. Suppression of the epithelial-mesenchymal transition by SHARP1 is linked to the NOTCH1 signaling pathway in metastasis of endometrial cancer. BMC Cancer. 2014;14:487. https://doi.org/10.1186/1471-2407-14-487.
Chen JC, Erikson DW, Piltonen TT, Meyer MR, Barragan F, McIntire RH, et al. Coculturing human endometrial epithelial cells and stromal fibroblasts alters cell-specific gene expression and cytokine production. Fertil Steril. 2013;100(4):1132–43. https://doi.org/10.1016/j.fertnstert.2013.06.007.
Tamaresis JS, Irwin JC, Goldfien GA, Rabban JT, Burney RO, Nezhat C, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986–99. https://doi.org/10.1210/en.2014-1490.
Patterson AL, Zhang L, Arango NA, Teixeira J, Pru JK. Mesenchymal-to-epithelial transition contributes to endometrial regeneration following natural and artificial decidualization. Stem Cells Dev. 2013;22(6):964–50. https://doi.org/10.1089/scd.2012.0435.
Cousins FL, Murray A, Esnal A, Gibson DA, Critchley HO, Saunders PT. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS One. 2014;9(1):e86378. https://doi.org/10.1371/journal.pone.0086378.
Deane JA, Gualano RC, Gargett CE. Regenerating endometrium from stem/progenitor cells: is it abnormal in endometriosis, Asherman’s syndrome and infertility? Curr Opin Obstet Gynecol. 2013;25(3):193–200. https://doi.org/10.1097/GCO.0b013e32836024e7.
Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. https://doi.org/10.1038/nature07039.
van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA. Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006;12(11):3067–73. https://doi.org/10.1089/ten.2006.12.3067.
Lee JK, Lee S, Han SA, Seong SC, Lee MC. The effect of platelet-rich plasma on the differentiation of synovium-derived mesenchymal stem cells. J Orthop Res. 2014;32(10):1317–25. https://doi.org/10.1002/jor.22668.
Creeper F, Ivanovski S. Effect of autologous and allogenic platelet-rich plasma on human gingival fibroblast function. Oral Dis. 2012;18(5):494–500. https://doi.org/10.1111/j.1601-0825.2011.01897.x.
Dvorakova M, Nenutil R, Bouchal P. Transgelins, cytoskeletal proteins implicated in different aspects of cancer development. Expert Rev Proteomics. 2014;11(2):149–65. https://doi.org/10.1586/14789450.2014.860358.
Dos Santos Hidalgo G, Meola J, Rosa ESJC, Paro de Paz CC, Ferriani RA. TAGLN expression is deregulated in endometriosis and may be involved in cell invasion, migration, and differentiation. Fertil Steril. 2011;96(3):700–3. https://doi.org/10.1016/j.fertnstert.2011.06.052.
Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol Cell Endocrinol. 2016;428:1–16. https://doi.org/10.1016/j.mce.2016.03.015.
Liu X, Shen M, Qi Q, Zhang H, Guo SW. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum Reprod. 2016;31(4):734–49. https://doi.org/10.1093/humrep/dew018.
Zhu B, Chen Y, Shen X, Liu X, Guo SW. Anti-platelet therapy holds promises in treating adenomyosis: experimental evidence. Reprod Biol Endocrinol. 2016;14(1):66. https://doi.org/10.1186/s12958-016-0198-1.
Zhang Q, Liu X, Guo SW. Progressive development of endometriosis and its hindrance by anti-platelet treatment in mice with induced endometriosis. Reprod BioMed Online. 2017;34(2):124–36. https://doi.org/10.1016/j.rbmo.2016.11.006.
Herndon CN, Aghajanova L, Balayan S, Erikson D, Barragan F, Goldfien G, et al. Global transcriptome abnormalities of the eutopic endometrium from women with adenomyosis. Reprod Sci. 2016;23(10):1289–303. https://doi.org/10.1177/1933719116650758.
Wang CC, Lee CH, Peng YJ, Salter DM, Lee HS. Platelet-rich plasma attenuates 30-kDa fibronectin fragment-induced chemokine and matrix metalloproteinase expression by meniscocytes and articular chondrocytes. Am J Sports Med. 2015;43(10):2481–9. https://doi.org/10.1177/0363546515597489.
Marini MG, Perrini C, Esposti P, Corradetti B, Bizzaro D, Riccaboni P, et al. Effects of platelet-rich plasma in a model of bovine endometrial inflammation in vitro. Reprod Biol Endocrinol. 2016;14(1):58. https://doi.org/10.1186/s12958-016-0195-4.
Reghini MF, Ramires Neto C, Segabinazzi LG, Castro Chaves MM, Dell’Aqua Cde P, Bussiere MC, et al. Inflammatory response in chronic degenerative endometritis mares treated with platelet-rich plasma. Theriogenology. 2016;86(2):516–22. https://doi.org/10.1016/j.theriogenology.2016.01.029.
Broughton G II, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):12S–34S. https://doi.org/10.1097/01.prs.0000225430.42531.c2.
Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7(8):e44285. https://doi.org/10.1371/journal.pone.0044285.
Caley MP, Martins VL, O'Toole EA. Metalloproteinases and wound healing. Adv Wound Care (New Rochelle). 2015;4(4):225–34. https://doi.org/10.1089/wound.2014.0581.
Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211(1):19–26. https://doi.org/10.1002/jcp.20948.
de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171–8. https://doi.org/10.1177/0363546508314430.
Shin MK, Lee JW, Kim YI, Kim YO, Seok H, Kim NI. The effects of platelet-rich clot releasate on the expression of MMP-1 and type I collagen in human adult dermal fibroblasts: PRP is a stronger MMP-1 stimulator. Mol Biol Rep. 2014;41(1):3–8. https://doi.org/10.1007/s11033-013-2718-9.
Lange-Consiglio A, Cazzaniga N, Garlappi R, Spelta C, Pollera C, Perrini C, et al. Platelet concentrate in bovine reproduction: effects on in vitro embryo production and after intrauterine administration in repeat breeder cows. Reprod Biol Endocrinol. 2015;13:65. https://doi.org/10.1186/s12958-015-0064-6.
Zhang Q, Ding D, Liu X, Guo SW. Activated platelets induce estrogen receptor beta expression in endometriotic stromal cells. Gynecol Obstet Investig. 2015;80(3):187–92. https://doi.org/10.1159/000377629.
Acknowledgments
We thank Dr. Joshua Robinson, PhD (UCSF), for his help with time-lapse microscopy.
Financial support
Funding was provided by IntegraMed Fertility 2016 Research Grant (LA), NIH NCTRI P50HD055764 (LCG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplemental Figure 1
Wound-healing assays for endometrial stromal fibroblasts (eSF, A), endometrial mesenchymal stem cells (eMSC, B), bone marrow-derived mesenchymal stem cells (BM-MSC, C) and Ishikawa cells (D). Representative images were obtained at 100× magnification. Images show the relative migration distance after incubation at different time points. aPRP = activated platelet rich plasma, naPRP = non-activated platelet rich plasma, aPPP = activated platelet poor plasma, naPPP = non-activated platelet poor plasma. (PDF 3982 kb)
Supplemental Figure 2
Transwell migration assays for endometrial stromal fibroblasts (eSF, A), endometrial mesenchymal stem cells (eMSC, B), bone marrow-derived mesenchymal stem cells (BM-MSC, C) and Ishikawa cells (D) at different time points. Representative images were obtained at 200× magnification. aPRP = activated platelet rich plasma, naPRP = non-activated platelet rich plasma, aPPP = activated platelet poor plasma, naPPP = non-activated platelet poor plasma. (PDF 2600 kb)
Rights and permissions
About this article
Cite this article
Aghajanova, L., Houshdaran, S., Balayan, S. et al. In vitro evidence that platelet-rich plasma stimulates cellular processes involved in endometrial regeneration. J Assist Reprod Genet 35, 757–770 (2018). https://doi.org/10.1007/s10815-018-1130-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1130-8