Abstract
Purpose
The aim of the study was to investigate the maturation rate of immature oocytes collected from ovarian medulla tissue normally discarded during preparation of ovarian cortical tissue for fertility preservation. Further we evaluated survival of derived MII oocytes following vitrification and warming.
Methods
36 patients aged from 8 to 41 years who had one ovary excised for fertility preservation were included. Oocytes were collected from the medulla tissue and matured in vitro 44–48 h followed by vitrification. Number of oocytes collected, the rates of maturation and post-warming survival were assessed.
Results
On average, 11 immature oocytes were collected per patient. The overall maturation rate was 29 % irrespective of whether the ovary was transported 4–5 h on ice or obtained immediately after oophorectomy. The maturation rate in patients below 20 years of age (55 %) was significantly higher than that of patients aged 20–30 years (29 %) and above 30 years (26 %). The post-warming survival rate was 64 %. No significant relationship was observed between the number of collected oocytes and the age of patients.
Conclusions
Approximately three MII oocytes were obtained per patient following in vitro maturation (IVM) of immature oocytes collected from medulla tissue, of which two survived vitrification and warming. This approach represents an add-on method to potentially augment the fertility opportunity for cancer patients, especially in young women with cancer where transplantation of cortical tissue may pose a risk of relapse, but the IVM approach is currently too inefficient to be the only method used for fertility preservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the overall number of patients getting cancer remains relatively stable over time, the survival rate from cancer diseases has increased and is currently exceeding 80 percent for many diagnoses. The growing number of long-term cancer survivors increasingly focus on quality-of-life aspects after the cancer treatment. One important side effect of modern cancer treatment is its negative effect on fertility. The possibility of having biological children after recovery is important to most young women facing a gonadotoxic treatment, but also avoiding ovarian insufficiency at an early age is considered of value. Fertility preservation methods in young female cancer patients facing a potential gonadotoxic treatment have over the last decade proved valuable in securing fertility and continuous steroid production. Currently, almost 60 healthy children have been born worldwide as a result of transplanting frozen/thawed ovarian tissue [1]. These methods are currently entering clinical practice in many countries.
One method for fertility preservation potentially securing both fertility and continuous hormone production is cryopreservation of ovarian tissue [2]. Currently practice usually involves the excision of one ovary while the other is left in situ. The cortical tissue with its dense population of resting follicles is frozen while the medulla tissue is normally discarded. However, the disposed medulla tissue contains a considerable pool of growing follicles, especially in young girls [3]. The small antral follicles in the excised ovary may span diameters from less than one millimeter to around 10 millimeters. A number of studies have shown that immature oocytes can be aspirated from small antral follicles visible on the surface of the ovary after excision usually with diameters exceeding 3 millimeter [4, 5]. Two pregnancies resulting from cryopreserved embryos obtained from in vitro maturation (IVM) oocytes have recently been reported from women who recovered from cancer [6, 7].
Antral follicles with small diameters are not easily detected macroscopically and the corresponding oocytes may mature less frequently than oocytes collected from FSH primed polycystic ovary (PCO) patients, who constitute the population where IVM is most often applied. Small antral follicles collected from surplus medulla tissue are present in relatively large number and the oocytes may represent a potentially valuable source for fertility preservation, providing that they undergo proper IVM. However, the maturation rate of immature oocytes collected at the time of ovarian cortex cryopreservation ranged from 3 % to 100 % [7–11]. Thus, more data are needed to estimate the overall success rate and safety of this method.
The aim of the present study was to isolate immature oocytes from surplus medulla tissue and evaluate the competence to mature the MII stage in relation to the age of the patient and to transport time of the ovary. Furthermore, the ability of the MII oocytes to sustain vitrification and warming was evaluated.
Materials and methods
Patients
A total of 36 patients with a mean age of 26 years (range 8 – 41 years) who received fertility preservation treatment from January 2015 to August 2015 were included in the study. One ovary was excised prior to gonadotoxic treatment and the cortical tissue was prepared and cryopreserved as previously described [12, 13]. The indications for fertility preservation were breast cancer (n = 20), Hodgkin lymphoma (n = 2), non-Hodgkin lymphoma (n = 2), cervix cancer (n = 4), sarcoma (n = 3), hematological disease (n = 3), and autoimmune disease (n = 1) and Premature menopause (n = 1) (Supplementary Table 1). The menstrual history and fertility of the women were not recorded.
The ethical committee of the municipalities approved the project (H-2-2011-044), and the patients or their parents signed an informed consent form.
Oocyte Collection and IVM
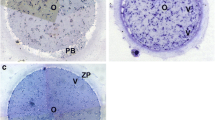
Ovaries were transported in IVF-flushing medium (Origio A/S, Maaløv, Denmark) to the laboratory either from the local hospital (less than 10 min, at 37 °C) or from collaborating hospitals (2 to 5 hours transport on crushed ice). Before isolation of the ovarian cortex tissue, all visible antral follicles were aspirated using 23-gauge syringe and used for other research projects. After isolating the cortex to a thickness of 1–2 mm for cryopreservation, the remaining ovarian tissue was defined as the medulla. It cannot be excluded that small amounts of residual ovarian cortex was present within this tissue [3]. During the process of cortex tissue isolation, medulla tissue usually was minced into many small fragments. The saline solution containing the normally discarded medulla fragments was examined for oocytes under stereomicroscope. Oocytes were washed three times in pre-warmed McCoy‘s 5α medium containing 25 mM HEPES (Invitrogen, GIBCO) supplemented with 5 mg/ml human serum albumin (HSA, CSL Behring 20 %, Marburg, Germany), 2 mM Glutamax (Invitrogen, GIBCO), 0.05 mg/ml penicillin/streptomycin (Invitrogen, GIBCO), 10 mg/ml insulin, 5.5 mg/ml transferrin, 6.7 ng/ml selenium (ITS, Invitrogen, GIBCO), which served as a holding medium during the process of oocyte collection (Fig. 1a). The oocytes were subsequently washed twice in LAG medium (Origio A/S) and then transferred to IVM medium (Origio A/S) containing 75 mIU/ml rFSH (Puregon; MSD, Oss, the Netherlands) and 100 mIU/ml rLH (Luveris, Merck, Copenhagen, Denmark) and 10 mg/ml HSA. The LAG medium and this final IVM medium were pre-equilibrated in 5 % CO2 at 37 °C for at least 4 h and kept at 5 % CO2 at 37 °C during work in the flow-bench. Two to three immature oocytes were co-cultured in 30 μl drops overlaid with culture oil (COOK, Australia) at 37 °C in 5 % CO2 for 44 to 48 h. According to the manufacturer’s instruction, the LAG and IVM medium is to be used within 7 days after opening. The IVM final medium was freshly prepared every week (i.e. “freshly prepared medium”) contrasting the first period of the study, where IVM medium was only prepared every two to three months (i.e. “IVM medium – long shelf-life”) due to the low frequency of ovarian tissue cryopreservation. And our previous study based on the “long shelf-life medium” showed the IVM rate was only 3.1 %, therefore, the IVM rates from these two groups were compared in the present study.
After 44 to 48 h of culture, all oocyte-cumulus-complexes were denuded using a 130–133 μm denudation pipette (Vitrolife, Gothenburg, Sweden) following one minute of exposure to 80 IU/ml hyaluronidase solution (Sigma Aldrich, USA) (Fig. 1b). Oocyte maturity was determined microscopically by the extrusion of the first polar body (i.e. MII oocyte).
Vitrification/Warming
MII oocytes were cryopreserved by vitrification using a commercially available vitrification kit (Kitazato Biophama Co.Ltd. Shizuoka, Japan). Briefly, oocytes were placed in equilibration solution (ES) for 9–12 min at room temperature, then transferred to vitrification solution (VS) and loaded to the tip of McGill Cryoleaf (Origio A/S) in a very small volume within one minute, then immediately plunged into the liquid nitrogen and protected by the plastic cover. For warming, the cryoleafs were transferred to 1 ml thawing solution (TS) (pre-warmed to 37 °C) for 1 min, followed by diluent solution (DS) for 3 min and washing solution (WS) for 5 min twice at room temperature. All vitrification freezing/warming steps were carried out according to the manufacturer’s protocol.
Viability Assessment of warmed Oocytes
Oocyte viability after warming was assessed by double fluorescent labeling with calcein AM and ethidium homodimer (EthD-1) dyes (Live/Dead® Viability/Cytotoxi-city kit, L-3224, Invitrogen, USA). Briefly, oocytes were washed in DPBS at 37 °C and then incubated in 1.6 μM calcein AM and 5.5 μM EthD-1 for 30–45 min at 37 °C in the dark. Non-fluorescent cell-permeant calcein AM entered the cell and was cleaved by esterase in live cells producing an intense uniform green fluorescence. EthD-1 entered the damaged cells and combined to DNA, producing a bright red fluorescence. Oocytes were defined as being alive when both oocyte and polar body displayed green fluorescence. On the contrary, if the oocyte was without green fluorescence, it was considered dead (Supplementary Figure 1).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 6. Data were analyzed by one-way ANOVA and multiple comparisons, and Chi-square test as appropriate. Linear correlations between the number of collected oocytes and age, or and the weight of medulla tissue were analyzed by Pearson’s correlation test. Value of P < 0.05 indicated statistical significance.
Results
A total of 393 oocytes from 36 patients were retrieved from saline solutions containing surplus medulla tissue. One oocyte was at the MII stage and 16 were degenerated (4 %) at retrieval. The average number of oocytes collected per patient was 10.9 ± 9.4 (mean ± SD), ranging from 0 to 43. Patient characteristics and outcome parameters regarding the rates of maturation and post-warming survival of oocytes are shown in Supplementary Table 1. No significant correlation was observed between the number of oocytes collected and the age of patients, as well as the number of oocytes collected and the weight of medulla tissue. No oocytes were found from the patient diagnosed with premature menopause. All three oocytes obtained from an 8-year-old girl were degenerated at retrieval.
The overall IVM rate was 29 % (99/339), range 0–100 %. The IVM rate from patients below 20 years of age (55 %) was significantly higher than that of patients both aged 20–30 years (29 %) and above 30 years (26 %) (P < 0.05) (Table 1).
Freshly prepared medium resulted in a significantly higher IVM rate than medium with long shelf-life (33 % vs. 12 %, P < 0.05) (Table 2). Moreover, cooling of ovarian tissue on crushed ice during transportation did not affect the IVM rate as compared to collection of oocytes immediately after the operation (Table 3).
Base on the live-dead assay the survival rate of vitrified MII oocytes was 64 % (45/70).
Discussion
This study demonstrates that surprisingly many immature oocytes can be collected from discarded medulla tissue during the process of ovarian cortex preparation. Oocytes from small antral follicles not visible on the surface of the ovary and with the majority of them having a diameter below approximately three millimeter possess competence to mature to the MII stage during proper culture conditions irrespective of whether the ovary was transported on ice prior to the isolation of immature oocytes. Furthermore, approximately two thirds of the MII oocytes survive vitrification and warming. Due to Danish legislation we have been unable to check the fertilizability of the warmed oocytes and prior to widespread clinical use oocyte competence should be evaluated closer. However, this study found on average approximately eleven oocytes being collected per patient, which translated into an average of two surviving MII oocytes after warming.
Currently it is estimated that around 20 MII oocytes are required per child [14–16], which means that only a minority of these patients may benefit from this option. Therefore, IVM matured oocytes in this way may serve as an add-on method to ovarian tissue freezing, especially in young patients and in those centers choosing to excise one whole ovary for fertility preservation, but IVM in this manner cannot be recommended as a single method of fertility preservation. If additional immature oocytes collected from antral follicles visible on the surface of the ovary are also included, which will be logical in a clinical setting, the number of MII oocytes will undoubtedly increase and augment efficacy [17]. Taken together, immature oocytes obtained as a by-product during preparation of ovarian cortical tissue for freezing, irrespective of whether the ovary has been cooled off to zero degrees during transportation, may be used as an additional method to improve chances for fertility later on. However, IVM maturation as described here is still too ineffective as the only method for fertility preservation.
In our previous study comprising more than 600 immature oocytes isolated from surplus medulla tissue we found an overall maturation rate of just a few percent using long shelf-life medium [11]. The present study has shown a considerable higher maturation rate of around 30–35 percent. This dramatic increase appears to be related to the use of freshly prepared culture medium including the addition of gonadotropins and HSA and a much quicker handling time of the immature oocytes reducing the period for oocyte identification in the left-over solution containing medulla tissue as compared to the previous study.
The IVM rate in the present study was significantly higher in younger patients as compared to patients above 20 years of age. This observation confirmed and extended that of Shirasawa and co-workers [18], who found a maturation rate of only 13 percent in patients aged 35–44 years. Therefore collection of immature oocytes especially in young women, who for instance may suffer from leukemia and where transplantation of the ovarian tissue presently is contraindicated due to the risk of relapse, may represent a group of patients who potentially could benefit from this method. However, it is important to point out that the present IVM method is currently too ineffective to be used as the only method. Furthermore, only a few oocytes are collected from a number of patients who therefore only have a small chance of benefitting from these oocytes later on. Actually, 42 % of the patients only produced zero or one MII oocyte and is therefore a priori unlikely to benefit from the procedure. On the other hand, 25 % of the patients produced at least five MII oocytes and have a higher chance of benefitting from the IVM procedure. In order to improve the efficacy of the procedure collection of oocytes from visible antral follicles from the surface of the ovary should be included. Further the number of immature oocytes may be augmented if visible follicles can be aspirated from the contralateral ovary, which remain in situ, at the time of unilateral ovariectomy.
Our study also demonstrates that immature oocytes from ovaries that have been transported on ice for four to five hours prior to freezing undergo IVM with a similar frequency to immature oocytes collected from ovaries that are not cooled. However, evaluation of matured oocytes from ovaries that have been chilled to zero degree during transportation prior to cryopreservation requires additional studies to secure the chromosomal status of the warmed oocytes. In the present study the percentage of MII with a round and regular polar body was 61 % (60/99). In addition, a subset of MII oocytes we also found either a small- or big-sized first polar body, or a bipolar body or fragmented polar body in the perivitelline space (data not shown). It is unclear whether this reflect that oocyte competence within antral follicles deteriorate at cold temperature. However, our results showed no significant difference in IVM rates among oocytes from different hospitals with or without cooling to zero degrees prior to processing. These findings were consistent with a study in domestic cats, which demonstrated that while 2 or 24 h storage of ovaries at 4 °C did not affect the meiotic competence of oocytes in vitro, whereas 48 h storage reduced results dramatically [19]. Therefore, as the human meiotic spindle is sensitive to alteration in temperature [20], this may reflect that IVM oocytes are more prone to chromosomal errors, which a recent study have confirmed [21]. In the context of the current study the chromosomal status of IVM matured should be investigated further before widespread clinical use.
There were two prepubertal girls included in the present study. In one patient all three oocytes collected were degenerated at collection, while in another patient three of five oocytes matured during IVM. A low MII rate was reported in prepubertal girls in previous study [7, 10], but the collective experience with IVM of oocytes from prepubertal girls does not allow any conclusions to be drawn and should be expanded on in future studies.
In conclusion, the present study demonstrates that oocytes can be retrieved from the saline solution of discarded ovarian medulla tissue, matured in vitro, and cryopreserved by vitrification. On average approximate three MII oocytes per patient would be obtained from this approach of which two survived vitrification and warming. Ovaries transported on ice for up to 5 hours do not appear to affect the meiotic competence of the immature oocytes, but further evaluation is required to justify widespread clinical utilization. Vitrification of in vitro matured oocytes combined with ovarian tissue cryopreservation may expand the fertility opportunity for cancer patients but is too ineffective to be used as a stand-alone method.
References
Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32(8):1167–70.
von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, Montag M, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy--a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45(9):1547–53.
Kristensen SG, Rasmussen A, Byskov AG, Andersen CY. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum Reprod. 2011;26(1):157–66.
Gonzalez C, Devesa M, Boada M, Coroleu B, Veiga A, Barri PN. Combined strategy for fertility preservation in an oncologic patient: vitrification of in vitro matured oocytes and ovarian tissue freezing. J Assist Reprod Genet. 2011;28(12):1147–9.
Imesch P, Scheiner D, Xie M, Fink D, Macas E, Dubey R, et al. Developmental potential of human oocytes matured in vitro followed by vitrification and activation. J Ovarian Res. 2013;6:30.
Prasath EB, Chan ML, Wong WH, Lim CJ, Tharmalingam MD, Hendricks M, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29(2):276–8.
Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32(8):1221–31.
Escriba MJ, Grau N, Escrich L, Novella-Maestre E, Sanchez-Serrano M. Spontaneous in vitro maturation of oocytes prior to ovarian tissue cryopreservation in natural cycles of oncologic patients. J Assist Reprod Genet. 2012;29(11):1261–5.
Revel A, Koler M, Simon A, Lewin A, Laufer N, Safran A. Oocyte collection during cryopreservation of the ovarian cortex. Fertil Steril. 2003;79(5):1237–9.
Fasano G, Moffa F, Dechene J, Englert Y, Demeestere I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod Biol Endocrinol. 2011;9:150.
Wilken-Jensen HN, Kristensen SG, Jeppesen JV, Yding AC. Developmental competence of oocytes isolated from surplus medulla tissue in connection with cryopreservation of ovarian tissue for fertility preservation. Acta Obstet Gynecol Scand. 2014;93(1):32–7.
Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11(7):1487–91.
Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23(10):2266–72.
Sole M, Santalo J, Boada M, Clua E, Rodriguez I, Martinez F, et al. How does vitrification affect oocyte viability in oocyte donation cycles? A prospective studto compare outcomes achieved with fresh versus vitrifed sibling oocytes. Hum Reprod. 2013;28(8):2087–92.
Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR, Tucker MJ. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril. 2016;105(2):459–466.e2.
Anzola AB, Pauly V, Geoffroy-Siraudin C, Gervoise-Boyer MJ, Montjean D, Boyer P. The first 50 live births after autologous oocyte vitrification in France. J Assist Reprod Genet. 2015;32(12):1781–7.
Shalom-Paz E, Almog B, Shehata F, Huang J, Holzer H, Chian RC, et al. Fertility preservation for breast-cancer patients using IVM followed by oocyte or embryo vitrification. Reprod Biomed Online. 2010;21(4):566–71.
Shirasawa H, Kumagai J, Sato W, Kumazawa Y, Sato N, Terada Y. Retrieval and in vitro maturation of human oocytes from ovaries removed during surgery for endometrial carcinoma: a novel strategy for human oocyte research. J Assist Reprod Genet. 2013;30(9):1227–30.
Evecen M, Cirit U, Demir K, Karaman E, Hamzaoglu AI, Bakirer G. Developmental competence of domestic cat oocytes from ovaries stored at various durations at 4 degrees C temperature. Anim Reprod Sci. 2009;116(1–2):169–72.
Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. Limited recovery of meiotic spindles in living human oocytes after cooling-rewarming observed using polarized light microscopy. Hum Reprod. 2001;16(11):2374–8.
Lei T, Guo N, Liu JQ, Tan MH, Li YF. Vitrification of in vitro matured oocytes: effects on meiotic spindle configuration and mitochondrial function. Int J Clin Exp Pathol. 2014;7(3):1159–65.
Funding
No fund supported the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest statement
None of the authors has any conflicts of interest.
Additional information
Capsule IVM of oocytes collected from surplus medualla tissue may be a valuable add-on-method for fertility preservation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Patient characteristics, IVM rate and survival of oocytes undergoing vitrification and warming. (DOC 74 kb)
ESM 2
Viability assessment of post-warming MII oocyte. (A) Survived MII and (B) dead MII from the patient (No.18) aged 22 years. (C) Negative control, MII from the patient (No.15) aged 27 years underwent 70 % alcohol treatment for 10 min and showed all cells are dead. Upper panel: bight field; lower panel: confocal field (×100 fold). (TIFF 1978 kb)
Rights and permissions
About this article
Cite this article
Yin, H., Jiang, H., Kristensen, S.G. et al. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet 33, 741–746 (2016). https://doi.org/10.1007/s10815-016-0691-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0691-7