Abstract
Purpose
The purpose of this study was to summarize the latest advances and successes in the field of ovarian tissue cryopreservation while identifying gaps in current knowledge that suggest opportunities for future research.
Methods
A systematic review was performed according to PRISMA guidelines for all relevant full-text articles in PubMed published in English that reviewed or studied historical or current advancements in ovarian tissue cryopreservation and auto-transplantation techniques.
Results
Ovarian tissue auto-transplantation in post-pubertal women is capable of restoring fertility with over 80 live births currently reported with a corresponding pregnancy rate of 23 to 37%. The recently reported successes of live births from transplants, both in orthotopic and heterotopic locations, as well as the emerging methods of in vitro maturation (IVM), in vitro culture of primordial follicles, and possibility of in vitro activation (IVA) suggest new fertility options for many women and girls. Vitrification, as an ovarian tissue cryopreservation technique, has also demonstrated successful live births and may be a more cost-effective method to freezing with less tissue injury. Further, transplantation via the artificial ovary with an extracellular tissue matrix (ECTM) scaffolding as well as the effects of sphingosine-1-phosphate (SIP) and fibrin modified with heparin-binding peptide (HBP), heparin, and a vascular endothelial growth factor (VEGF) have demonstrated important advancements in fertility preservation. As a fertility preservation method, ovarian tissue cryopreservation and auto-transplantation are currently considered experimental, but future research may pave the way for these modalities to become a standard of care for women facing the prospect of sterility from ovarian damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
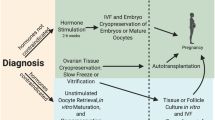
Recent reports indicate that approximately 300,000 young children, adolescents, and teens under the age of 19 will be diagnosed with cancer worldwide with the most common childhood malignancies being leukemia, brain and central nervous system cancers, and lymphoma [1, 2]. Improvements in the diagnostic and treatment modalities in prepubertal girls with cancer have led to increased survivorship among reproductive age females [3–5]. Given this increased life expectancy, greater emphasis has been placed on improved quality of life, with a specific focus on future fertility [4, 6]. As such, the field of oncofertility was established with a dedicated purpose to preserve, expand, and restore the reproductive future of individuals whose cancer treatments may compromise fertility [6, 7]. Although it is currently considered experimental, ovarian tissue cryopreservation and auto-transplantation is the only fertility preservation treatment modality available to prepubertal girls. In this review, we examine the current status of ovarian tissue cryopreservation, highlighting significant historical landmarks and recent major advancements while identifying gaps in current knowledge and their implications for research endeavors and further advancements.
Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a systematic review of the literature was performed from inception until January of 2017 for all relevant full-text articles published in PubMed in English to evaluate both the historical events and current advancements for ovarian tissue cryopreservation. The following electronic search strategy was performed in PubMed: ((Ovarian tissue cryopreservation [MESH]) AND ovarian tissue auto-transplantation) AND English [lang]). The published and peer-reviewed full-text articles identified from this search were evaluated by reviewing the titles and abstracts. Only the full-text articles which reviewed or studied historical or current advancements in ovarian tissue cryopreservation and auto-transplantation techniques which aided in identifying gaps in our current knowledge were included. In addition, the bibliographies of the included articles were reviewed for further studies. Multiple reviewers (C.L., A.M., and N.D.) independently reviewed the included reports. Data from the text, graphs, and tables were analyzed.
Results
A total of 204 full-text articles were identified from the initial search. During the initial review of titles and abstracts of all 204 articles, 77 were excluded while the remaining 127 which met our inclusion criteria were evaluated. An additional 18 citations were included based on bibliographies from originally included articles in addition to relevant studies, organizational guidelines, and epidemiological data that were not identified in our initial search (Fig. 1).
Effects of chemotherapy and radiotherapy on reproductive potential
Both chemotherapy and radiotherapy can cause gonadotoxicity with oocyte depletion and ovarian damage, thereby decreasing reproductive potential. The chemotherapeutic effects on reproductive potential are largely based on the type, dose, and patient age at the time of administration [4, 6]. Although most chemotherapeutic agents are relatively gonadotoxic, the commonly used alkylating agents, such as cyclophosphamide, procarbazine, and busulfan, are particularly damaging [4, 8]. These agents are associated with ovarian toxicity regardless of cell cycle stage and are therefore associated with a higher risk of primordial follicle death compared to platinum-based drugs, plant alkaloids, and other anti-metabolites [9]. Regarding vulnerability to irradiation, factors placing a woman at increased risk include age, dose, and irradiation field [10]. Radiation-induced loss of primordial follicles results in premature ovarian failure with decreased hormone levels, early menopause, and uterine dysfunction [11]. A woman’s fertility depends on a functional neuroendocrine system that controls the menstrual cycle, promotes oocyte maturation, and can maintain a pregnancy [12, 13]. When the hypothalamic-pituitary axis is within the irradiation field, reproductive function may be decreased. Dependent on patient age, individuals may demonstrate alterations in the timing of puberty, gonadotropin deficiency, and hyperprolactinemia, thereby creating oligomenorrhea or amenorrhea [14]. At the gonadal level, direct toxicity to estrogen-producing follicles can disrupt normal menstrual cycling. Furthermore, radiation and chemotherapy have been found to disrupt uterine function. Specifically, radiation has been shown to reduce uterine volume and elasticity, induce arrested growth in prepubertal girls, inhibit uterine expansion during pregnancy, damage the uterine endometrium and myometrium, and decrease the vasculature required for embryo implantation and fetal development [11, 14, 15]. Studies have shown that some women who conceive after radiotherapy have increased risk of adverse outcomes, including placental anomalies, preterm birth, fetal growth restriction, and miscarriage [11].
Preserving reproductive potential
With funding from a National Institute of Health Roadmap Grant [16], the Oncofertility Consortium was established. Clinicians, scientists, and ethicists were brought together to create an interdisciplinary committee working to navigate the considerable ethical, legal, and religious concerns surrounding scientific advances in reproductive technologies. Recommendations state that healthcare providers need to address the probability of infertility after administration of gonadotoxic drugs and radiotherapy and specifically identify available methods of fertility preservation [16, 17] .
Although oocyte and embryo cryopreservation have demonstrated success, ovarian tissue cryopreservation and auto-transplantation may have advantages for fertility preservation in female cancer patients. Ovarian tissue cryopreservation does not require prior ovarian stimulation thereby allowing cancer treatments to begin immediately and is the only option available to prepubertal girls [5, 17–20].
Oocyte cryopreservation
At present, embryo and mature oocyte cryopreservation following egg retrieval are the only established methods of fertility preservation endorsed by the American Society for Reproductive Medicine [17]. Early studies reported difficulty with oocyte cryopreservation, due to their low surface area to volume ratio and high susceptibility to intracellular ice crystal formation [21]. Early research further highlighted difficulties in predicting the membrane permeability characteristics of human oocytes along with other biophysical parameters [22]. Studies also revealed the adverse effects of cryopreservation on the stability of microtubules and microfilaments in human oocytes, which are vital for normal chromosomal segregation [23–25]. Hardening of the zona pellucida and subsequent low fertilization rates were further difficulties initially associated with cryopreservation [26]. Later reports suggested that human oocytes had the potential to retain their morphology and chromosomal integrity post-cryopreservation [27]. Research into oocyte cryopreservation was accelerated by legislative restrictions surrounding the storage of embryos, particularly those in Italy that prevented the cryopreservation of excess embryos [28]. The introduction of vitrification as an alternative to slow freezing reduced damage to internal structures and led to improved success rates [29–32]. Further, the use of intracytoplasmic sperm injection (ICSI) as an insemination method for vitrified oocytes was found to rectify fertilization issues due to zona pellucida hardening [33–35]. The earliest report of a pregnancy achieved with cryopreserved oocytes was published by Chen in 1986 [36]. Oocyte cryopreservation has now become the main treatment modality for infertility patients [17].
The quiescent ovaries of prepubertal girls pose a unique fertility preservation challenge. Prepubertal girls who require high doses of systemic chemotherapy for cancer treatment are likely to lose their future ovarian function and fertility potential. Oocyte and embryo cryopreservation may not be an option for prepubertal girls for several reasons including the time requirement to begin cancer treatment, hormone-sensitive malignancies which may progress with ovarian stimulation protocols, and lack of a partner. Moreover, prepubertal ovaries may not respond to stimulating drugs used in assisted reproductive technology and therefore cannot undergo ovarian stimulation to have their oocytes cryopreserved. Currently, the only option to preserve future fertility in prepubertal girls is ovarian tissue cryopreservation [3, 4].
Ovarian tissue cryopreservation
Ovarian tissue cryopreservation and transplantation have been studied in animals for more than 60 years [37]. In 1954, Deanesly [38] demonstrated that rat ovarian tissue could be removed, frozen, and implanted subcutaneously in a different ovariectomized rat female, while still retaining its functionality. Given the larger size and fibrous character of human ovarian tissue, further animal studies were needed before techniques could be applied to humans. In 1996, after successful transplantations in sheep, whose ovaries are similar in character to humans, Newton and colleagues [39] grafted human ovarian cortical tissue into mice. Several experiments with human ovarian tissue transplanted into mice were further carried out with promising results [40, 41]. The first human ovarian transplantation with cryopreserved ovarian tissue was later performed by Dr. Oktay in 1999 [42]. In 2004, Donnez and colleagues reported the first live human birth from frozen ovarian tissue via the slow freeze method, followed by orthotopic auto-transplantation [43]. The first birth after ovarian tissue transplantation from tissue stored overnight at a central cryopreservation bank was performed by a center of the Fertiprotekt network in 2011 [44].
The majority of ovarian tissue cryopreservation procedures have been performed on prepubertal girls before undergoing gonadotoxic chemotherapy and or radiotherapy treatments. Cryopreserved ovarian tissue followed by auto-transplantation illustrates that resumption of endocrine function with initiation of puberty is possible [45]. Two such cases by Poirot et al. [46] and Ernest et al. [47] reported return of endocrine function with pubertal development after ovarian tissue transplantation in prepubertal girls. These reports, together with earlier studies in post-pubertal women, indicate that auto-transplantation of frozen-thawed ovarian tissue is useful for the restoration of endocrine function before and after puberty [48, 49].
Live births following cryopreservation of prepubertal ovarian tissue had not been described until the 2015 case report by Demeestere et al. [7] which documented the first live birth following an auto-transplantation of ovarian tissue extracted before the onset of menarche. Heterotopic and orthotopic ovarian fragments were auto-transplanted after which the patient conceived spontaneously [7]. To our knowledge, this is the only report providing evidence of in vivo spontaneous maturation of oocytes in thawed human ovarian tissue extracted during puberty and before the onset of menarche.
Given the experimental state of ovarian tissue cryopreservation and auto-transplantation, a significant portion of the literature demonstrating live births have been reported via case reports [7, 42, 50–62]. More recently, larger retrospective cohorts have reported success with both restoration of endocrine function and additional live births [18, 19, 63–67]. Two of the largest centers in the world performing transplantations of frozen-thawed ovarian tissue have reported a number of successful live births in women following transplantations. In 2015, Jensen et al. [68] published a retrospective cohort study of 41 women which demonstrated a 31% live birth rate after orthotopic transplanted ovarian tissue. Ovarian grafted tissue showed a life span of close to 10 years in some cases. The authors do note, however, the importance of further studies with longer observation periods to demonstrate the actual efficacy of ovarian function and lack of malignant relapse. Subsequently, Van der Ven et al. [69] performed a retrospective analysis of 74 orthotopic transplantation surgeries. The authors showed that following transplantations, the ovaries were active in 67% of cases after 1 year with clinical pregnancy and live birth rates of 33 and 25%, respectively. The authors were able to perform a subgroup analysis of patients undergoing a first transplantation with primary ovarian insufficiency with tissue activity of 63% after 1 year, with pregnancy and live birth rates of 28 and 23%, respectively [69]. To date, at least 80 live births have been reported globally following the transplantation of cryopreserved ovarian tissue with pregnancies achieved in 23 to 37% of cases [68–72].
Whole ovary cryopreservation
The first reports on human whole-ovary retrieval and freezing were not followed by active research in that area [73–75]. Similar to ovarian cortical tissue cryopreservation, whole ovary cryopreservation does not require ovarian stimulation prior to extraction, allowing cancer treatments to begin immediately. Further, whole ovary cryopreservation allows for spontaneous pregnancy or the possibility to pursue ovarian stimulation using in vitro fertilization within a paracrine milieu that resembles normal follicle and oocyte development. Tissue ischemia with follicular destruction, observed when ovarian cortical fragments are transplanted, may be overcome with whole ovary auto-transplantation, given the re-anastomosis of the transplanted vascular pedicle [20]. Disadvantages include possible re-implantation of malignancy, technical difficulties with vascular re-anastomosis and greater risk for cryoinjury. Whole ovary auto-transplantation may be challenging in humans given the larger surface area of ovarian tissue and the related difficulty with adequate diffusion of cryoprotective agents (CPAs) [20]. In 2014, however, Campbell et al. [76] demonstrated that full restoration of ovarian function with high rates of fertility in sheep could be obtained after whole-ovary cryopreservation and transplantation by optimizing cryopreservation penetration during perfusion, along with the use of anti-thrombotic agents to prevent post-operative clot formation in the ovarian vasculature. Martinez-Madrid et al. [73] described a cryopreservation protocol for an intact human ovary with its vascular pedicle involving a cryoprotective solution with slow freezing in a cryofreezing container. The authors demonstrated high survival rates of follicles and small vessels with a 75% survival after thawing. A 2008 report by Silber et al. [77] described a successful pregnancy in monozygotic twins after fresh whole ovary transplantation using microsurgical techniques for anastomosis. Although healthy offspring have been demonstrated after frozen-thawed whole ovary transplantation in sheep, to date auto-transplantation of frozen-thawed human whole ovary has not resulted in any live births. Future research identifying new cryochambers, protocols for administering cryoprotectants, and further surgical techniques for microvascular anastomosis may lead to this fertility sparing option in the future.
Ovarian tissue freezing and respective results
Cryopreservation preserves cells and tissues by decreasing the temperature, thereby slowing down metabolic reactions in the cells. However, independent of the freezing technique, cryoinjury may occur during cooling and warming, particularly in the temperature range of 0 to −15 °C, thereby compromising cell or tissue viability [78, 79]. Cryopreservation of ovarian tissue can be performed using one of two established techniques: slow freezing or rapid freezing (vitrification). The slow freezing method has already resulted in dozens of live births worldwide, whereas vitrified tissue has only led to a few reported live births to date [18, 67, 80, 81]. There is, however, a recent resurgence in research dedicated to establishing the technique of vitrification [63, 67, 82, 83].
Controlled slow freezing which was favored in earlier protocols takes the entire specimen and storage medium from liquid to solid. This well-established procedure begins with exposing cells to low concentrations of CPAs with slow decreases in temperatures. Oocytes are first cooled to a temperature of −5 to −7 °C, after which equilibration and seeding take place. This initial equilibration of cell/tissue in human serum albumin-containing medium typically utilizes one of three common CPAs: propanediol, dimethyl sulphoxide (DMSO), or ethylene glycol (EF). Multiple studies have demonstrated success using slow freezing techniques to cryopreserve oocytes [34, 84–88].
Slow freezing is a straightforward and efficient method; however, the cooling component of the procedure is time-consuming and requires expensive equipment in clinical practice in comparison to vitrification. Vitrification of the human ovarian tissue has been extensively studied [89–92]. In contrast to slow freezing, vitrification requires higher concentrations of CPA, which lowers the risk of ice nucleation and is significantly faster, with cooling rates of close to 5000 °C per minute before submersion into liquid nitrogen [93, 94]. In vitrification, a high concentration of CPA is added to the medium and therefore the viscosity of intracellular and extracellular solutions progressively increases, which decreases the diffusivity of water. When the solution is rapidly cooled, the tissue turns into a glassy, vitrified state, avoiding extracellular and intracellular ice crystallization (as in slow freezing) [95]. The disadvantage of vitrification is related to the relatively high concentrations of CPA. To reduce toxicity and excessive CPA permeation into the cells, recent studies reviewed by Amorim et al. have combined low concentrations of different CPAs to obtain a vitrifiable solution without compromising its cryoprotective capacity [96]. According to Amorim et al. [96], the most common permeable CPA for human ovarian tissue vitrification is ethylene glycol due to its low toxic effect and rapid diffusion into cells. On the other hand, non-permeable CPAs can increase viscosity and prevent water molecules from forming into ice crystals. Since CPAs do not penetrate the cells, cellular toxicity is theoretically decreased. Most studies of human ovarian tissue vitrification have added sucrose or another simple sugar (i.e., impermeable CPA) to their equilibration solutions since sugar molecules facilitate cellular dehydration to prevent intracellular ice crystallization and form hydrogen bonds with the phospholipids on the outer cellular membranes to reduce exposure to CPAs [96].
The first live birth following vitrification was achieved in 1999 [81] with Kuwayama et al. [97] later developing the widely used Cryotop® vitrification method in 2005. To date, the clinical outcomes of tissue vitrification are not inferior to those of the conventional slow freezing technique [96]. Recently, Sanfilippo et al. [98] compared slow freezing and vitrification protocols in ovarian tissue samples harvested from the same women. The investigators measured follicle density, morphology, and DNA fragmentation and found no difference between the two methods. However, due to its overall simplicity, the vitrification method is becoming more popular in clinical practice, as happened for vitrification of oocytes.
Orthotopic versus heterotopic transplantation
Traditionally, frozen-thawed ovarian cortical fragments have been transplanted orthotopically (into the remaining ovary, ovarian fossa, or broad ligament). Orthotopic transplantation may provide the ability to achieve a natural pregnancy; however, it requires abdominal surgery with general anesthesia [99]. In 2000, Oktay et al. [41] reported the first laparoscopic orthotopic transplantation with ovarian tissue placed into the pelvic peritoneum. Follicular development and ovulation resulted 15 weeks later with ovarian function continued for 9 months post-grafting. The first pregnancies resulting in live births after frozen-thawed orthotopic transplantation of ovarian tissue were reported by Donnez et al. [43] and Meirow et al. [54] in 2004 and 2005, respectively. Donnez and colleagues grafted ovarian tissue fragments onto preexisting ovarian tissue [43]. The findings by Donnez and colleagues were promising; however, questions later arose regarding whether the fertilized oocyte had ovulated from the grafted tissue, versus the preexisting ovarian tissue [99]. In a 2008 systematic review, Bedaiwy et al. [71] reported improved success with fresh ovarian tissue grafts versus frozen-thawed grafts. In their study, Bedaiwy et al. [71] reported an increased likelihood of ovarian function and a decreased likelihood of recurrent ovarian failure. Over 80 live births in addition to the restoration of endocrine function have been achieved with orthotopic transplantation by several centers worldwide [72].
Heterotopic transplantation (into the subcutaneous space of the forearm, subcutaneous tissue of the abdomen, anterior wall of the abdomen, just beneath the peritoneum, or in the rectus muscle) is advantageous in cases of severe pelvic adhesions, distorted pelvic anatomy, and poor pelvic vasculature due to previous irradiation (Table 1). Heterotopic transplantation has the possibility of creating long-term ovarian endocrine function with a less invasive surgical approach for transplantation which does not require general anesthesia thereby creating a more cost-effective option [100]. Heterotopic transplantation, however, may produce oocytes and therefore embryos with reduced quality as compared to orthotopic transplantation sites [100]. This outcome is likely related to the suboptimal environment of heterotopic sites in regards to pressure, temperature, decreased blood supply, and reduced paracrine factors. Schmidt et al. [101] reported findings after 12 auto-transplantations of cryopreserved ovarian tissue. Although the heterotopic site generated mature oocytes after stimulation, no pregnancies were reported compared to two live births obtained from orthotopic sites. Based on the current reports of live births after ovarian tissue transplantation, orthotopic locations are likely to produce improved fertility in comparison to heterotopic locations [18, 100]. In fact, only a small number of live births after a heterotopic transplantation of cryopreserved ovarian tissue have been reported. In two studies, Oktay et al. [102, 103] reported three live births from the same patient. These live births took place following a heterotopic transplantation in a woman who previously underwent preconditioning chemotherapy before hematologic stem cell transplantation. As the authors point out, caution must be exercised when interpreting the source of pregnancies because conception is possible following recovery of (in situ) ovarian function after aggressive chemotherapy [103]. However, despite the follicular activity and mittelschmerz-type pain observed in the in situ ovary, conception may have been aided by the heterotopic auto-transplanted graft [103]. Further studies are needed to assess the possible augmentation of fertility following heterotopic auto-transplantation.
Although restoration of ovarian function has been reported for both approaches, live births following bilateral oophorectomy had only been documented from orthotopic transplantations [104, 105] until the report by Stern et al. in 2013 [62]. Slices of cryopreserved frozen-thawed ovarian tissue were grafted into the right and left anterior abdominal walls after the slow freezing technique. Following ovarian stimulation and ICSI, the patient became pregnant which resulted in the healthy live birth of twins. The manuscript by Stern et al. [62] provides evidence that cryopreservation preserves follicular development and that standard ovarian function and pregnancy can occur from a “non-ovarian” transplantation site. Although these results are optimistic for women who do not have a suitable orthotopic site for grafting, future randomized controlled trials comparing transplantation sites are needed.
Tissue cryopreservation combined with other assisted reproductive technologies
Oocytes can be aspirated from antral follicles either in vivo or ex vivo from removed ovarian tissue. Vitrification after in vitro maturation (IVM) of immature oocytes obtained at the time of ovarian tissue cryopreservation may further enhance fertility preservation. Segers et al. [106] and Fasano et al. [107] successfully retrieved immature oocytes ex vivo from ovarian tissue, with maturation rates between 31 and 36%. In 2016, Park et al. [108] reported maturation rates of 67.9% after retrieval of immature oocytes ex vivo. Yin et al. [109] demonstrated an overall maturation rate of 29% after IVM of immature oocytes with maturation rates directly proportional to patient age. In contrast, Revel et al. [110] found that the maturation rate, as well as the number of oocytes obtained and cryopreserved, was not age dependent. Yin et al. [109] further demonstrated a 64% survival after vitrification and warming of the mature oocytes. Differences in maturation rates may be due to ischemic time, culture medium, cancer type, and ovarian tissue volume. Maman and Meirow et al. [111] recently evaluated IVM during the follicular and luteal phases specifically examining the number of oocytes, maturation and fertilization rates, and the number of oocytes and embryos frozen. The authors found no difference between the two groups, indicating that luteal phase IVM of immature oocytes may be offered when urgent fertility preservation is warranted.
Abir and colleagues [112] carried out IVM of immature oocytes and oocyte cryopreservation after ovarian tissue removal in female pediatric cancer patients both before and after chemotherapy. Antral follicles were manually aspirated for immature oocytes from the excised ovarian tissue after oophorectomy. Retrieved oocytes underwent incubation in a maturation medium. The ovarian tissue was slowly frozen, and the mature, intact oocytes were cryopreserved [112]. Abir et al. [112] demonstrated maturation rates of 32 and 26.4% after IVM before and after chemotherapy, indicating that fertility preservation may be possible after chemotherapy. Frozen mature oocytes as a gamete source for cancer survivors decreases the risk of reintroducing malignancy upon transfer [112–114].
In 2014, Prasath and colleagues [115] reported the first live birth from an immature oocyte that was aspirated ex vivo from extracted ovarian tissue and matured and fertilized in vitro. This patient had undergone a bilateral oophorectomy for ovarian cancer. Both of her ovaries were found to have cancer cells, and therefore, ovarian tissue auto-transplantation would not be an acceptable option. Recent reports from in vitro fertility laboratories have shown success with IVM with a 20 to 35% live birth rate from frozen IVM oocytes [108, 116–119]. Further studies evaluating the protocols and culture media may improve maturation rates and possibly live birth rates. Reports have indicated that in vivo collected immature oocytes have improved quality, compared to ex vivo collection, likely due to meiotic disturbances after temperature shifts during removal [120]. A better understanding of the variables involved will help guide when and how immature oocytes can be collected.
To maximize their fertility potential, cancer patients with may undergo a modified ovarian stimulation regimen followed by egg retrieval before the extraction of ovarian tissue for cryopreservation and the initiation of chemotherapy. Hourvitz and colleagues reviewed the records of their institution and concluded that oocyte aspiration just prior to ovarian tissue cryopreservation yielded more oocytes, with a better maturation rate, than oocytes retrieved from ex vivo ovarian tissue [121]. The combination of ovarian tissue stimulation, egg retrieval, and cryopreservation can optimize the endocrine function and fertility potential. These recent advancements in the field of reproductive technology raise the question: which combination of procedures and techniques might offer the best chance of success for our patients?
With the integration of IVA (currently experimental) following ovarian tissue freezing, Kawamura and colleagues have recently demonstrated that some women with primary ovarian insufficiency (POI) can have live births following the activation of immature follicles from ovarian tissue formerly considered menopausal [67, 80]. The first live birth was reported in 2013 in a 29-year-old woman with idiopathic POI [80]. The IVA protocol is based on a considerable body of work by Dr. Hsueh and colleagues, which includes mechanical fragmentation of ovarian tissue and the incubation with Akt-stimulating drugs [122, 123]. In this method, both ovarian tissue fragmentation (leading to disruption of Hippo signaling) and the incubation with Akt-stimulating compounds were used to stimulate the growth of resting/primordial follicles. The mechanism of action of the Hippo signaling and Akt activation has been reviewed [124]. To date, more than 20 women with POI have had their frozen-thawed ovarian tissue treated with Akt-stimulating compounds. Following auto-transplantation in nine of them, the ovarian tissue responded to IVA treatment, and two out of these nine women had a successful live birth [67]. Additionally, according to a recent review by Kawamura et al. [125], two more patients had undergone similar treatments and subsequently conceived. These results suggest that the remaining follicles from vitrified menopausal ovarian tissue can be stimulated in vitro, reach maturity in vivo, regain sufficient endocrine function, and produce live births. While the idea is promising, uncertainty remains regarding potential negative effects of the drugs involved on oocyte quality. In addition, in interpreting successful live births following IVA in patients with POI, one must also consider the 5% spontaneous pregnancy rate in women with confirmed POI [126].
Potential complications/adverse events
Dysfunctional folliculogenesis following ovarian transplantation has been described [127–130]. Reasons for the dysfunction include asynchrony between granulosa cells and oocyte maturation, oocyte damage, impaired hormonal balance due to elevated follicular stimulating hormone levels, reduced ovarian reserve, a delay before efficient revascularization of the graft, and specific post-grafting activation. Ischemia is likely responsible for follicular loss, as revascularization after transplantation may take up to 48 h in rodents and up to 5 days in humans [128].
Soleimani et al. [127] evaluated the effects of SIP on follicular loss during revascularization after ovarian tissue transplantation. They specifically addressed whether SIP could enhance neo-angiogenesis and follicular survival in a xenograft model. Human tissue xenografts were placed in severe combined immunodeficient mice and treated with SIP. The authors found an increased vascular density, accelerated angiogenic process, significant proliferation of ovarian stromal cells, and reduced necrosis and tissue hypoxia which resulted in a lower percentage of apoptotic follicles compared to controls [127]. Shikanov et al. [128] concurrently evaluated the decreased follicular pool seen after ovarian tissue transplantation due to ischemic death. The authors promoted angiogenesis in a mouse model via a biomaterial-based system. Vitrified/thawed ovarian tissue was encapsulated in fibrin modified with heparin-binding peptide (HBP), heparin, and a vascular endothelial growth factor (VEGF). The treatment group demonstrated twice as many functioning primordial follicles, increased blood vessels, and natural conception with live offspring [128]. Further research identifying other methods to promote angiogenesis will likely serve to increase reproductive potential by decreasing ischemia and follicular loss in the immediate period after transplantation.
Oktay et al. [129] recently reported a live birth after ovarian tissue transplantation with a human extracellular matrix scaffold. This framework is thought to aid in the revascularization of the cryopreserved ovarian tissue and therefore further enhance fertility after tissue freezing. The authors extensively studied the viability of the ECTM with ovarian tissue in a series of preclinical evaluations. They first determined the appropriate ECTM thickness by comparing different sizes in thawing media while evaluating its effectiveness with ovarian cortical tissue from organ donor cadavers. They later xenografted ovarian tissue with ECTM to immunodeficient mice. After 10 days, the ovarian stroma had integrated into the ECTM. Finally, they cultured mouse oocytes with ECTM and compared them to controls. There was no difference in oocyte survival when ECTM was used. In this translational work, they evaluated two subjects over a 14-year follow-up period. Each patient had a laparoscopic oophorectomy with cryopreservation of ovarian cortical strips via the slow freeze protocol. Transplantation with ECTM was later carried out laparoscopically. Both patients underwent in vitro fertilization with one ongoing pregnancy at the time of their writing and one reported live birth [129]. Given that this is the first live birth reported to date using this protocol, more research is needed to demonstrate continued success.
Re-implantation of malignant cells together with the grafted ovarian tissue remains a serious concern (Table 1). A review published in 2013 [131] examined all available evidence of malignancy risk, particularly in patients with leukemia, which is the most common hematological cancer in women below 20 years of age, followed by Hodgkin’s lymphoma and non-Hodgkin’s lymphoma. Polymerase chain reaction (PCR) has demonstrated genetic material consistent with leukemia in ovarian tissue, leading researchers to adopt a restrictive approach regarding transplantation. Although further study is required, leukemia patients who are in complete remission may be candidates for ovarian tissue transplantation. In animal studies, mice that were transplanted with PCR-negative tissue showed no evidence of malignancy [132]. The concept of an artificial ovary may be useful in cases in which malignant cells are suspected to be in the harvested ovarian tissue. Selective transplantation and maturation of healthy follicles in an artificial ovary could reduce or eliminate the possibility of reintroducing malignant cells to a patient who has been cured and wishes to conceive.
An additional fertility sparing approach involves in vitro culture of primordial follicles with ovarian tissue cryopreservation. Theoretically, ovarian follicles are cultured to produce mature oocytes capable of fertilization [133]. Obrien et al. have shown successful live births following in vitro development of murine primordial follicles [134]. Telfer et al. [135] demonstrated accelerated follicular growth of pre-antral follicles when cultured in the presence of activin A compared with controls. The same group further demonstrated that 30% of the surviving follicles cultured in activin A exhibited normal morphology with intact oocytes [135]. A later study by Hornick et al. [136] evaluating primordial follicles demonstrated further progress. Their study demonstrated that follicular survival and morphology were optimal when cultured in 2% alginate. Ovarian tissue was maintained for up to 24 h at 4 °C without compromising follicular health. In 2013, Lerer-Serfaty et al. [137] suggested improvements may be possible with a two-step culturing system as discussed by Telfer et al. [135, 137]. A short-term culture of ovarian tissue with PEG-fibrinogen hydrogels to develop secondary follicles for later isolation and culture may improve results. Further developments in culture media and strategies for in vitro maturation of primordial human follicles may offer unique fertility sparing options.
Discussion
In 2013 the American Society of Clinical Oncology (ASCO) updated their guidelines stating that healthcare providers need to address the probability of infertility after administration of gonadotoxic drugs and radiotherapy and specifically identify available methods of fertility preservation [138]. In this review, we summarized the latest advances and successes in the field of ovarian tissue cryopreservation, highlighted gaps in current knowledge, and pointed to opportunities for future research. Ovarian tissue auto-transplantation in post-pubertal women is capable of restoring fertility and has resulted in spontaneous conceptions, with over 80 live births currently reported with pregnancy rates of 23 to 37% [68–72, 82]. In contrast to oocyte cryopreservation, ovarian tissue freezing and transplantation are still considered experimental [17]. Nonetheless, the recently reported successes with live births from transplants both in orthotopic and heterotopic locations as well as the emerging methods of IVM, in vitro culture of primordial follicles, and possibility of IVA suggest new fertility options for many women and girls [7, 50–62, 138]. Further, transplantation via the artificial ovary with an ECTM scaffolding to improve revascularization as well as the effects of SIP and fibrin-HBP-VEGF to promote neo-angiogenesis and follicular survival are promising important advancements in fertility preservation. Further research addressing whole ovary cryopreservation and auto-transplantation with focused attention on CPAs and avoidance of cryoinjury may lead to improved graft survival. Further evaluation of in vivo versus ex vivo aspiration of immature oocytes for IVM to identify risk factors for decreased oocyte quality may lead to improved maturation rates. Given the limited live births reported in the literature, additional research reviewing the factors affecting heterotopic transplantation sites may lead to increased ovarian (graft) function post transplantation. Additional studies evaluating culture media and techniques for in vitro culturing of primordial follicles and drugs used in the IVA protocols may lead to new fertility preservation options for young women. Research identifying those factors which effect post-transplantation tissue ischemia and follicular loss may lead to long-term ovarian function with increased reproductive potential. Evaluating indications for ovarian tissue cryopreservation as well as the possibility of ovarian tissue cryopreservation after chemotherapy will expand the number of patients who may benefit from this treatment modality. Further studies addressing these identified gaps are likely to improve ovarian tissue cryopreservation and auto-transplantation, thereby enhancing the endocrine and reproductive functions of women with conditions which cause ovarian damage.
Although the primary goal of ovarian cryopreservation is fertility preservation, transplantation of frozen-thawed ovarian tissue may be used for other purposes as well. Bedaiwy et al. [71] reported success after ovarian tissue transplantation for primary ovarian failure. Other indications may include autoimmune and hematological diseases which are treated with chemotherapy, endometriosis, benign ovarian lesions, or those undergoing a prophylactic oophorectomy [99].
The quantitative likelihood for a live birth following ovarian tissue cryopreservation and auto-transplantation has not yet been firmly established [83, 139–141]. The actual number of women who have undergone auto-transplantation and the number of transplantation attempts in each patient have not been systematically reported. Furthermore, the majority of live births that are reported come from ovarian tissues that were cryopreserved by the slow freezing method rather than vitrification. Additionally, the majority of women did not undergo bilateral oophorectomy. Therefore, although less likely, a conception from any functional ovarian tissue rather than from the transplant cannot be ruled out. Previous authors have suggested that a voluntary national registry would be a valuable resource as it would provide accurate statistics regarding ovarian tissue cryopreservation and transplantation results [18, 45].
The technique of ovarian tissue cryopreservation offers hope to preserve the fertility for the millions of girls and young women who will become cancer survivors. The field of tissue cryopreservation is evolving and is likely to provide additional achievements in the near future. Progress will likely be fueled in part by advances in angiogenesis and biomechanics of tissue engineering as evidenced by the first live birth after ovarian tissue transplantation utilizing a human de-cellularized extracellular tissue matrix as a scaffold. While ovarian tissue cryopreservation and auto-transplantation, as a fertility preservation method, is currently experimental, further research may allow this modality to become a standard of care for women facing the prospect of sterility from ovarian destruction caused by various diseases, including cancer. Improvements in techniques, aided by assisted reproduction, will likely fuel the development of highly efficient, patient-friendly approaches.
References
International Agency for Research on Cancer. https://www.iarc.fr. 2016.
American Cancer Society. https://www.cancer.org/research/cancer-facts-statistics.html. 2016.
Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6(4):209–18. doi:10.1016/S1470-2045(05)70092-9.
Levine JM, Kelvin JF, Quinn GP, Gracia CR. Infertility in reproductive-age female cancer survivors. Cancer. 2015;121(10):1532–9. doi:10.1002/cncr.29181.
Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod. 2014;29(9):1931–40. doi:10.1093/humrep/deu158.
Salama M, Isachenko V, Isachenko E, Rahimi G, Mallmann P. Updates in preserving reproductive potential of prepubertal girls with cancer: systematic review. Crit Rev Oncol Hematol. 2016;103:10–21. doi:10.1016/j.critrevonc.2016.04.002.
Demeestere I, Simon P, Dedeken L, Moffa F, Tsepelidis S, Brachet C, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30(9):2107–9. doi:10.1093/humrep/dev128.
Dunlop CE, Anderson RA. Uses of anti-Mullerian hormone (AMH) measurement before and after cancer treatment in women. Maturitas. 2015;80(3):245–50. doi:10.1016/j.maturitas.2014.12.005.
Gunasheela S, Gunasheela D. Preventative management of infertility caused by treatment of malignancy. In: Gunasheela S, editor. Practical management of gynecological problems. 2nd ed. New Delhi: Jaypee Brothers Medical Pub; 2011. pp. 233–49.
Meirow D, Biederman H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53(4):727–39. doi:10.1097/GRF.0b013e3181f96b54.
Critchley HO, Wallace WH. Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr. 2005;34:64–8. doi:10.1093/jncimonographs/lgi022.
McCartney C, Marshall J. Neuroendocrinology of reproduction. In: Strauss JF, Barbieri RL, editors. Yen & Jaffe’s reproductive endocrinology: Physiology, pathophysiology, and clinical management. Philadelphia, PA: Elsevier/Saunders; 2014. pp. 3–26.
Hall J. Neuroendocrine control of the menstrual cycle. In: Strauss JF, Barbieri RL, editors. Yen & Jaffe’s reproductive endocrinology: Physiology, pathophysiology, and clinical management. Philadelphia, PA: Elsevier/Saunders; 2014. pp. 141–56.
Ogilvy-Stuart AL, Shalet SM. Effect of radiation on the human reproductive system. Environ Health Perspect. 1993;101(Suppl 2):109–16.
Critchley HO, Bath LE, Wallace WH. Radiation damage to the uterus—review of the effects of treatment of childhood cancer. Hum Fertil (Camb). 2002;5(2):61–6.
Waimey KE, Duncan FE, Su HI, Smith K, Wallach H, Jona K, et al. Future directions in oncofertility and fertility preservation: a report from the 2011 oncofertility consortium conference. J Adolesc Young Adult Oncol. 2013;2(1):25–30. doi:10.1089/jayao.2012.0035.
Practice Committee of American Society for Reproductive M. Ovarian tissue cryopreservation: a committee opinion. Fertil Steril. 2014;101(5):1237–43. doi:10.1016/j.fertnstert.2014.02.052.
Salama M, Woodruff TK. New advances in ovarian autotransplantation to restore fertility in cancer patients. Cancer Metastasis Rev. 2015;34(4):807–22. doi:10.1007/s10555-015-9600-2.
Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–13. doi:10.1016/j.fertnstert.2013.03.030.
Donnez J, Jadoul P, Squifflet J, Van Langendonckt A, Donnez O, Van Eyck AS, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):87–100. doi:10.1016/j.bpobgyn.2009.09.003.
Paynter SJ, Cooper A, Fuller BJ, Shaw RW. Cryopreservation of bovine ovarian tissue: structural normality of follicles after thawing and culture in vitro. Cryobiology. 1999;38(4):301–9. doi:10.1006/cryo.1999.2170.
Hunter J, Bernard A, Fuller B, McGrath J, Shaw RW. Plasma membrane water permeabilities of human oocytes: the temperature dependence of water movement in individual cells. J Cell Physiol. 1992;150(1):175–9. doi:10.1002/jcp.1041500123.
Pickering SJ, Braude PR, Johnson MH, Cant A, Currie J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril. 1990;54(1):102–8.
Chen SU, Lien YR, Chao KH, Ho HN, Yang YS, Lee TY. Effects of cryopreservation on meiotic spindles of oocytes and its dynamics after thawing: clinical implications in oocyte freezing—a review article. Mol Cell Endocrinol. 2003;202(1–2):101–7.
Glenister PH, Wood MJ, Kirby C, Whittingham DG. Incidence of chromosome anomalies in first-cleavage mouse embryos obtained from frozen-thawed oocytes fertilized in vitro. Gamete Res. 1987;16(3):205–16. doi:10.1002/mrd.1120160303.
Johnson MH, Pickering SJ, George MA. The influence of cooling on the properties of the zona pellucida of the mouse oocyte. Hum Reprod. 1988;3(3):383–7.
Gook DA, Osborn SM, Bourne H, Johnston WI. Fertilization of human oocytes following cryopreservation; normal karyotypes and absence of stray chromosomes. Hum Reprod. 1994;9(4):684–91.
Ragni G, Allegra A, Anserini P, Causio F, Ferraretti AP, Greco E, et al. The 2004 Italian legislation regulating assisted reproduction technology: a multicentre survey on the results of IVF cycles. Hum Reprod. 2005;20(8):2224–8. doi:10.1093/humrep/dei011.
Antinori M, Licata E, Dani G, Cerusico F, Versaci C, Antinori S. Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod BioMed Online. 2007;14(1):72–9.
Cao YX, Xing Q, Li L, Cong L, Zhang ZG, Wei ZL, et al. Comparison of survival and embryonic development in human oocytes cryopreserved by slow-freezing and vitrification. Fertil Steril. 2009;92(4):1306–11. doi:10.1016/j.fertnstert.2008.08.069.
Fadini R, Brambillasca F, Renzini MM, Merola M, Comi R, De Ponti E, et al. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod BioMed Online. 2009;19(2):171–80.
Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–95. doi:10.1016/j.fertnstert.2009.12.065.
Kazem R, Thompson LA, Srikantharajah A, Laing MA, Hamilton MP, Templeton A. Cryopreservation of human oocytes and fertilization by two techniques: in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1995;10(10):2650–4.
Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997;68(4):724–6.
Palermo GD, Cohen J, Rosenwaks Z. Intracytoplasmic sperm injection: a powerful tool to overcome fertilization failure. Fertil Steril. 1996;65(5):899–908.
Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–6.
Parrott DM. The fertility of mice with orthotopic ovarian grafts derived from frozen tissue. J Reprod Fertil. 1960;1:230–41.
Deanesly R. Immature rat ovaries grafted after freezing and thawing. J Endocrinol. 1954;11(2):197–200.
Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11(7):1487–91.
Oktay K, Newton H, Mullan J, Gosden RG. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod. 1998;13(5):1133–8.
Oktay K, Karlikaya GG, Aydin BA. Ovarian cryopreservation and transplantation: basic aspects. Mol Cell Endocrinol. 2000;169(1–2):105–8.
Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342(25):1919. doi:10.1056/NEJM200006223422516.
Donnez J, Dolmans MM, Demylle D, Jadout P, Pirad C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–10.
Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97 (2):387–90. doi:10.1016/j.fertnstert.2011.11.047.
Kolp LA, Hubayter Z. Autotransplantation of cryopreserved ovarian tissue: a procedure with promise, risks, and a need for a registry. Fertil Steril. 2011;95(6):1879–86. doi:10.1016/j.fertnstert.2011.02.049.
Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379(9815):588. doi:10.1016/S0140-6736(11)61781-9.
Ernst E, Kjaersgaard M, Birkebaek NH, Clausen N, Andersen CY. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer. 2013;49(4):911–4. doi:10.1016/j.ejca.2012.09.028.
Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18(2):59–67. doi:10.1093/molehr/gar082.
Resetkova N, Hayashi M, Kolp LA, Christianson MS. Fertility preservation for prepubertal girls: update and current challenges. Curr Obstet Gynecol Rep. 2013;2(4):218–25. doi:10.1007/s13669-013-0060-9.
Ernst E, Bergholdt S, Jorgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25(5):1280–1. doi:10.1093/humrep/deq033.
Demeestere I, Simon P, Moffa F, Delbaere A, Englert Y. Birth of a second healthy girl more than 3 years after cryopreserved ovarian graft. Hum Reprod. 2010;25(6):1590–1. doi:10.1093/humrep/deq096.
Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Van Langendonckt A, et al. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril. 2011a;95(5):1787 e1–4. doi:10.1016/j.fertnstert.2010.11.041.
Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98(3):720–5. doi:10.1016/j.fertnstert.2012.05.017.
Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–21. doi:10.1056/NEJMc055237.
Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Yemini Z, et al. Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: endocrine studies, in vitro fertilization cycles, and live birth. Fertil Steril. 2007;87(2):418 e7–e15. doi:10.1016/j.fertnstert.2006.05.086.
Burmeister L, Kovacs GT, Osianlis T. First Australian pregnancy after ovarian tissue cryopreservation and subsequent autotransplantation. Med J Aust. 2013;198(3):158–9.
Sanchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escriba MJ, Simon C, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93(1):268 e11–3. doi:10.1016/j.fertnstert.2009.09.046.
Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93(7):2413 e15–9. doi:10.1016/j.fertnstert.2009.12.022.
Revelli A, Marchino G, Dolfin E, Molinari E, Delle Piane L, Salvagno F, et al. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertil Steril. 2013;99(1):227–30. doi:10.1016/j.fertnstert.2012.09.029.
Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008a;23(7):1531–7. doi:10.1093/humrep/den032.
Rodriguez-Wallberg KA, Karlstrom PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, et al. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet Gynecol Scand. 2015;94(3):324–8. doi:10.1111/aogs.12568.
Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Hum Reprod. 2014;29(8):1828. doi:10.1093/humrep/deu119.
Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. J Assist Reprod Genet. 2014;31(11):1557–64. doi:10.1007/s10815-014-0331-z.
Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011b;43(6):437–50. doi:10.3109/07853890.2010.546807.
Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertil Steril. 2015;103(2):462–8. doi:10.1016/j.fertnstert.2014.10.045.
Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94(6):2191–6. doi:10.1016/j.fertnstert.2009.12.073.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–15. doi:10.1093/humrep/deu353.
Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedders, Ernest E et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum reprod. 2015;30(12):2838–45. doi:10.1093/humrep/dev230.
Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krussel J, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;31(9):2031–41. doi:10.1093/humrep/dew165.
Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet. 2016;33(12):1595–603. doi:10.1007/s10815-016-0814-1.
Bedaiwy MA, El-Nashar SA, El Saman AM, Evers JL, Sandadi S, Desai N, et al. Reproductive outcome after transplantation of ovarian tissue: a systematic review. Hum Reprod. 2008;23(12):2709–17. doi:10.1093/humrep/den301.
Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2016; doi:10.1007/s10815-016-0843-9.
Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82(5):1390–4. doi:10.1016/j.fertnstert.2004.06.036.
Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12(5):519–35. doi:10.1093/humupd/dml032.
Jadoul P, Donnez J, Dolmans MM, Squifflet J, Lengele B, Martinez-Madrid B. Laparoscopic ovariectomy for whole human ovary cryopreservation: technical aspects. Fertil Steril. 2007;87(4):971–5. doi:10.1016/j.fertnstert.2006.10.012.
Campbell BK, Hernandez-Medrano J, Onions V, Pincott-Allen C, Aljaser F, Fisher J, et al. Restoration of ovarian function and natural fertility following the cryopreservation and autotransplantation of whole adult sheep ovaries. Hum Reprod. 2014;29(8):1749–63. doi:10.1093/humrep/deu144.
Silber SJ, Grudzinskas G, Gosden RG. Successful pregnancy after microsurgical transplantation of an intact ovary. N Engl J Med. 2008b;359(24):2617–8. doi:10.1056/NEJMc0804321.
Mullen SF, Critser JK. The science of cryobiology. Cancer Treat Res. 2007;138:83–109.
Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17(3):243–56.
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110(43):17474–9. doi:10.1073/pnas.1312830110.
Kuleshova L, Gianaroli L, Magli C, Ferraretti A, Trounson A. Birth following vitrification of a small number of human oocytes: case report. Hum Reprod. 1999;14(12):3077–9.
Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32(8):1167–70.
Stoop D, Cobo A, Silber S. Fertility preservation for age-related fertility decline. Lancet. 2014;384(9950):1311–9. doi:10.1016/S0140-6736(14)61261-7.
Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16(3):411–6.
Borini A, Bianchi V, Bonu MA, Sciajno R, Sereni E, Cattoli M, et al. Evidence-based clinical outcome of oocyte slow cooling. Reprod BioMed Online. 2007;15(2):175–81.
Borini A, Bonu MA, Coticchio G, Bianchi V, Cattoli M, Flamigni C. Pregnancies and births after oocyte cryopreservation. Fertil Steril. 2004;82(3):601–5. doi:10.1016/j.fertnstert.2004.04.025.
Bianchi V, Coticchio G, Distratis V, Di Giusto N, Flamigni C, Borini A. Differential sucrose concentration during dehydration (0.2 mol/l) and rehydration (0.3 mol/l) increases the implantation rate of frozen human oocytes. Reprod BioMed Online. 2007;14(1):64–71.
Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93(2):391–6. doi:10.1016/j.fertnstert.2009.02.067.
Isachenko V, Isachenko E. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen. Cryo Letters. 2002;23(5):333–44.
Isachenko E, Isachenko V. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen Eur J Obstet Gynecol Reprod Biol. 2003;108(2):186–93.
Rahimi G, Isachenko E. Effect of different vitrification protocols for human ovarian tissue on reactive oxygen species and apoptosis. Reprod Fertil Dev. 2003;15(6):343–9.
Rahimi G, Isachenko E. Comparision of necrosis in human ovarian tissue after conventional slow freezing or vitrification. Reprod Biomed Online. 2004;9(2):187–93.
Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141(1):1–19. doi:10.1530/REP-10-0236.
Fahy GM. Vitrification: a new approach to organ cryopreservation. Prog Clin Biol Res. 1986;224:305–35.
Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21(4):407–26.
Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod BioMed Online. 2011;23(2):160–86. doi:10.1016/j.rbmo.2011.04.005.
Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod BioMed Online. 2005;11(5):608–14.
Sanfilippo S, Canis M, Smitz J, Sion B, Darcha C, Janny L, et al. Vitrification of human ovarian tissue: a practical and relevant alternative to slow freezing. Reprod Biol Endocrinol. 2015;13:67. doi:10.1186/s12958-015-0065-5.
Sonmezer M, Oktay K. Orthotopic and heterotopic ovarian tissue transplantation. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):113–26. doi:10.1016/j.bpobgyn.2009.09.002.
Soares M, Dolmans MM, Donnez J. Heterotopic ovarian tissue transplantation. In: Suzuki N, Donnez J, editors. Gonadal tissue cryopreservation in fertility preservation. Japan: Springer. 2016. pp. 105–23.
Schmidt KT, Rosendahl M, Ernst E, Loft A, Andersen AN, Dueholm M, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril. 2011;95(2):695–701. doi:10.1016/j.fertnstert.2010.07.1080.
Oktay K. Spontaneous conceptions and live birth after heterotopic ovarian transplantation: is there a germline stem cell connection? Hum Reprod. 2006;21(6):1345–8. doi:10.1093/humrep/del007.
Oktay K, Turkcuoglu I, Rodriguez-Wallberg KA. Four spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: what is the explanation? Fertil Steril. 2011;95(2):804 e7–10. doi:10.1016/j.fertnstert.2010.07.1072.
Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15(6):649–65. doi:10.1093/humupd/dmp021.
Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best Pract Res Clin Obstet Gynaecol. 2014;28(8):1188–97. doi:10.1016/j.bpobgyn.2014.09.003.
Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32(8):1221–31. doi:10.1007/s10815-015-0528-9.
Fasano G, Moffa F, Dechene J, Englert Y, Demeestere I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod Biol Endocrinol. 2011;9:150. doi:10.1186/1477-7827-9-150.
Park CW, Lee SH, Yang KM, Lee IH, Lim KT, Lee KH, et al. Cryopreservation of in vitro matured oocytes after ex vivo oocyte retrieval from gynecologic cancer patients undergoing radical surgery. Clin Exp Reprod Med. 2016;43(2):119–25. doi:10.5653/cerm.2016.43.2.119.
Yin H, Jiang H, Kristensen SG, Andersen CY. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet. 2016;33(6):741–6. doi:10.1007/s10815-016-0691-7.
Revel A, Revel-Vilk S, Aizenman E, Porat-Katz A, Safran A, Ben-Meir A, et al. At what age can human oocytes be obtained? Fertil Steril. 2009;92(2):458–63. doi:10.1016/j.fertnstert.2008.07.013.
Maman E, Meirow D, Brengauz M, Raanani H, Dor J, Hourvitz A. Luteal phase oocyte retrieval and in vitro maturation is an optional procedure for urgent fertility preservation. Fertil Steril. 2011;95(1):64–7. doi:10.1016/j.fertnstert.2010.06.064.
Abir R, Ben-Aharon I, Garor R, Yaniv I, Ash S, Stemmer SM, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod. 2016;31(4):750–62. doi:10.1093/humrep/dew007.
Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23(5):1007–13. doi:10.1093/humrep/den055.
Abir R, Feinmesser M, Yaniv I, Fisch B, Cohen IJ, Ben-Haroush A, et al. Occasional involvement of the ovary in Ewing sarcoma. Hum Reprod. 2010;25(7):1708–12. doi:10.1093/humrep/deq121.
Prasath EB, Chan ML, Wong WH, Lim CJ, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29(2):276–8. doi:10.1093/humrep/det420.
Ellenbogen A, Shavit T, Shalom-Paz E. IVM results are comparable and may have advantages over standard IVF. Facts Views Vis Obgyn. 2014;6(2):77–80.
Uzelac PS, Delaney AA, Christensen GL, Bohler HC, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104(5):1258–60. doi:10.1016/j.fertnstert.2015.07.1148.
Chian RC, Gilbert L, Huang JY, Demirtas E, Holzer H, Benjamin A, et al. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril. 2009a;91(2):372–6. doi:10.1016/j.fertnstert.2007.11.088.
Chian RC, Huang JY, Gilbert L, Son WY, Holzer H, Cui SJ, et al. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009b;91(6):2391–8. doi:10.1016/j.fertnstert.2008.04.014.
Huang JY, Tulandi T, Holzer H, Tan SL, Chian RC. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89(3):567–72. doi:10.1016/j.fertnstert.2007.03.090.
Hourvitz A, Yerushalmi GM, Maman E, Raanani H, Elizur S, Brengauz M, et al. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod BioMed Online. 2015;31(4):497–505. doi:10.1016/j.rbmo.2015.06.025.
Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36(1):1–24. doi:10.1210/er.2014-1020,10.1210/er.2015.36.issue-1.edboard.
Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A. 2010;107(22):10280–4. doi:10.1073/pnas.1001198107.
Cordeiro CN, Christianson MS, Selter JH, Segars Jr JH. In vitro activation: a possible new frontier for treatment of primary ovarian insufficiency. Reprod Sci. 2016;23(4):429–38. doi:10.1177/1933719115625842.
Kawamura K, Kawamura N, Hsueh AJ. Activation of dormant follicles: a new treatment for premature ovarian failure? Curr Opin Obstet Gynecol. 2016;28(3):217–22. doi:10.1097/GCO.0000000000000268.
van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5(5):483–92.
Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6(4):e19475. doi:10.1371/journal.pone.0019475.
Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, et al. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17(23–24):3095–104. doi:10.1089/ten.TEA.2011.0204.
Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94 e1–9. doi:10.1016/j.ajog.2015.10.001.
Dolmans MM, Donnez J, Camboni A, Demylle D, Amorim C, Van Langendonckt A, et al. IVF outcome in patients with orthotopically transplanted ovarian tissue. Hum Reprod. 2009;24(11):2778–87. doi:10.1093/humrep/dep289.
Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30(1):11–24. doi:10.1007/s10815-012-9912-x.
Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sorensen SD, Rosendahl M, et al. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood. 2012;120(22):4311–6. doi:10.1182/blood-2012-01-403022.
Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology. 2013;67(1):64–9. doi:10.1016/j.cryobiol.2013.05.002.
O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68(5):1682–6. doi:10.1095/biolreprod.102.013029.
Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23(5):1151–8. doi:10.1093/humrep/den070.
Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27(6):1801–10. doi:10.1093/humrep/der468.
Lerer-Serfaty G, Samara N, Fisch B, Shachar M, Kossover O, Seliktar D, et al. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J Assist Reprod Genet. 2013;30(10):1279–88. doi:10.1007/s10815-013-0052-8.
Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23(10):2266–72. doi:10.1093/humrep/den244.
Mol BW, Zoll M. Fertility preservation for age-related fertility decline. Lancet. 2015;385(9967):507. doi:10.1016/S0140-6736(15)60199-4.
Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, et al. Fertility preservation for age-related fertility decline. Lancet. 2015;385(9967):506–7. doi:10.1016/S0140-6736(15)60198-2.
Stoop D, Silber S, Cobo A. Fertility preservation for age-related fertility decline—authors’ reply. Lancet. 2015;385(9967):507–8. doi:10.1016/S0140-6736(15)60200-8.
Acknowledgements
This work was supported by the Howard W. and Georgeanna Seegar Jones Endowment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ladanyi, C., Mor, A., Christianson, M.S. et al. Recent advances in the field of ovarian tissue cryopreservation and opportunities for research. J Assist Reprod Genet 34, 709–722 (2017). https://doi.org/10.1007/s10815-017-0899-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0899-1