Abstract
Fragile X premutation carriers have 55–200 CGG repeats in the 5’ untranslated region of the FMR1 gene. Women with this premutation face many physical and emotional challenges in their life. Approximately 20% of these women will develop fragile X-associated primary ovarian insufficiency (FXPOI). In addition, they suffer from increased rates of menstrual dysfunction, diminished ovarian reserve, reduction in age of menopause, infertility, dizygotic twinning, and risk of having an offspring with a premutation or full mutation. Consequent chronic hypoestrogenism may result in impaired bone health and increased cardiovascular risk. Neuropsychiatric issues include risk of developing fragile X-associated tremor/ataxia syndrome, neuropathy, musculoskeletal problems, increased prevalence of anxiety, depression, and sleep disturbances independent of the stress of raising an offspring with fragile X syndrome and higher risk of postpartum depression. Some studies have reported a higher prevalence of thyroid abnormalities and hypertension in these women. Reproductive health providers play an important role in the health supervision of women with fragile X premutation. Awareness of these risks and correlation of the various manifestations could help in early diagnosis and coordination of care and services for these women and their families. This paper reviews current evidence regarding the possible conditions that may present in women with premutation-sized repeats beyond FXPOI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mutations in the FMR1 gene cause fragile X syndrome (FXS), the most common form of inherited mental retardation [1]. The spectrum of disorders associated with abnormalities in this gene includes fragile X-associated primary ovarian insufficiency (FXPOI), a condition frequently recognized by reproductive health care providers.
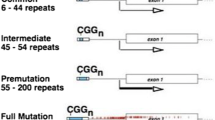
The FMR1 gene has a CGG repeat tract in the 5’ untranslated region which varies in number of repeats throughout the population, 29 being the most frequent number of repeats encountered [1]. Depending on the number of CGG repeats present, this DNA region becomes meiotically unstable and has a risk of expansion upon maternal transmission that increases with the number of trinucleotide repeats, with larger alleles expanding into the full mutation more often than that with shorter alleles [1]. The risk of expansion into the full mutation has been used to classify the number of CGG repeats into normal, intermediate (“gray zone”), premutation, and full mutation. Both the American College of Obstetrics and Gynecology (ACOG) and the American College of Medical Genetics and Genomic (ACMGG) have classified the limits of the CGG alleles of the FMR1 gene into normal (5–44 repeats), intermediate (45–54 repeats), premutation (55–200 repeats), and full mutation (>200 repeats) [2–4]. If the number of repeats goes above 200, hypermethylation of the repeat sequence and an adjacent CpG island occurs, which leads to silencing of the gene and therefore an absence of fragile X mental retardation protein (FMRP) [5, 6]. This protein is an RNA-binding protein with nuclear localization and exports signals that has been proven to associate with translating polyribosomes and messenger RNAs (mRNAs), which suggests that it may shuttle between the nucleus and cytoplasm and act as a translational modulator of specific mRNAs [7–9]. Absence of FMRP is the cause of FXS; the suggested mechanism involves a misregulation of protein synthesis during synapse development, which affects this process and brain plasticity, thus ultimately leading to mental retardation [7].
On the other hand, carriers of the premutation (55–200 repeats) do not have their FMR1 gene silenced. Kenneson et al. (2001) [10] found that these patients have an increase in FMR1 RNA transcription proportionally related to the number of CGG repeats and a decrease in FMRP translation inversely related to the number of CGG repeats. The excess of FMR1 mRNA seen in carriers leads to dysregulation of several proteins and its deposition, along with FMR1 mRNA, in the form of cellular inclusions in several parts of the body including the central nervous system, peripheral nervous system (especially autonomic ganglia), Leydig cells, and pituitary among others [11–13]. Therefore, any pathology associated with FMR1 premutations would not be caused by a complete absence of FMRP like FXS is, but probably due to partial FMRP deficiency and/or RNA toxicity.
The estimated carrier frequency of women with the fragile X premutation in the USA is 1:178 [14], and recently, Tassone et al. (2012) [15] through a blood-spot newborn screening study in CA estimated the premutation prevalence in females to be 1:209. This relatively high prevalence of carriers of the premutation has several implications for reproductive health care providers who frequently serve as the primary provider for women. Most physicians are aware of the fact that FMR1 premutation is the leading single-gene cause of primary ovarian insufficiency (POI) [16]. However, there is growing evidence that female carriers of the fragile X premutation are at risk for several comorbidities spanning diverse systems throughout the body including a variety of neurologic, reproductive, endocrine, autoimmune, and psychiatric conditions, besides FXPOI (Fig. 1).
The American College of Obstetrics and Gynecology (ACOG) recommends fragile X premutation carrier screening and genetic counseling for women with a family history of fragile X-related disorders, POI, autism, unexplained mental retardation, or developmental delay. In addition, women may request screening regardless of family history [4]. Polymerase chain reaction (PCR) and Southern blot analysis are used in testing to determine the individual’s genotype [3].
The primary objective of this review is to familiarize reproductive health care providers including reproductive endocrinology and infertility specialists with the different phenotypes encompassing FMR1 premutation carriers. It explores the current evidence regarding all the possible conditions that may arise in women with premutation-sized repeats beyond FXPOI.
Reproductive involvement
The reproductive system is commonly involved, and manifestations of its compromise are frequently encountered among women carriers of the premutation. Overall, carriers have been proven to go through menopause approximately 5 years earlier than controls [17] and also to be at increased risk for primary ovarian insufficiency [18]. Approximately 3% of female carriers have their final menses before age 29 and 1% prior to age 18 [19].
Primary ovarian insufficiency was one of the first conditions ever to be associated with carrier women. It has an approximate prevalence of 16% [20], and the exact pathophysiologic mechanism underlying its cause is presently unknown, although it is suggested that it might be caused by an RNA-toxic effect [18, 21].
What has been established regarding ovarian function and FMR1 premutations is the intimate relation between the number of CGG repeats present and the ovarian phenotype. It has been found that the risk of POI and the ovarian manifestations present on a specific patient may have a non-linear association with the number of CGG repeats, with the risk of developing ovarian insufficiency increasing with the number of repeats and then reaching a plateau or even decreasing after 80–100 CGG repeats are reached [21–24].
Allen et al. (2007) [24] did a study with 948 carriers with varying repeat sizes in order to get a better view of the reproductive aging milestones among these women and its relation with the number of CGG repeats present. To accomplish this, the author classified the subjects into non-carriers (<59), low (59–79), medium (80–100), and high premutation alleles (>100 repeats) and compared the prevalence of POI between non-carriers and the other premutation groups. They found that all the carrier groups had an increased prevalence of POI compared to non-carriers, but the highest odds ratio for POI was that of the medium-sized premutation group. The same is true for menopausal age, with all groups of carriers having a decreased mean menopausal age, but with the medium-sized group presenting with the lowest mean age of menopause overall. Regarding menstrual cycle characteristics, they reported that women in the low- and medium-sized premutation groups were more likely to report short cycles of less than 27 days and to have gone six or more weeks without a period when compared to controls, whereas those in the medium-sized group were more likely to report irregular cycles. The latter group of patients, those with medium-sized repeats, also had lower fertility compared to non-carriers and the other groups in addition to an increased rate of dizygotic twinning. However, there was no increase in the rate of spontaneous abortions, which indirectly suggests that oocyte quality is not compromised in these patients. Their overall conclusion was that those carriers of premutations ranging from 80–99 CGG repeats have increased rates of menstrual dysfunction, reduction in age of menopause, infertility, dizygotic twinning, and POI when compared to non-carriers, but most importantly, carriers of low and high premutation repeats also suffer from certain degrees of ovarian insufficiency, just not to such an extent as medium-sized carriers. This is important since it leads to the hypothesis that women with premutations may have a continuum of impaired ovarian function [18], being POI one tail of the distribution and relating to the number of CGG repeats present in the FMR1 gene.
Besides Allen et al. (2007) [24], other investigators have also reported a continuum of impaired ovarian function. Welt et al. (2004) [25] recruited 11 women with the premutation and compared them with age-matched controls in order to show differences in menstrual cycles and hormonal changes. Premutation carriers had shorter duration of their follicular phase length, which consequently reflected in a decreased total cycle length. The carriers also had increased follicular and luteal FSH, whereas inhibin B was decreased in the follicular phase, and inhibin A and progesterone were decreased in the luteal phase, with no difference found in LH and estradiol. All women included in this study were having regular ovulatory cycles; therefore, this finding supports the idea that carriers are not only affected by POI but also have impaired ovarian function despite having regular periods. An important weakness of this study was the small number of carriers included, the fact that the size of the CGG repeat was not ascertained and that these measurements were only done through one menstrual cycle. Premutation carriers with repeat lengths beyond 70 also have lower AMH levels when compared to carriers with repeat lengths shorter than 70 [26].
In addition to impaired ovarian function and all the potential consequences of chronic hypoestrogenism (i.e., impaired bone health, increased cardiovascular risk, etc.), an additional reported finding has been that of a significantly higher odds of difficulty in achieving orgasm among premutation carriers when compared to controls (12.87 vs. 0%; P = 0.002) [27].
Outcomes of assisted reproduction
Women with a premutation tend to present to infertility clinics, not infrequently, seeking assisted reproduction either because of subfertility or to undergo preimplantation genetic diagnosis (PGD) due to expansion concerns. Several management issues arise during the care of these women starting with the obstacle posed by a diminished ovarian reserve even when the patient does not have a history of infertility. As a matter of fact, a positive correlation between the number of CGG repeats and parameters of ovarian response in addition to a negative correlation between the former and the dose of rFSH among patients undergoing controlled ovarian stimulation (COH) for PGD has been reported [28], which is concordant with the previously described effect of the premutation on ovarian reserve. Patients with <100 CGG repeats have significantly lower milestones of ovarian response and fertilization rates than those with ≥100 CGG repeats [28], and it has even been postulated that distinct FMR1 genotypes might explain differences in IVF outcome between races [29]. Kushnir et al. [30] suggested that the FMR1 gene may negatively impact oocyte/embryo quality and thus IVF pregnancy rates particularly among those patients that have an allele with low repeats independently from ploidy. Therefore, the number of repeats found on the FMR1 gene may have a role not only in the prognosis of IVF success through embryo quality expectations but also in treatment adjustments since some patients may be initially managed as low responders [28–30]. In the future, infertility clinics might become one of the main places where affected families are identified. This identification has wide implications and from the reproductive standpoint could involve the possibility of fertility preservation with subsequent preimplantation genetic diagnosis for young women with fragile X premutation. In addition, testing of poor responders, particularly if they are young, could potentially have a role in the management of these subsets of patients.
Neuropsychiatric and muscular involvement
It is evident that the FMR1 gene has a vital function in the nervous system, as expected from the abundant gene expression in the neural tissue [31] and from the clinical phenotype seen among patients with FXS. But not only the absence of the protein product may have consequences on the nervous system; deviations from normal expression may also have critical effects on nervous functioning, as exemplified by fragile X-associated tremor/ataxia syndrome (FXTAS).
FXTAS was among the first conditions associated with carriers of the premutation [32]. This is a neurodegenerative disease mainly characterized by intention tremor and cerebellar ataxia that tends to occur as a function of age, as evidenced by Jacquemont et al. (2004) [33] who found an increased prevalence of FXTAS among men with the premutation in each subsequent decade of life after age 50. Besides ataxia and tremor, FXTAS may also present with short-term memory loss, executive function deficits, cognitive decline, parkinsonism, peripheral neuropathy, lower limb proximal muscle weakness, and autonomic dysfunction [34].
The most accepted theory regarding the pathologic mechanism involving FXTAS is an RNA gain of function toxicity mechanism [35]. The latter would help explain why the prevalence and symptoms of FXTAS worsen with age as the toxic effect accumulates over time.
Differences in penetrance and expression have been observed between the two genders. FXTAS has been classically considered to be more prevalent in men than women [33]; Coffey et al. (2008) [36] found a FXTAS prevalence of 8.3% over age 40 in women carriers of the premutation, and Rodriguez-Revenga et al. (2009) [37] found a penetrance of FXTAS symptoms of 16.5% among women carriers older than 50 years, whereas the prevalence in men is 40%. A possible explanation for the decreased prevalence of FXTAS in women is the X chromosome inactivation process which would lead to some cells only expressing the normal allele and not the premutated one [35, 38].
However, neurological involvement in premutation carriers does not only include FXTAS; it should be looked at as a spectrum, with full-blown FXTAS on an end of the spectrum and an absence of neurological symptoms on the other, which would correlate with a high and a low expression of the premutated allele, respectively.
This variation in the expression of the premutated allele among women carriers of the premutation linked to the X inactivation process may explain why they are less affected by FXTAS and why they are presented with a variety of neurological symptoms not diagnostic of FXTAS. It would also explain why women who fulfill the FXTAS diagnostic criteria demonstrate milder neuroradiological brain changes than affected men [39] and possibly a milder phenotype and older age of onset.
Several studies addressing the extent of neurological compromise in women carriers have been published. For example, Jacquemont et al. (2004) [33] failed to report patients with definite or probable FXTAS among his population of carrier women, but they did find mild intention tremor and/or gait ataxia in some of them, besides finding significantly higher scores of the cerebellar dysfunction, International Cooperative Ataxia Rating Scale (ICARS), parkinsonism, Unified Parkinson’s Disease Rating Scale (UPDRS), and scales when carriers were compared to controls. At that time, the author concluded that this subclinical neurological involvement of women carriers needed further evaluation. After that, several studies that have addressed the expanded phenotype seen among the specific population of women carriers of the FMR1 premutation have revealed some evidence regarding the neurological compromise of these patients.
Coffey et al. (2008) [36] studied 146 women carriers of the premutation trying to determine their overall phenotype and divided them into a non-FXTAS (n = 128) and a FXTAS (n = 18) group, the latter being composed of patients with probable or definite FXTAS according to the classification criteria in Jacquemont et al. (2003) [34]. The author found that those patients in the FXTAS group had a greater comorbidity when compared with controls. Among neuromuscular manifestations of disease, they found a significant difference in prevalence of seizures, peripheral neuropathy, and fibromyalgia, in addition to the typical symptoms of FXTAS (tremor and ataxia). When comparing the non-FXTAS group with controls, there was a significantly higher history of muscle pain, persistent paresthesias in extremities, and history of tremors. A difference in neuropathy was also found among the non-FXTAS group, but this difference was not statistically significant when compared to controls. With this data, the author proposed that the mRNA toxicity seen in premutation carriers might produce a continuum of neurological effects directly related to the neurodegenerative phenotype associated with FXTAS, and that this would explain why non-FXTAS carriers present with more muscle pain, tremors, and paresthesias and why FXTAS carriers tend to have more history of seizures besides the components of the FXTAS complex.
Rodriguez-Revenga et al. (2009) [37] also studied the prevalence of different phenotypes associated with women with the premutation. Their study included a total of 280 women carriers, 195 of them between the age of 40 and 50 years and 85 of them older than 50 years. They found that among patients older than 50 years of age, the prevalence of FXTAS was 16.5% and the penetrance of its symptoms was age-related increasing together with the age of the individuals, with the only exception being those patients older than 80 years of age, which showed a slight decrease in prevalence. The author speculated that this was probably due to an early death of these patients when compared to their non-FXTAS age counterparts and that FXTAS was the probable cause of their death. The prevalence of chronic muscle pain in this population was 24.4%, which, when compared to the prevalence of this condition among the general population, yields a statistically significant difference (P = 0.00002).
Hunter et al. (2010) [40] recruited a study population of 334 women with the premutation aged 18–50 years, and sought to determine which other diagnoses were being reported in these patients. The author found that overall mental health disorders were being reported significantly more often in carriers than in non-carriers. Among them, attention-deficit/hyperactivity disorder (ADHD), anxiety, and depression were initially statistically different when comparing the crude data between the two. When adjustment for covariates were made, this trend changed and the significant difference was lost for mental health disorders as a whole, anxiety and depression, but was maintained for ADHD. Another finding was a marginally increased reporting of learning disabilities for carriers of the premutation which was statistically significant, and the fact that raising a child with FXS was not found to be a significant predictor of anxiety, depression, ADHD, or all mental health disorders combined. This last outcome is important, since there has been much debate regarding the psychological phenotype of these patients being a consequence of the fact of having to raise a child with FXS and not inherent to the premutation itself.
In this study, no difference was found regarding fibromyalgia, chronic muscle pain, peripheral neuropathy, and seizures between carriers and non-carriers. Among these conditions, seizures were the only one directly addressed in the questionnaire given to the participants. For the other conditions, the way the author found this outcome was by analyzing self-reported additional diagnoses not specifically queried, which may have led to a decreased power of the study to find such associations, although the younger age of the participants (18–50 years) and the absence of women with either probable or definite FXTAS in this population, unlike the populations recruited by Coffey et al. (2008) [36] and Rodriguez-Revenga et al. (2009) [37], may have also influenced such outcomes.
Despite Hunter et al. (2010) [40] not finding an association with either fibromyalgia or chronic muscle pain among carriers, this has been a consistent finding in the literature, and both fibromyalgia and chronic muscle pain are biologically plausible in our current understanding of the mechanism of disease.
Just like FXTAS, the presence of muscle pain among carriers should also be looked at as a spectrum of neurological compromise, being fibromyalgia on one end of the distribution and no muscle pain on the other, with some patients lying between them and expressing only some chronic muscle pain without fulfilling the diagnostic criteria for fibromyalgia.
Chonchaiya et al. (2010) [27] examined the prevalence and the age onset of neurological symptoms among other variables in 110 carriers between the ages of 30 and 65 with FXTAS fathers vs. 36 carriers between the age of 35 and 66 whose fathers did not have FXTAS and 43 controls between the ages of 30 and 55. They found that daughters of men with FXTAS had significantly higher odds of neurological problems when compared to non-carrier controls including tremor (13.6 vs. 0%; P = 0.0065), balance problems (27.3 vs. 0%; P = <0.001), memory problems (38.9 vs. 7.0%; P < 0.001), and dizziness (28.4 vs. 5.1%; P = 0.0026). When these patients were compared to carriers whose fathers have not developed FXTAS, only a statistically significant difference in balance problems (27.7 vs. 5.6%; P = 0.0075) and an increased trend with respect to memory problems (37.6 vs. 16.7%; P = 0.0222) were found even though the observed percentages of neurological problems were typically higher in the first group. Although no significant difference was observed between carrier daughters of men with FXTAS and controls in terms of neuropathy, unlike other studies, they tended to report these symptoms at an earlier age when compared to controls, usually in their early 40s.
Au et al. (2013) [41] assessed the prevalence and risk of migraine headaches in premutation carriers comparing 203 women carriers of the premutation vs. 83 control women and found a significantly higher prevalence of migraines in carriers (54.2 vs. 25.3%; P = 0.0001) than in controls.
Carriers have also been long known to be at increased risk for psychopathology. This is not surprising since we have proven that a common denominator for those carriers of the premutation is compromise of the nervous system, and as such an impact on the psychological and psychiatric features of these patients may be expected. The main example of the psychiatric compromise of carriers occurs in those that develop FXTAS, which is not only a neurodegenerative disease as previously stated, but it is also considered to be a neuropsychiatric condition that often courses with dementia especially in male patients [42] which although previously believed to be rare in women with the premutation, increasing evidence is proving otherwise [43–46].
Besides dementia in FXTAS, carriers of the premutation also develop several other psychopathologic manifestations. Franke et al. (1998) [47] reported that these individuals have an increased risk for social phobia, schizotypal, and avoidant personality disorder when compared to controls. Johnston et al. (2001) [48] reported schizotypal traits and emotional difficulties including social anxiety and depressed mood.
Roberts et al. (2009) [49] found a high frequency of lifetime major depressive disorder, lifetime panic disorder without agoraphobia, and current agoraphobia without panic at the time of the study. The author stated that a large proportion of women carriers are affected by a major depressive disorder at some point in their lives, but few of them reported recurrent episodes. They hypothesized that this might be due to the high psychotropic medication use found in this population which may account not only for the low recurrence rate of major depressive disorder but also for the low frequency of other disorders found at the time of the study.
This group also stated that the number of FXS children and children’s behavioral problems was not associated with major depressive disorder in their population of women with the premutation, and most importantly that 48% of the sample that had a mood disorder, had it before the birth of their first child with FXS. Therefore, they concluded that the elevated rate of major depressive disorder could not be explained by either raising a child with FXS or receiving the diagnosis of FXS.
An interesting finding done by this same group is the fact that the relationship between CGG repeat and major depressive disorder was non-linear, with women with mid-range repeat lengths having more risk to develop a major depressive disorder than women with the highest repeat numbers. The interesting fact about this finding is that it has also been reported for ovarian insufficiency [21–24].
Unlike the findings of Roberts et al. (2009) [49], Obadia et al. (2013) [50], when evaluating the characteristics of postpartum depression (PPD) during a pilot study done in carriers, found that mothers of children with FXS are at higher risk for PPD than female carriers without affected children and that for each additional child with FXS, the risk more than doubled. PPD episodes in this study were linked to having children with FXS since all PPD episodes occurred after carriers had a child who was eventually diagnosed with FXS, even though the syndrome’s diagnosis was usually done years later.
Bourgeois et al. (2011) [51] also revealed a significantly higher lifetime prevalence of a major depressive disorder and specific phobia in carriers with FXTAS and a higher prevalence of social phobia in those without FXTAS when compared to the general population.
Regarding anxiety disorders, Roberts et al. (2009) [49] found that overall, these women displayed a lower frequency of anxiety disorders, although as mentioned before, specific entities such as lifetime panic disorder without agoraphobia and agoraphobia without panic at the time of the study were more likely among carriers. This lower frequency of anxiety disorders goes against previous findings by other groups who have found that women carriers tend to have an increased risk of anxiety disorders; in fact, Franke et al. (1998) [47] reported a 41% lifetime diagnosis rate of anxiety disorder among carriers with FXS children. Nevertheless, the key to this difference may be related to the presence of affected children. Roberts et al. (2009) [49] found that anxiety disorders in this population were strongly related to child variables, including the number of children with FXS and/or child problem behavior.
One could argue that this was even suggested in the data provided by Franke et al. (1998) [47] who reported that only 11.8% of women carriers without FXS children had anxiety disorders vs. 41% of women carriers with FXS children. Chonchaiya et al. (2010) [27], although did not include the number of FXS children as a variable, did find a higher rate of anxiety among carrier daughters of men with FXTAS (65.1 vs. 34.9%; P < 0.001) when compared to non-carrier controls. This group also found a significant difference when comparing sleep problems to non-carrier controls (62.7 vs. 32.6%; P = 0.001).
Specifically, about social phobia, Roberts et al. (2009) [49] did not find an increase among carriers of the premutation even though authors such as Franke et al. (1998) [47] reported an increased risk independent of having children with FXS. The weakness of the data provided by Franke et al. (1998) [47] to draw such conclusion lies on the small number of carriers without FXS children recruited.
Carriers of the premutation have also been associated with the development of autistic disorder and autism spectrum disorders, however, most of the studies have been done in boys. In girls, Clifford et al. (2007) [52] found that 4.7% of carriers met the Autism Diagnostic Observation Schedule-Generic (ADOS-G) criteria for autism spectrum disorders vs. 14.3% of boys. Other conditions reported in the literature include eating disorders, polysubstance abuse, and somatoform disorders independent of the presence or absence of FXTAS [53].
Involvement of other systems
Several studies attempting to describe the full extent of phenotypical involvement among women carriers of the premutation have encountered certain degree of endocrine, vascular, and autoimmune involvement.
Coffey et al. (2008) [36] reported that among those carriers with definite or probable FXTAS, there was an increased prevalence of thyroid disease (50%) and hypertension (61.1%) which was statistically significant when compared to controls. An increased prevalence of thyroid disease (17%) and hypertension (16.4%) was also found in the carrier group without FXTAS, but this difference was not statistically significant when compared to controls. Among the thyroid problems reported, both hypothyroidism and hyperthyroidism were present.
Hunter et al. (2010) [40] performed an exploratory analysis using self-reported medical histories in a sample of 334 women carriers of the premutation and found a slightly higher prevalence of thyroid disease which was not significantly elevated. However, the author relied on self-reported diagnosis by participants in order to estimate the frequency of thyroid problems, which may have led to an underestimation of the real prevalence since many of these patients may have had undiagnosed thyroid disease.
Regarding autoimmune diseases, recently, Winarni et al. (2012) [54] studying 344 women with the premutation aged 19–81 found that among study participants 44.77% had at least one immune-mediated disorder, being autoimmune thyroid disorder the most common (24.4%) followed by fibromyalgia (10.2%). The authors found a strong association between FXTAS, FXPOI, and immune-mediated disorders among premutation carriers; the latter means that statistically significant differences for immune-mediated disorders as a group were found when comparing carriers with FXTAS and carriers without FXTAS, as well as only carriers with FXTAS with controls; however, no difference was found between carriers without FXTAS and controls. The same statistically significant difference was found when comparing only autoimmune thyroid disease and fibromyalgia as individual entities. When FXPOI was used as the variable instead of FXTAS, the same reported difference was found between groups for autoimmune disorders as a whole; however, no difference was found for individual autoimmune entities, including autoimmune thyroid disease and fibromyalgia.
Discussion
Female fragile X premutation carriers have variable expression of disease and differ regarding the frequencies of each condition. Frequencies of certain conditions may increase when women are affected by FXTAS and/or FXPOI; this may either reflect a direct consequence of the premutation or a direct effect of FXTAS and/or FXPOI and not of the premutation itself.
For example, Coffey et al. (2008) [36] reported that women with probable or definite FXTAS had greater medical comorbidity, including a significantly increased prevalence of thyroid disease, hypertension, seizures, peripheral neuropathy, and fibromyalgia. Instead, non-FXTAS carriers had a significantly increased prevalence of muscle pain, tremor, numbness, and tingling in the extremities, whereas, although there was an increased prevalence of thyroid problems, hypertension, and neuropathy, this difference was not statistically significant when compared to controls. Similarly, Winarni et al. (2012) [54] found that among carriers affected by either FXTAS or FXPOI, immune-mediated disorders were significantly more common compared to controls; however, this significant difference was not found when carriers without those conditions were used as the comparison group.
Now, why do these patients report significantly more comorbidities? Maybe the mRNA toxicity produces a continuum of phenotypical manifestations directly related to its toxic effects. This deleterious effect may be such that when women have reached the extreme of toxicity leading to almost or complete fulfillment of the FXTAS criteria, for example, more systems throughout the body become compromised, leading to all of the reported comorbidities.
One downfall for this hypothesis would be the fact that for this to be true, carrier men would also need to have more comorbidities, and Hunter et al. (2010) [40] did not find a higher rate of medical conditions among carrier men when compared to controls. Also, if this were to be true, and the pathologic mechanism underlying FXPOI was RNA toxicity, then those women affected by FXTAS who have reached the tail of the distribution of RNA toxicity would be more affected by FXPOI than women carriers without FXTAS. This was not found by Coffey et al. (2008) [36] who reported a prevalence of POI in the non-FXTAS group of 19% and of 13% in the FXTAS group. This difference was not significant, which may suggest that either POI is not caused by the same mechanism of FXTAS or its penetrance may be influenced by other factors.
Women with fragile X premutation face many challenges in their life, including menstrual abnormalities, infertility, menopausal abnormalities, risk of having an offspring with a premutation or full mutation, and neuromuscular and emotional problems. Awareness of these risks and correlation of the various manifestations could help in early diagnosis and coordination of care and services for these women. In addition, reproductive health providers could also assist in facilitating genetic counseling and testing of family members. If confirmed, the patients might benefit for an integral and collaborative approach with other medical specialties including medical genetics, neurology, psychiatry, endocrinology, and rheumatology. From the reproductive point of view, finding an FMR1 premutation before signs or symptoms of POI gives us the option to offer patients fertility preservation options including oocyte cryopreservation or embryo freezing, or the fact of knowing that there is an increased subfertility risk in the future might lead patients to change their immediate family plans and attempt to start a family earlier than planned without the aid of assisted reproductive technologies.
In conclusion, women carriers of the FMR1 premutation are at risk for diverse conditions other than POI including FXTAS, dementia, hypothyroidism, hypertension, seizures, fibromyalgia, autoimmune diseases, neuropathies, migraines, and psychiatric conditions including postpartum depression. It is likely that the studies done so far have not been able to capture these patients’ whole phenotypical spectrum; however, the information that we have to date is strong enough to build a collaborative network of care for these women involving multiple specialties, with a “look-out” role for reproductive health care specialists.
References
Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–58.
Maddalena A, Richards CS, McGinniss MJ, Brothman A, Desnick RJ, Grier RE, et al. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001;3(3):200–5.
Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7(8):584–7.
Genetics ACoOaGCo. ACOG Committee Opinion No 469: carrier screening for fragile X syndrome. Obstet Gynecol. 2010;116(4):1008–10.
Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, et al. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1(6):397–400.
Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–22.
Jin P, Warren ST. Understanding the molecular basis of fragile X syndrome. Hum Mol Genet. 2000;9(6):901–8.
Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, et al. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112(3):317–27.
Kim M, Bellini M, Ceman S. Fragile X mental retardation protein FMRP binds mRNAs in the nucleus. Mol Cell Biol. 2009;29(1):214–28.
Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10(14):1449–54.
Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain. 2006;129(Pt 1):243–55.
Gokden M, Al-Hinti JT, Harik SI. Peripheral nervous system pathology in fragile X tremor/ataxia syndrome (FXTAS). Neuropathology. 2009;29(3):280–4.
Greco CM, Soontrapornchai K, Wirojanan J, Gould JE, Hagerman PJ, Hagerman RJ. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. J Urol. 2007;177(4):1434–7.
Hantash FM, Goos DM, Crossley B, Anderson B, Zhang K, Sun W, et al. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet Med. 2011;13(1):39–45.
Tassone F, Iong KP, Tong TH, Lo J, Gane LW, Berry-Kravis E, et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012;4(12):100.
Oostra BA, Willemsen R. FMR1: a gene with three faces. Biochim Biophys Acta. 2009;1790(6):467–77.
Murray A, Ennis S, MacSwiney F, Webb J, Morton NE. Reproductive and menstrual history of females with fragile X expansions. Eur J Hum Genet. 2000;8(4):247–52.
Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007;87(3):456–65.
De Caro JJ, Dominguez C, Sherman SL. Reproductive health of adolescent girls who carry the FMR1 premutation: expected phenotype based on current knowledge of fragile x-associated primary ovarian insufficiency. Ann N Y Acad Sci. 2008;1135:99–111.
Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in fragile X study—preliminary data. Am J Med Genet. 1999;83(4):322–5.
Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2006;14(2):253–5.
Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–12.
Tejada MI, García-Alegría E, Bilbao A, Martínez-Bouzas C, Beristain E, Poch M, et al. Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause. 2008;15(5):945–9.
Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22(8):2142–52.
Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004;89(9):4569–74.
Rohr J, Allen EG, Charen K, Giles J, He W, Dominguez C, et al. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod. 2008;23(5):1220–5.
Chonchaiya W, Nguyen DV, Au J, Campos L, Berry-Kravis EM, Lohse K, et al. Clinical involvement in daughters of men with fragile X-associated tremor ataxia syndrome. Clin Genet. 2010;78(1):38–46.
Bibi G, Malcov M, Yuval Y, Reches A, Ben-Yosef D, Almog B, et al. The effect of CGG repeat number on ovarian response among fragile X premutation carriers undergoing preimplantation genetic diagnosis. Fertil Steril. 2010;94(3):869–74.
Gleicher N, Weghofer A, Lee IH, Barad DH. Association of FMR1 genotypes with in vitro fertilization (IVF) outcomes based on ethnicity/race. PLoS One. 2011;6(4):e18781.
Kushnir VA, Yu Y, Barad DH, Weghofer A, Himaya E, Lee HJ, et al. Utilizing FMR1 gene mutations as predictors of treatment success in human in vitro fertilization. PLoS One. 2014;9(7):e102274.
Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, et al. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet. 1993;3(1):36–43.
Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57(1):127–30.
Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291(4):460–9.
Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72(4):869–78.
Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74(5):805–16.
Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A(8):1009–16.
Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xunclà M, Badenas C, Kulisevsky J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009;17(10):1359–62.
Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, et al. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22(14):2018–30. quiz 140.
Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS). Neurology. 2007;69(9):851–9.
Hunter JE, Rohr JK, Sherman SL. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet. 2010;77(4):374–81.
Au J, Akins R, Berkowitz-Sutherland L, Tang HT, Chen Y, Boyd A, et al. Prevalence and risk of migraine headaches in adult fragile X premutation carriers. Clin Genet. 2013;84:546–51.
Bourgeois JA, Coffey SM, Rivera SM, Hessl D, Gane LW, Tassone F, et al. A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry. 2009;70(6):852–62.
Karmon Y, Gadoth N. Fragile X associated tremor/ataxia syndrome (FXTAS) with dementia in a female harbouring FMR1 premutation. J Neurol Neurosurg Psychiatry. 2008;79(6):738–9.
Al-Hinti JT, Nagan N, Harik SI. Fragile X premutation in a woman with cognitive impairment, tremor, and history of premature ovarian failure. Alzheimer Dis Assoc Disord. 2007;21(3):262–4.
Rodriguez-Revenga L, Pagonabarraga J, Gómez-Anson B, López-Mourelo O, Madrigal I, Xunclà M, et al. Motor and mental dysfunction in mother-daughter transmitted FXTAS. Neurology. 2010;75(15):1370–6.
Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, Gane LW, et al. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012;11(5):577–85.
Franke P, Leboyer M, Gänsicke M, Weiffenbach O, Biancalana V, Cornillet-Lefebre P, et al. Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Res. 1998;80(2):113–27.
Johnston C, Eliez S, Dyer-Friedman J, Hessl D, Glaser B, Blasey C, et al. Neurobehavioral phenotype in carriers of the fragile X premutation. Am J Med Genet. 2001;103(4):314–9.
Roberts JE, Bailey DB, Mankowski J, Ford A, Sideris J, Weisenfeld LA, et al. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(1):130–9.
Obadia RW, Iosif A-M, Seritan AL. Postpartum depression in women with the FMR1 premutation. Curr Psychiatr Rev. 2013;9(1):72–6.
Bourgeois JA, Seritan AL, Casillas EM, Hessl D, Schneider A, Yang Y, et al. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2011;72(2):175–82.
Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J Autism Dev Disord. 2007;37(4):738–47.
Seritan AL, Ortigas M, Seritan S, Bourgeois JA, Hagerman RJ. Psychiatric disorders associated with FXTAS. Curr Psychiatr Rev. 2013;9(1):59–64(6).
Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, et al. Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A. 2012;158A(10):2473–81.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule This paper reviews current evidence regarding the possible conditions that may present in women with premutation-sized repeats beyond FXPOI.
Rights and permissions
About this article
Cite this article
Hoyos, L.R., Thakur, M. Fragile X premutation in women: recognizing the health challenges beyond primary ovarian insufficiency. J Assist Reprod Genet 34, 315–323 (2017). https://doi.org/10.1007/s10815-016-0854-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0854-6