Abstract
Fragile X-associated primary ovarian insufficiency (FXPOI) is among a family of disorders caused by the expansion of a CGG repeat sequence located in the 5′ untranslated region of the X-linked gene FMR1. About 20 % of women who carry a premutation have cessation of menses for at least 1 year prior to age 40, a 20-fold increased risk compared with the general population. Further, the frequency of women with the premutation attending reproductive endocrinology clinics for infertility is significantly increased compared with the carrier frequency expected in the general population, ~3 % compared with 1/150–1/250. This makes FXPOI the leading known inherited cause of idiopathic POI. Cross-sectional studies clearly show the health burden related to the clinical outcomes associated with FXPOI. Despite the significant reduction in reproductive life span and increased frequency of estrogen deficiency-related medical conditions among women with the premutation who are diagnosed with FXPOI, little is known about the underlying molecular etiology. In this chapter, we focus on describing the clinical manifestation of FXPOI, the risk factors that potentially lead to earlier onset and severity, and the clinical management that can help ameliorate symptoms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Overview and Focus
Reproductive health is a strong predictor of overall health and well-being. One marker of reproductive health in women is the age at natural menopause. The median age of menopause is ~51 ± 1 years, with 1 % of women experiencing menopause prematurely (Palacios et al. 2010). In the late 1990s and early 2000s, the FMR1 premutation was established as an important genetic cause of premature ovarian failure (POF) (reviewed in Sherman 2000). At that time, the term POF was defined as 12 months of secondary amenorrhea before age 40 and the majority of studies were based on this phenotype. The clinical definition and the term used for the observed ovarian dysfunction have changed. The term primary ovarian insufficiency (POI) is clinically defined as 4 months of oligo/amenorrhea before age 40 with two follicle stimulating hormone (FSH) levels in the menopausal range (as defined by the measuring laboratory) 1 month apart (Nelson 2009). In the current field of fragile X, FXPOI is used to encompass the spectrum of reproductive outcomes that includes not only cessation of regular menses before age 40, but also occult indicators of impaired ovarian function, which are manifest by a reduced ovarian response to stimulation, but no alteration in menstrual cyclicity.
FXPOI significantly impacts a woman’s quality of life. The most immediate and significant consequence of diminished ovarian function is reduced fertility (Allen et al. 2007; Streuli et al. 2009; Wheeler et al. 2014). If we define POI here as cessation of menses for at least 1 year prior to age 40, POI occurs in ~20 % of women with the premutation, about ~20 times higher than the general population (for reviews, see De Caro et al. 2008; Sherman 2000; Sullivan et al. 2011). Taking all women who carry the premutation, they go through menopause on average about 5 years earlier than those without the premutation (Murray 2000; Seltzer et al. 2012; Sullivan et al. 2005). Since diminished ovarian reserve precedes POI by 10 or more years, impaired fertility may be seen in women with the premutation before the more typical decline in fertility observed after age 35.
The premutation occurs at a significantly higher frequency among women with ovarian dysfunction (e.g., Barasoain et al. 2013; Karimov et al. 2011; Mallolas et al. 2001; Murray et al. 2014; Pu et al. 2014; Streuli et al. 2009; Tosh et al. 2014; Ye et al. 2014). In a recent study of over 2000 women who experienced menopause before the age of 46 years, the prevalence of the premutation was 2.0 % among women with cessation of menses prior to age 40, 0.7 % in those with cessation between 40 and 45 years, and 0.4 % in controls. Combining women with cessation of menses prior to age 46, the odds ratio for carrying the premutation was 2.4 (95 % confidence interval = 1.02–5.8; p = 0.04). In studies that separated isolated and familial cases of POI, about 3.2 and 11 % of women carried the premutation (reviewed in Sherman et al. 2007).
Notably, the state of early estrogen deficiency observed among women with POI has significant clinical consequences, including an increased risk for low bone mineral density, earlier onset osteoporosis and bone fractures (Gallagher 2007), impaired endothelial function (Kalantaridou et al. 2004), earlier onset of coronary heart disease (Atsma et al. 2006), and increased cardiovascular mortality and overall mortality (e.g., Jacobsen et al. 2003; Mondul et al. 2005). In addition, women with an earlier age at menopause are reported to have more anxiety, depression, somatization, sensitivity, hostility, and psychological distress than women with normal ovarian function (e.g., Van Der Stege et al. 2008). Hypoestrogenism has cognitive consequences as well. Estrogen is a neuroprotective agent that plays an important role in brain functioning, and changes in estrogen levels during aging are associated with reduced cognitive function and an increased risk of Alzheimer disease (Janicki and Schupf 2010).
Thus, women with FXPOI are at risk for these clinical disorders associated with prolonged estrogen deficiency. But it is important to emphasize that they are also at risk for other premutation-associated symptoms, most importantly neurodegenerative symptoms associated with fragile X-associated tremor/ataxia syndrome (FXTAS) . As such, they may be more vulnerable to effects of early estrogen deficiency. The clinical manifestation of FXTAS in general is discussed in Chaps. 1–4 and FXTAS and other premutation-associated disorders specifically in women are reviewed in Chap. 12. At this point, a natural history study has not been conducted to determine whether women with FXPOI are at a higher risk for FXTAS than those without FXPOI.
Little is known about the mechanism that underlies FXPOI. Other repeat expansion disorders , including FXTAS, complemented by model systems of these disorders, have provided important insights into the possible etiology underlying FXPOI. These are described in detail in Chap. 11. As noted, not all women with the premutation experience POI —some have complete cessation of menses in their 20s while others go through menopause at the typical age of 51 (reviewed in De Caro et al. 2008). Why there is such extensive variation in the clinical outcomes of the FMR1 premutation is unknown. In this chapter, we will focus on the clinical manifestation of FXPOI, the risk factors that potentially lead to earlier onset and severity, and the clinical management that can help ameliorate symptoms.
Primary Ovarian Insufficiency
A woman’s reproductive life is based on the complex orchestration of the hypothalamic-pituitary-ovarian (HPO) axis. During early ovarian development, oocytes are organized into functional units called follicles. Each follicle consists of a single oocyte and associated granulosa cells. A woman’s maximum endowment of oocytes (about six to seven million) occurs early in gestation, around 16–20 weeks (Baker 1963; Houmard et al. 2001). Through a natural process of follicular atresia , only about one to two million oocytes remain at birth and only 300,000–400,000 remain at menarche (Baker 1963; Block 1952). Thus, in the natural processes of ovarian development and age-related decline in ovarian function, a woman’s pool of oocytes starts to decline well before she achieves reproductive maturity.
During a woman’s reproductive years, primordial follicles are recruited and mature through a hormonally regulated process. The result of this process is a single oocyte selected for monthly ovulation. Due to the monthly ovulation and continued follicular atresia, the number of primordial follicles declines until about 1000 remain and the menopause ensues (Faddy 2000; Richardson et al. 1987). Age-related changes in both the quantity and quality of oocytes/follicles are responsible for the typical features of the age-related decline in ovarian function in women. These include characteristic hormonal fluctuations (elevated gonadotropins and decreased ovarian hormones), menstrual cycle changes (amenorrhea, oligomenorrhea, and dysfunctional uterine bleeding), diminished fertility and higher rates of twinning, aneuploidy and miscarriage.
The term “ovarian reserve” refers to a woman’s remaining fertility potential attributable to her ovaries. However, this term is misleading and scientifically inaccurate because the biological measures to assess “ovarian reserve” are all based on ovarian response, not reserve. In this chapter, we will use “ovarian response” to refer to the state of ovarian function. As described below, many measures of ovarian response in women with FXPOI are consistent with significantly impaired ovarian function. However, we emphasize that FXPOI is not equivalent to an early natural menopause (Nelson et al. 2005). Menopause is defined as the permanent cessation of menses, and with this, the end of fertility. Women sometimes conceive naturally after getting the diagnosis of FXPOI.
As noted previously, POI is a more general term that is now used to encompass diagnostic conditions including what in the past has been referred to as premature menopause or POF, hypergonadotropic hypogonadism, and ovarian dysgenesis. Overall, this term describes impaired ovarian function on a continuum, rather than a specific endpoint (Welt 2008). The diagnosis and experience of POI is difficult as it can be transient and progressive (Nelson 2009). By the age of 20 years, POI affects about 1/10,000 women and 1/100 by the age of 40 years (Coulam et al. 1986; Cramer and Xu 1996; Goswami and Conway 2005; Kim et al. 1997). These estimates appear to differ by ethnic/racial group, indicating modifying factors (Luborsky et al. 2003). Both genetic and environmental causes of POI are known and have been extensively reviewed (e.g., Santoro, 2003; Cox and Liu 2014); however, the cause of POI in most women remains unknown.
The diagnosis of POI depends on the clinical picture (at least 4 months of oligo/amenorrhea) as well as the hormonal profile showing increased levels of FSH. Women with POI often go many years before a correct diagnosis is made. In fact, women with POI may have clinical symptoms wax and wane with periods of regular menses and amenorrhea further masking the underlying dysfunction . Natural menopause occurring after age 45 is often accompanied by hot flashes, night sweats, sleep problems, mood changes, and vaginal dryness (National Institute of Health 2005). POI occurs earlier in life and can be associated with residual ovarian function including return of menses, ovulation, and possible pregnancy (Hubayter et al. 2010; Nelson et al. 1994; Van Kasteren and Schoemaker 1999). The full experience of symptoms for natural age at menopause is becoming well recognized; however for women with POI, more work needs to be done. Studies suggest that the unexpected and, sometimes intractable, infertility at a young age, along with early and prolonged exposure to estrogen deficiency can be traumatic and can exacerbate typical menopause-related symptoms. Indeed, Allshouse et al. (2015) have shown that menopause symptoms in women with POI are not adequately captured by the typical menopause symptom checklist. Their symptoms do not appear to diminish over time in contrast to women going through natural age at menopause. For example, in their sample, women with POI experienced depression prior to their diagnosis and hypothyroidism appeared to be more frequent compared with aged-norms.
Three potential mechanisms can underlie POI. These include: (1) a congenital decrease in primordial follicles, (2) accelerated follicular atresia, and (3) impaired ovarian follicle function. The various causes of POI are sometimes categorized as genetic, autoimmune, metabolic dysfunction, infectious, and iatrogenic; however, these groups clearly overlap as some are descriptive and some are etiologic based. Irrespective, FXPOI is a leading cause of the genetic forms of POI.
FXPOI: Clinical Description
The primary FXPOI-related trait that has been measured among women who carry the premutation is age at menopause, defined as cessation of menses for at least 1 year. As with all epidemiological studies of menopause, self-reported age at menopause is problematic. Pinpointing an event that has a long transition period and is somewhat ambiguous depending on symptoms is not easy. This is particularly true for cross-sectional surveys that involve recall. Moreover, many women are prescribed hormone medication as soon as cycle traits become variable or symptoms of menopause occur. Some women on hormone treatment may have had the ability to continue cycling naturally and some may not; hormone treatment masks this distinction. This, of course, complicates the ability to define a specific age at menopause, but if measured using the same definition within or among studies, significant patterns emerge among women who do and do not carry the premutation.
Increased Rate of POI Associated with the FMR1 Premutation
Schwartz et al. (1994) were the first to provide preliminary evidence for a premutation-associated reproductive disorder after the FMR1 mutation was identified. Subsequently, a collaborative, multisite study was conducted to specifically confirm that women with the premutation, not the full mutation, were at risk for POI (Allingham-Hawkins et al. 1999). Although definitions of premutation allele sizes differed as well as the criteria for POI along with study protocols, the estimates of prevalence of POI, here defined as cessation of menses for at least 1 year prior to age 40, among women who carry the premutation in subsequent studies all exceeded the expected 1 % in the general population (Allen et al. 2007; Coffey et al. 2008; Hundscheid et al. 2003a; Machado-Ferreira et al. 2004; Mallolas et al. 2001; Murray et al. 2000; Rodriguez-Revenga et al. 2009b; Sullivan et al. 2005; Uzielli et al. 1999a; Vianna-Morgante et al. 1999b; Wheeler et al. 2014). Based on a review of studies that interviewed women with the premutation at age 40 and older, the best estimate of the penetrance of POI was 15.5 % (70/451) (95 % CI: 12.3–19.2 %) and women without the premutation was 1.7 % (6/359) (95 % CI: 0.6–3.6 %). The estimate of early menopause, or menopause prior to the age of 45 years, was 20.0 % (47/235) (95 % CI: 15.1–25.7 %) and 4.0 % (6/149) (95 % CI: 1.5–8.6 %) among women with and without the premutation, respectively (Sherman et al. 2007). In a more recent study of 88 women with the premutation who reported experiencing menopause and were mothers of children with FXS, 35 % reported POI but only 18 % had been medically diagnosed with FXPOI (Mailick et al. 2014).
Defining a POI phenotype during adolescence is difficult due to the high variability in menstrual cycle characteristics that occur naturally during this reproductive stage (Chiazze et al. 1968; Treloar et al. 1967; Vollman 1977), in addition to the reduced ability to assess fertility. Combining data from two studies that obtained self-reported cessation of menses for at least 1 year prior to age 40 (Allingham-Hawkins et al. 1999; Uzielli et al. 1999b), 3 of 106 (~3 %) women with the premutation enrolled at ages 18–29 had already experienced POI. Combining data from two additional studies (Murray et al. 2000; Vianna-Morgante et al. 1999a), 7 of 217 (~3 %) women with the premutation reported the onset of menopause at or before age 29 and 3 of 217 (~1.4 %) reported menopause prior to age 18. These estimates among women with the premutation suggest a significant increase in risk compared with that in the general population, in which the incidence of menopause between ages 15 and 29 is estimated to be only 1 in 1000 (Goswami and Conway 2005).
Decreased Age at Menopause
An important question is to ask whether only a subset of women with the premutation are affected with FXPOI (incomplete penetrance) or whether the age at menopause distribution is decreased among all women with the premutation (variable expressivity). Although these alternatives are not mutually exclusive, they may provide insights toward mechanism and treatment. Previous studies estimated the age at menopause among women with the premutation using survival models. Sullivan et al. (2005) incorporated hormone use into their age at menopause definitions as follows: (1) age at her last menses irrespective of hormone use; (2) age at her last menses, but if she had started hormone medication prior to that event, she was censored (or dropped from the analysis) at the age she started hormone medication; or (3) age at last menses or age at the start of hormone medication. They found that the mean age at menopause among all women with the premutation given the definitions above was 47.5 ± 0.7, 47.7 ± 0.8, and 46.6 ± 0.7 and among women without the premutation 52.2 ± 0.6, 53.1 ± 0.7, and 51.5 ± 0.6, respectively. These results show a consistent 5-year reduction in the age at menopause between women with and without the premutation, irrespective of the definition. Murray et al. (2000) found similar results using the more conservative definition of age at menopause, censoring women when they began hormone medication (definition #2 as defined above) and using survival analyses found similar results: 47.9 years (SEmean = 0.9) among women with the premutation compared with 53.0 (SEmean = 0.8) in women without the premutation. In a population-based sample of older adults in Wisconsin, 20 premutation carrier women had an average age at menopause (defined as last day of menstrual period) of 48.1 years compared with 50.8 years among women without the premutation (n = 1893) (Seltzer et al. 2012). This smaller difference may be due to the average repeat size identified among women with the premutation in this screened sample (see below).
Allen et al. (2014) tested the hypothesis that there is a subset of women who are at risk for ovarian dysfunction (i.e., incomplete penetrance). To do this, they simply examined the age at menopause distribution among women with the premutation, including and excluding those who reported cessation of menses for at least 1 year prior to age 40. They used the first definition described above (i.e., age at her last menses irrespective of hormone use). They considered this to provide a conservative estimate, as hormone use during transition may mask menopause and thus, lead a woman to report a later age at menopause. To test whether the differences in mean menopause age between women with and without the premutation were statistically significant, they estimated hazard ratios using a Cox proportional hazard model, adjusting for age at interview, racial/ethnic group, body mass index, and ever having smoked. For each premutation repeat group (55–79 repeats, 80–99 repeats and 100–200 repeats), the hazard ratio was significantly increased, indicating a higher risk for reaching menopause earlier among women with the premutation compared with women without the premutation, irrespective of the inclusion or exclusion of women who reported having FXPOI . This suggests that the majority of women with the premutation, not just a subset, experience ovarian insufficiency earlier than those without the premutation. This idea is supported by the results of Wheeler et al. (2014) who compared women with the premutation who did or did not have a diagnosis of FXPOI using a self-reported survey. They found that about 20 % of women without FXPOI also reported irregular or absent periods (18.4 %), early menopause (21.4 %), and 12.6 % also had difficulty getting pregnant.
Altered Hormonal Profile Among Women with Menstrual Cycles
There are many biomarkers that are used to indirectly measure ovarian response. These include the pituitary hormones (i.e., follicle stimulating hormone (FSH) and luteinizing hormone (LH)) and the ovarian hormones (i.e., estradiol (E2), progesterone (P4), inhibin B, inhibin A, and anti-müllerian hormone (AMH)). FSH, a gonadotropin hormone, is the most established measure of ovarian response. AMH, also called müllerian-inhibiting substance (MIS), is a promising biomarker as many studies have reported a strong association between AMH levels and surrogate measures of ovarian response (including chronological age, antral follicle counts, time to menopausal transition and response to IVF) (e.g., De Vet et al. 2002; Fanchin et al. 2003; Seifer et al. 2002; Tremellen et al. 2005; Van Rooij et al. 2004). Also, several studies have found AMH to be more sensitive than the other commonly used markers (FSH, inhibin B, E2) (e.g., Fanchin et al. 2003; Hazout et al. 2004).
The most comprehensive study of the hormonal profile of women with the premutation was conducted by Welt et al. (2004). They hypothesized that women with the premutation with menstrual cycles would show hormonal changes characteristic of early age-related decline in ovarian function. They identified 11 women who carried the premutation who were still cycling, ages 24–41, and drew daily blood samples across one menstrual cycle. LH, FSH, E2, P4, inhibin A, and inhibin B levels were compared with levels in 22 age-matched, women without the premutation who were still cycling. They found that FSH was elevated across the follicular and luteal phases in women with the premutation compared to controls. Inhibin B in the follicular phase and inhibin A and progesterone in the luteal phase were all decreased in women with the premutation compared to controls. There was no difference in E2 or LH between groups. Thus, despite regular ovulatory cycles, FSH was increased in women with the premutation compared to controls and was accompanied by decreased inhibin B in the follicular phase and inhibin A and P4 in the luteal phase. These data are consistent with the hypothesis that women with the premutation show early age-related decline in ovarian function despite regular menstrual cycles . This profile may result from decreased follicle number and/or function, as reflected by lower inhibin B, inhibin A, and P4 levels.
In addition to this comprehensive study of a small number of women who carry the premutation, other studies have measured single serum FSH measurement taken during the early follicular phase of the cycle (day 2–5) among large study samples. This design is not ideal, as a woman’s hormone profile can fluctuate significantly from month to month, particularly during the transition into menopause. Nevertheless, it allows a relatively noninvasive assessment of ovarian response and valid comparisons of means from large groups of women. Based on this design, the mean level of FSH among women who carried the premutation and were still cycling was significantly higher than that of noncarriers and of full mutation relative controls (Hundscheid et al. 2001; Murray et al. 1999) (adjusting for age and familial effects in the study of Murray et al. 1999 and adjusting for age, familial effects, smoking behavior, and use of oral contraceptives in the study of Hundscheid et al. 2001). In the study of Sullivan et al. (2005), they found increased levels of FSH only among women with the premutation aged 30–39 compared to women without the premutation. They did not find this same increase among women with the premutation in the younger or older age groups. They suggested that the lack of a difference among the older group (40–50 years) was due to a selection bias: those with POI (i.e., those who were not cycling) were not included in the study.
Rohr et al. (2008) measured both AMH and FSH in a cross-sectional study of women between ages 18 and 50 years. They found that serum AMH was reduced compared with women who do not carry the premutation, even at early ages (18–30 years). In contrast, elevated FSH indicative of early impaired ovarian response was only evident in older women who carried the premutation (31–40 years). These data suggested that AMH may be a better marker than FSH in identifying early stages of impaired ovarian response.
In a larger sample of 240 women ascertained through families with FXS (n = 127 women who carried the premutation and n = 113 women without the premutation; this sample includes that of Rohr et al. 2008), Spath et al. (2011a) found that women who carried the premutation showed reduced levels of AMH compared to women without the premutation at all ages. For all women, AMH was found to decrease by 10 % per year. The added effect of carrying a premutation decreased AMH levels by 54 %. They also modeled the decline in AMH levels and, using the subset of longitudinal samples (n = 41), found that it may be possible to create a standardized AMH using carrier status and age to potentially serve as a predictor for FXPOI.
Altered Menstrual Traits
Alteration of menstrual cycle traits can be the first indicator of POI and can provide insight into underlying pathology; thus, it is important to characterize these traits among women with the premutation. With respect to age at menarche, girls with the premutation appear to begin menses at the same time as girls without the premutation (Allen et al. 2007; Burgess et al. 1996; Hundscheid et al. 2003a). With respect to menstrual cycle characteristics, Schwartz et al. (1994) were the first to provide evidence that women with the premutation reported irregular menses more often than those without the premutation. Welt et al. (2004) showed that the length of the total cycle and of the follicular phase were significantly decreased in women with the premutation compared to their controls, whereas luteal phase length was similar. Subsequently, Allen et al. (2007) found that women with the premutation, and most significantly those with 80–100 repeats premutation alleles, reported shorter, irregular length and skipped cycles more often than women without the premutation. They found no difference in menstrual cycle bleeding length by carrier status. In the survey of Wheeler et al. (2014) women with FXPOI reported high rates of irregular or absent periods, early menopause, and difficulty getting pregnant at the time of the survey . The presence of these and of menstrual cycle symptoms were, of course, dependent on the diagnosis at the time the survey was taken.
Pre-, Peri-, and Postpartum Experiences
Kallinen et al. (2000) studied singleton pregnancies of 63 women with the FMR1 expansions (55 with the premutation and 8 with the full mutation). Overall, they found that the pregnancy outcomes were favorable. The only complication was that women who carried the mutation experienced more bleeding late in pregnancy than did the reference group. In the study of Wheeler et al. (2014), rates of preeclampsia or high blood pressure during at least one pregnancy were found to be higher among women with the premutation compared with the general population: about 16 % and 13 % of women with the premutation, with and without a diagnosis of FXPOI, respectively, reported these findings. Although rates did not differ among those with and without a diagnosis of FXPOI, this clinically significant medical condition should be explored further to determine the underlying cause of the increased rates. Similar to Kallinen et al., no other experiences with pregnancy, birth, or labor outcomes were different among women with the premutation compared with that expected in the general population.
Rates of Miscarriages Not Increased
In addition to hormonal fluctuations and changes in menstrual cycle characteristics, the age-related decline in ovarian function in women is associated with increased pregnancy loss resulting from aneuploidy. This age-related increase in aneuploidy results from an increased rate of chromosome nondisjunction in the ovary of older women (for review, see Sherman et al. 2013). Among women with the premutation, there is no evidence of an increased rate of miscarriages or aneuploidy (Allen et al. 2007; Hundscheid et al. 2003a; Murray et al. 2000).
Increased Rates of Twinning
Dizygotic twinning (DZ) is associated with advanced maternal age, ethnicity/race, and genetic factors. The association with maternal age is thought to be due to the elevated FSH levels and reduced ovarian feedback associated with diminished ovarian response, leading to an increased chance of multiple ovulations (reviewed in Lambalk et al. 1998). There have been conflicting reports of increased twinning among women who carry the premutation compared with women without the premutation (Allen et al. 2007; Fryns 1986; Hundscheid et al. 2003b; Murray et al. 2000; Sherman et al. 1996; Turner et al. 1994; Vianna-Morgante 1999). These variable results are probably due to the age and repeat-size characteristics of the study samples. For example, Allen et al. (2007) found increased twinning among only women with mid-range premutation repeat sizes who have the highest risk for FXPOI (see below), not among low and high premutation repeat groups.
Infertility and Subfertility
Perhaps the most significant clinical manifestation of FXPOI is infertility or subfertility early in reproductive life. The most comprehensive study to estimate the frequency of fertility problems is that of Wheeler et al. (2014). They found that 46.6 % of women with FXPOI (n = 73) had difficulty getting pregnant, a significantly increased rate over those women with a premutation without a diagnosis of FXPOI (n = 365). However, among those women without FXPOI, about 12.6 % reported difficulty getting pregnant. They also asked about reproductive assistance before and after they received a diagnosis of having a premutation. They found that all women with a premutation reported an increased frequency for fertility assistance, irrespective of their diagnosis of POI. Among women with and without a diagnosis of FXPOI, 31.1 and 8.5 % used some form of reproductive assistance prior to knowing their carrier status . Fertility drugs (e.g., Clomid) were most commonly used prior to carrier status diagnosis.
Early Symptoms of FXPOI
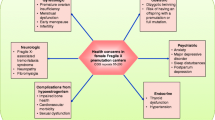
Few studies have assessed early symptoms of FXPOI. These are expected to be similar to those experienced by women transitioning into menopause, such as hot flashes (flushes), night sweats, irritability, poor concentration, depression, decreased interest in sex, and vaginal dryness. These symptoms may be particularly difficult to cope with for a young woman who is not anticipating such menopause-related symptoms early in life. In a descriptive assessment of 88 women with the premutation who had a child with FXS, the majority of women reported having at least some of these menopause-related symptoms (Mailick et al. 2014). The most common symptom was hot flushes/flashes (30.7 % experiencing this symptom “a lot”). In addition, night sweats (26.1 % experienced this symptom “a lot”), sleep disturbance (22.7 %), and depression (19.3 %) were reported.
Wheeler et al. (2014) found that headaches, menstrual symptoms, and hot flashes were reported by women with the premutation, irrespective of their diagnosis of FXPOI. The frequency of reporting tended to decrease in the older age groups, while joint pain, for example, tended to be reported at higher rates by older women. There is some evidence that experiencing such symptoms or reporting of such symptoms may be increased among women with increased stress. For example, Smith et al. (2012) surveyed mothers of adolescents and adults with FXS (n = 112), mothers of adolescents and adults with autism spectrum disorders (ASD, n = 96) and compared them to a nationally representative sample of mothers with similarly aged children without disabilities (n = 230). They found that both groups of women with children who had disabilities reported a significantly increased frequency of hot flashes/flushes compared to controls.
Other Associated Disorders
Women with the premutation are more likely to experience estrogen deficiency at an earlier age than women in the general population. As such, the premutation becomes a biomarker for a population of women who are at risk of early estrogen deficiency-associated morbidity and mortality. Hundscheid et al. (2003a, b) were the first to investigate medical conditions associated with estrogen deficiency among 152 women with the premutation and 112 controls (women with the full mutation or women with a normal FMR1 genotype), all ascertained from families with FXS. They asked about the following medical conditions: hypertension, hypercholesterolemia, cardiovascular disease, thrombosis, reduction of bone mineral density, subfertility, uterus extirpation, rheumatoid arthritis, type 2 diabetes, hyperthyroidism, hypothyroidism, and reproductive-associated cancers (breast, ovarian, endometrial). The only statistically significant difference between groups was the increase in bone loss among women with the premutation. Among the women with the premutation who reported bone loss, all went through menopause before the age of 44 years. Allen et al. (2007) also found an increase in self-reported osteoporosis, but only among the highest risk group for FXPOI (those with 80–100 repeats). Other health conditions specifically reported (autoimmune disorders, lupus, diabetes, Graves disease, breast cancer, ovarian cancer, hysterectomy) were not increased among women with the premutation. One limitation of both studies was that the study population was relatively young for these later-onset complications.
Several studies compared health conditions among women with and without indications of POI (e.g., irregular cycles) or a diagnosis of FXPOI. Among 325 women with the premutation, Hunter et al. (2010) found that women who were experiencing irregular cycles at the time of their evaluation reported higher rates of thyroid problems and depression/anxiety compared with those with no indication of ovarian dysfunction. Kenna et al. (2013) studied a small sample of 46 mothers who had at least one child with FXS. They found that women with the premutation had an earlier mean age of menopause (mean age = 45.6 years) and a high rate (76 %) of lifetime depressive or anxiety history, with 43 % of the reporting a comorbid history of both diagnoses. However, they found no evidence that their psychiatric history was related to their ovarian dysfunction.
In the largest study to date, Wheeler et al. (2014) separated women by their diagnosis of FXPOI (n = 75 with and 365 without FXPOI) and compared their self-reported diagnostic history of seven medical conditions, some of which are associated with estrogen deficiency: thyroid disease, hypertension, autoimmune disease, heart disease, gastrointestinal (GI) issues, seizures, and diabetes. Although they found high reporting rates, especially thyroid (24 and 19 % among those with and without FXPOI) and GI problems (32 and 23 % among those with and without FXPOI), there was no difference based on their FXPOI diagnosis. Increasing age was a significant predictor of the frequency of most of these conditions. Winarni et al. (2012) focused on the risk of immune-mediated disorders (IMDs) among women with the premutation using a survey. Among women who were 40 years or more, there were increased rates of IMD among the 41 women with the premutation with a diagnosis of FXPOI (66 %) compared with 147 women with the premutation without FXPOI (46 %) and compared with 50 controls (34 %). Among women with the premutation, the most commonly reported IMD was autoimmune thyroid disorder (24.4 %).
Wheeler et al. (2014) also asked about five psychological or educational (depression, anxiety, ADHD, learning disabilities, speech/language disorder) diagnoses. As in Kenna et al. (2013), depression and anxiety were both reported at high rates: about 40 % of women with and without FXPOI reported a history of these diagnoses, but the presence of these conditions was not associated with FXPOI. Lastly, Wheeler et al. (2014) probed women about specific physical symptoms that have been reported in the literature (see Chap. 13 for details). These included questions on symptoms such as headache, fatigue, joint pain, and menstrual cycle problems experienced over the previous 30 days. They found that fatigue was the most common daily symptom endorsed: 49 and 35 % of women with and without FXPOI reported fatigue. Women with FXPOI were significantly more likely to report muscle weakness, dizziness, and nausea compared with women who were not diagnosed with FXPOI.
Risk Factors of FXPOI
Not all women with the premutation experience FXPOI ; some go through menopause at ages similar to women without the premutation. Four factors have been examined to try to explain the incomplete penetrance of POI among women with the premutation: (1) CGG repeat length, (2) skewed X-chromosome inactivation (XCI), (3) smoking, and (4) background genes. With respect to FMR1 repeat length, there is a strong nonlinear association of repeat size with severity of symptoms of FXPOI. For example, women with mid-range premutation repeats (~80–100 repeats) have the highest risk for FXPOI. Women who carry both smaller or larger premutation repeat alleles also have an increased risk of FXPOI compared to the general population, but not to the same extent as women with the mid-range premutation repeat length (Allen et al. 2007; Ennis et al. 2006; Mailick et al. 2014; Spath et al. 2011b; Sullivan et al. 2005; Tejada et al. 2008). Women with mid-range repeats have the lowest mean age at menopause compared to the other groups, an indication of increased severity of FXPOI. Using survival analysis, the unadjusted mean age at menopause for women without the premutation and for women in the three premutation categories, low (55–79 repeats), mid (80–100 repeats) and high (100–200 repeats), were 52.3 + 0.5, 48.5 + 0.7, 44.9 + 0.6, and 47.5 + 1.2, respectively (Allen et al. 2007). Similarly, symptoms of FXPOI were most frequently reported by women with the mid-range premutation. Such women had an increased risk for altered cycle traits (shortened cycle length, irregular cycles, and skipped cycles), subfertility, and dizygotic twinning. This increase in symptoms is not unexpected given the 7-year reduction in age at menopause among women with the mid-range premutation repeat length (Allen et al. 2007). These definitions of low, mid, and high premutation alleles do not have specific biological meaning. Indeed, in an analysis that did not predefine repeat size groups, women with alleles between about 65 and 90 repeats had the highest risk for FXPOI (as defined by a hazard ratio and the 95 % confidence intervals exceeding 2) (Spath et al. 2011b). Clearly, more work is needed to define the repeat size alleles that impose the highest risk and why.
As the FMR1 mutations are located on the X chromosome, it is important to consider skewed XCI is an important modifier of the risk for FXPOI. No study has found evidence for skewed XCI based on samples from fresh blood among women with the premutation (Bione et al. 2006; Mailick et al. 2014; Murray et al. 2000; Rodriguez-Revenga et al. 2009a; Spath et al. 2010; Sullivan et al. 2005; Tejada et al. 2008). Assuming that XCI in blood can be used as a proxy for the correct target tissue, one possible explanation for this observation is that the toxic effect of the premutation acts during a stage in development when both X chromosomes are active.
Lifestyle factors have been investigated as modifiable factors that may affect natural age at menopause (e.g., Sapre and Thakur 2014; Schoenaker et al. 2014). Smoking, an important modifiable risk factor, is known to reduce age at menopause. With respect to women with the premutation, smoking was also found to reduce age at menopause/FXPOI (Allen et al. 2007; Spath et al. 2011b). Importantly, the effect size of this modifiable factor appears to be similar in women with and without the premutation.
The influence of genetic variants on the onset of natural menopause is now well established (e.g., Chen et al. 2014; He et al. 2009; Shen et al. 2013; Stolk et al. 2012). Many of these variants are also found to influence age at menopause among those with early menopause or POI (e.g., Perry et al. 2013; Qin et al. 2012). An important question is whether these genetic variants also play a role in FXPOI (i.e., are such variants also important when in the presence of a single mutation of large effect size on ovarian function?). There is both indirect and preliminary direct evidence that background genes are important in defining the age of onset of menopause/FXPOI in women with a premutation.
The indirect evidence comes from two studies. Hunter et al. (2008) used a random-effects Cox proportional hazards model to analyze age at menopause on 680 women (carriers and noncarriers) drawn from 225 families with FXS and 321 women from 219 families drawn from the general population. They found a statistically significant residual additive genetic effect after adjustment for repeat length and other covariates. The study of Spath et al. (2011b) took a different approach using mean age at menopause of first-degree relatives with the same mutation status as a predictor variable for age at menopause along with smoking and repeat length. Their sample included data sets from the USA and from the Netherlands consisting of 1068 women. They found that the mean age at menopause of first-degree relatives was a statistically significant predictor of age at menopause, adjusting for repeat size and smoking status, only for women without the premutation, not for women with a premutation. This may be due to the lower power to detect such background gene effects in the presence of the major gene effect of the premutation or to the limited information in the definition of the predictor variable.
Direct evidence for the role of genes modifying the penetrance of FXPOI is mounting. First, in a preliminary study, five common single nucleotide polymorphisms (SNPs) known to influence age at menopause and age at POI (Perry et al. 2013; Stolk et al. 2012) were examined among 72 women with the premutation and known age at menopause (Allen et al. 2014). Because of the small sample size, only one primary statistical test that combined the effect of all five SNPs was conducted. They calculated “Total SNP Risk,” or the sum of the number of risk alleles among the five SNPs, weighted by the effect size per allele. They used linear regression with age at menopause as the outcome variable and repeat size and Total SNP Risk as predictor variables. The Total SNP Risk was significantly associated with age at menopause after adjusting for effect of the premutation repeat.
Others have taken a different approach to identify misregulated gene expression among carriers of the premutation. Mateu-Huertas et al. (2014) used gene expression profiling among men with and without the premutation and identified one differentially expressed gene, Early at Menopause 1 (EAP1), as a potential candidate for modifying the severity of FXPOI. This gene is a component in the hypothalamic control for the initiation of estrus in rodents and puberty and menstrual cycles in nonhuman primates (Heger et al. 2007; Lomniczi et al. 2012). To follow this lead, expression levels of EAP1 were compared among the following women: 12 women with a premutation and FXPOI, 13 women with a premutation without FXPOI, eight women without the premutation, and four women with POI who did not have a premutation. EAP1 was significantly downregulated in women with a premutation and FXPOI compared with those without FXPOI. In addition, EAP1 was also significantly downregulated in women with POI without a premutation compared with controls. Thus, decreased EAP1 levels may contribute to the ovarian dysfunction in women with FXPOI.
Alvarez-Mora et al. (2015) used whole genome expression arrays to study six women with the premutation and FXPOI and six premutation women without FXPOI along with four women without the premutation or POI. Although they did not find any gene with a significant differential gene expression, they noted pathways that showed significant alterations, primarily downregulation, of associated genes in women with FXPOI. Gene annotation and gene-set enrichment methods of the gene expression profiles suggested that FXPOI may be due to generalized deregulation of key signaling pathways involved in oocyte maturation.
As pointed out in both gene expression profiling studies, the limitation of this approach is based on the use of blood instead of the target tissue involved in FXPOI. Nonetheless, these studies provide clues that can complement results from model systems to identify important pathways on which to concentrate efforts to find therapeutic targets (see Chap. 11).
Management of FXPOI
FXPOI is one of the few conditions of POI for which a known risk factor exists, namely carrying the FMR1 premutation. This is a significant advantage, as there are ways to help manage the potential consequences of the condition prior to onset of symptoms. Layered onto this actionable situation is the complication of risk for having a child with significant behavioral problems and intellectual disability, i.e., having a child with FXS. Without trying to minimize this complex situation, here we will only outline management of FXPOI, acknowledging that the diagnosis of carrying the premutation brings challenges to a woman that are far beyond this scope of the following discussion.
Diagnosis
The difficulty for a woman getting the diagnosis of POI has been discussed thoughtfully and emphasizes the need to have health professionals spend the needed time to deliver the information to a woman in an appropriate setting, as this news may lead to shock, anxiety, depression, and the stigmatization of infertility (Sterling and Nelson 2011). For women in this situation, the diagnosis of POI typically comes first, followed by further work-up for the potential cause. The American College of Obstetricians and Gynecologists recommends that all women presenting with POI should be tested for the premutation, regardless of their family history (ACOG 2010). Pre-test counseling for FXPOI to inform a woman about the potential of a diagnosis of FXPOI and other associated risks could be helpful; however, as only about 3 % of women with POI will be found to carry the premutation, sometimes pre-test counseling may increase anxiety.
For a woman who carries the premutation, her carrier status is most often identified through the presence of symptoms of FXS in her offspring or another family member. At that time, she is seeking information about FXS and how to care for her child. She is most often in the hands of a pediatrician, clinical geneticist, or genetic counselor at this point. She may be seeking information about her risk for having a child with FXS, but she is most likely not seeking information about POI. Clinicians need to be aware that the discussion about the woman’s risk of FXPOI in this scenario may not be heard (Espinel et al. in press). Thus, follow-up with a reproductive endocrinologist or obstetrician/gynecologist is most likely necessary.
In either diagnostic situation, women will need significant health professional and social support assistance (Sterling and Nelson 2011). Women with a premutation may perceive or experience stigmatization resulting from carrying a gene that may lead to offspring with intellectual disability and possibly autism, to premutation-associated disorders of FXPOI and FXTAS , and to the specific outcome of infertility. Comments from others that infertility is beneficial to one who has a risk for passing the mutation for FXS are damaging. Stigma is associated with anxiety and depression (Davis et al. 2010; Slade et al. 2007). Health professionals can draw from the rich literature on ways to help reduce perceived loss of social support and low self-esteem that often come with stigmatization (Sterling and Nelson 2011).
Women with premutation or FXPOI may experience grief or loss or disruption of their life plan when they receive their diagnosis. A woman’s pursuit for childbearing and for children free from intellectual hardships is central to many. Sterling and Nelson discuss possible ways to help navigate through this transition towards a new life plan for women with a diagnosis of POI. These suggested ways to help build on a woman’s inherent positive psychological resources to redefine life goals are important and, again, must be considered in the context of both FXPOI and risk for FXS in offspring.
Management Prior to Onset of Symptoms of FXPOI
For FXPOI, self-management can begin prior to onset of symptoms. As soon as a woman is identified as carrying the premutation, she should become aware of possible medical concerns. As not all women with the premutation will experience FXPOI, it is important to identify biomarkers that can be monitored to identify those at high risk for a shortened reproductive life span and onset of estrogen deficiency. After establishing supportive evidence, biomarkers such as cycle variability, AMH, and FSH could be measured when a woman is first diagnosed as carrying the premutation and monitored on a regular basis. As noted above, AMH may be a better marker for early stages of POI compared with FSH. At this point, we know very little about the occult stages of FXPOI in adolescents. Based on the fact that age at menarche does not seem to be affected in FXPOI and that cycles can be highly variable, as well as measures of AMH during adolescents in all women, perhaps monitoring should begin around age 18 years. Although the predictive value of these biomarkers for FXPOI has not been studied longitudinally, they may be helpful in identifying which women with the premutation are at higher risk for FXPOI and may need more counseling from a reproductive endocrinologist about monitoring, potential fertility preservation options, and hormone therapy if cycles become irregular. It is important to stress, however, that having diminished ovarian response as evidenced by abnormally low AMH or abnormally high FSH does not necessarily equate with infertility or impending secondary amenorrhea.
Management at Onset of Symptoms of FXPOI
POI leads to early estrogen deficiency. As such, hormone treatment is important to the health of girls and young women experiencing FXPOI. Most experts agree that estrogen and progestin replacement is indicated for women with POI and that replacement should continue until they reach the typical age at menopause (Board of the International Menopause Society et al. 2007; Nelson 2009; Practice Committee of the American Society for Reproductive Medicine 2004; Rebar 2009; Welt 2008).
As noted above, loss of bone density is reported more often among women with the premutation. This is a consequence of the early estrogen deficiency, although a direct effect of the premutation on bone turnover has not been studied. A bone density study is indicated once a woman has confirmed FXPOI with associated oligo/amenorrhea. To maintain wellness once symptoms of FXPOI occur (e.g., cycle variability, altered hormone profile), women with a premutation should be aware of bone health maintenance, as outlined for perimenopausal women in the North American Menopause Society (North American Menopause Society 2006). This includes maintaining adequate vitamin D levels, supplementation of calcium intake, and establishing a regular mixed exercise program of loading exercise and resistance training. There is important new evidence from a controlled trial regarding hormone replacement therapy (HRT) for women with POI. The study compared bone mineral density over 3 years in a group of control women with normal ovarian function and a group of women with POI taking a physiologic HRT (100 μg/day estradiol patch, 10 mg per day oral medroxyprogesterone acetate for 12 days each month). At study entry, women with POI had significantly lower bone mineral density compared to controls. By study end, bone mineral density had increased markedly in women with POI to such a degree that it did not differ from bone density in the control group. Thus, the study showed that not only could this regimen of HRT reduce the rate at which women with POI lose bone mineral density, it could actually restore bone density to normal (Popat et al. 2014). This study sets the HRT standard by which to compare any other HRT regimens in women with POI. Evidence also suggests that this regimen promotes better bone health in women with POI than using an oral contraceptive for HRT. The oral contraceptive suppresses bone formation markers in these women, whereas transdermal estradiol significantly increases bone formation markers. Because of the uncertain effects of bisphosphonates on the fetus and their long skeletal half-life, these agents are not recommended in women who might conceive (Drake et al. 2008).
After recovering emotionally from being informed of the diagnosis of FXPOI, there is much to consider with respect to family planning. One important issue is to understand that some 5–10 % women with a diagnosis of POI can have spontaneous ovarian function and can conceive without medical intervention. Thus, for women who do not want to become pregnant, contraception must be considered. Because the effectiveness of oral contraception has not been evaluated among women with FXPOI, a barrier contraceptive method or intrauterine device should be considered.
For each woman with the premutation and potentially with FXPOI, her decision about family planning is highly personal. Options include making the decision not to have offspring, trying to conceive naturally, adoption, foster parenting, and medical intervention. The latter can include in vitro fertilization along with preimplantation genetic screening or egg or embryo donation . Chapter 13 provides more detail about the various options with respect to family planning in the context of fragile X-associated disorders. The important point to make is that each woman needs to make her own choices on her own time schedule. Both social and medical support should be available if she needs it.
Future Studies and Conclusions
The underlying etiology of FXPOI is still unknown. At this point, there are established guidelines to help manage the symptoms of FXPOI, but it is important to continue the search for the underlying etiology. FXPOI is one of three established fragile X-associated disorders that are the consequence of the repeat expansion in the 5′ untranslated region of the FMR1 gene. As noted above, FXPOI is limited to women who carry the premutation, not the full mutation. This suggests that reduction of FMRP, the protein that is not produced from full mutation alleles, is not the primary cause of FXPOI. Instead, some characteristic of the premutation allele is the culprit. There are important molecular consequences of the premutation: with increasing repeat length, there is increasing FMR1 mRNA levels and decreasing FMRP levels (Allen et al. 2004; Garcia-Alegria et al. 2007; Kenneson et al. 2001; Peprah et al. 2010; Tassone et al. 2007). Many have postulated that FMR1 mRNA toxicity may underlie FXPOI, as is the case for the other premutation-associated disorder, FXTAS. Chapter 11 reviews the evidence for the proposed mechanisms that may play a role in FXPOI. Two prevailing proposals include sequestration of important binding proteins or production of novel proteins with different homopolymeric or heteropolymeric amino acid tracts. Both of these outcomes are known to exist in FXTAS and are related to the unusual secondary structures that are formed as a result of the large repeat track in the premutation mRNA. Chapter 11 also describes the mouse models for FXPOI and shows evidence that, at least for these models, the premutation allele does not affect the primordial follicle pool. They also show that there is no specific block in follicular development or premature activation of follicles. Instead, data suggest that the problem may be related to abnormal granulosa cell proliferation. The importance of understanding the mechanism cannot be overstated. Once identified, possible interventions can be considered and well as possible biomarkers that indicate risk for a shortened reproductive life span.
In parallel, natural history studies of women with the premutation, starting as early as possible and continuing longitudinally, would be important to understand how early occult symptoms of FXPOI begin and to determine the comorbid associations of the disorder. These studies would help identify how best to manage outcomes of FXPOI in terms of timing and treatment regimes.
References
ACOG (2010) ACOG Committee Opinion No. 469: carrier screening for fragile X syndrome. Obstet Gynecol 116(4):1008–1010
Allen EG, He W, Yadav-Shah M, Sherman SL (2004) A study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum Genet 114(5):439–447
Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, Charen K, He W, Taylor K, Sherman SL (2007) Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod 22:2142–2152
Allen EG, Grus WE, Narayan S, Espinel W, Sherman SL (2014) Approaches to identify genetic variants that influence the risk for onset of fragile X-associated primary ovarian insufficiency (FXPOI): a preliminary study. Front Genet 5:260
Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A, Jenkins EC, Brown WT, Howard-Peebles PN, Becchi C, Cummings E, Fallon L, Seitz S, Black SH, Vianna-Morgante AM, Costa SS, Otto PA, Mingroni-Netto RC, Murray A, Webb J, Vieri F et al (1999) Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet 83(4):322–325
Allshouse AA, Semple AL, Santoro NF (2015) Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause 22(2):166–174
Alvarez-Mora MI, Rodriguez-Revenga L, Madrigal I, Garcia-Garcia F, Duran M, Dopazo J, Estivill X, Mila M (2015) Deregulation of key signaling pathways involved in oocyte maturation in FMR1 premutation carriers with Fragile X-associated primary ovarian insufficiency. Gene 571(1):52–57
Atsma F, Bartelink ML, Grobbee DE, Van Der Schouw YT (2006) Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause 13(2):265–279
Baker TG (1963) A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci 158:417–433
Barasoain M, Barrenetxea G, Huerta I, Telez M, Carrillo A, Perez C, Criado B, Arrieta I (2013) Study of FMR1 gene association with ovarian dysfunction in a sample from the Basque Country. Gene 521(1):145–149
Bione S, Benedetti S, Goegan M, Menditto I, Marozzi A, Ferrari M, Toniolo D (2006) Skewed X-chromosome inactivation is not associated with premature ovarian failure in a large cohort of Italian patients. Am J Med Genet A 140(12):1349–1351
Block E (1952) Quantitative morphological investigations of the follicular system in women; variations at different ages. Acta Anat (Basel) 14(1–2):108–123
Board of the International Menopause Society, Pines A, Sturdee DW, Birkhauser MH, Schneider HP, Gambacciani M, Panay N (2007) IMS updated recommendations on postmenopausal hormone therapy. Climacteric 10(3):181–194
Burgess B, Partington M, Turner G, Robinson H (1996) Normal age of menarche in fragile X syndrome [letter]. Am J Med Genet 64(2):376
Chen CT, Liu CT, Chen GK, Andrews JS, Arnold AM, Dreyfus J, Franceschini N, Garcia ME, Kerr KF, Li G, Lohman KK, Musani SK, Nalls MA, Raffel LJ, Smith J, Ambrosone CB, Bandera EV, Bernstein L, Britton A, Brzyski RG, Cappola A, Carlson CS, Couper D, Deming SL, Goodarzi MO, Heiss G, John EM, Lu X, Le Marchand L, Marciante K, Mcknight B, Millikan R, Nock NL, Olshan AF, Press MF, Vaiyda D, Woods NF, Taylor HA, Zhao W, Zheng W, Evans MK, Harris TB, Henderson BE, Kardia SL, Kooperberg C, Liu Y, Mosley TH, Psaty B, Wellons M, Windham BG, Zonderman AB, Cupples LA, Demerath EW, Haiman C, Murabito JM, Rajkovic A (2014) Meta-analysis of loci associated with age at natural menopause in African-American women. Hum Mol Genet 23(12):3327–3342
Chiazze L Jr, Brayer FT, Macisco JJ Jr, Parker MP, Duffy BJ (1968) The length and variability of the human menstrual cycle. JAMA 203(6):377–380
Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ (2008) Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A 146(8):1009–1016
Coulam CB, Adamson SC, Annegers JF (1986) Incidence of premature ovarian failure. Obstet Gynecol 67(4):604–606
Cox L, Liu JH (2014) Primary ovarian insufficiency: an update. Int J Womens Health 6:235–243
Cramer DW, Xu H (1996) Predicting age at menopause. Maturitas 23(3):319–326
Davis M, Ventura JL, Wieners M, Covington SN, Vanderhoof VH, Ryan ME, Koziol DE, Popat VB, Nelson LM (2010) The psychosocial transition associated with spontaneous 46, XX primary ovarian insufficiency: illness uncertainty, stigma, goal flexibility, and purpose in life as factors in emotional health. Fertil Steril 93(7):2321–2329
De Caro JJ, Dominguez C, Sherman SL (2008) Reproductive health of adolescent girls who carry the FMR1 premutation: expected phenotype based on current knowledge of fragile X-associated primary ovarian insufficiency. Ann N Y Acad Sci 1135:99–111
De Vet A, Laven JS, De Jong FH, Themmen AP, Fauser BC (2002) Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 77(2):357–362
Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 83(9):1032–1045
Ennis S, Ward D, Murray A (2006) Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet 14(2):253–255
Espinel W, Charen K, Huddleston L, Visootsak J, Sherman S (in press) Improving health education for women who carry an FMR1 premutation. J Genet Couns
Faddy MJ (2000) Follicle dynamics during ovarian ageing. Mol Cell Endocrinol 163(1–2):43–48
Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J (2003) Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod 18(2):323–327
Fryns JP (1986) The female and the fragile X. A study of 144 obligate female carriers. Am J Med Genet 23(1–2):157–169
Gallagher JC (2007) Effect of early menopause on bone mineral density and fractures. Menopause 14(3 Pt 2):567–571
Garcia-Alegria E, Ibanez B, Minguez M, Poch M, Valiente A, Sanz-Parra A, Martinez-Bouzas C, Beristain E, Tejada MI (2007) Analysis of FMR1 gene expression in female premutation carriers using robust segmented linear regression models. RNA 13(5):756–762
Goswami D, Conway GS (2005) Premature ovarian failure. Hum Reprod Update 11(4):391–410
Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P (2004) Serum antimullerian hormone/mullerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril 82(5):1323–1329
He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI (2009) Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet 41(6):724–728
Health NIO (2005) National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Ann Intern Med 142(12 Pt 1):1003–1013
Heger S, Mastronardi C, Dissen GA, Lomniczi A, Cabrera R, Roth CL, Jung H, Galimi F, Sippell W, Ojeda SR (2007) Enhanced at puberty 1 (EAP1) is a new transcriptional regulator of the female neuroendocrine reproductive axis. J Clin Invest 117(8):2145–2154
Houmard BS, Battaglia DE, Seifer DB (2001) Female reproductive aging. In: Seifer DB, Samuels P, Kniss DA (eds) The physiologic basis of gynecology and obstetrics. Lippincott Willieam and Wilkins, Philadelphia, pp 174–196
Hubayter ZR, Popat V, Vanderhoof VH, Ndubizu O, Johnson D, Mao E, Calis KA, Troendle JF, Nelson LM (2010) A prospective evaluation of antral follicle function in women with 46, XX spontaneous primary ovarian insufficiency. Fertil Steril 94(5):1769–1774
Hundscheid RD, Sistermans EA, Thomas CM, Braat DD, Straatman H, Kiemeney LA, Oostra BA, Smits AP (2000) Imprinting effect in premature ovarian failure confined to paternally inherited fragile X premutations. Am J Hum Genet 66:413–418
Hundscheid RD, Braat DD, Kiemeney LA, Smits AP, Thomas CM (2001) Increased serum FSH in female fragile X premutation carriers with either regular menstrual cycles or on oral contraceptives. Hum Reprod 16(3):457–462
Hundscheid RD, Smits AP, Thomas CM, Kiemeney LA, Braat DD (2003a) Female carriers of fragile X premutations have no increased risk for additional diseases other than premature ovarian failure. Am J Med Genet 117A(1):6–9
Hundscheid RD, Smits AP, Thomas CM, Kiemeney LA, Braat DD (2003b) Female carriers of fragile X premutations have no increased risk for additional diseases other than premature ovarian failure. Am J Med Genet A 117(1):6–9
Hunter JE, Epstein MP, Tinker SW, Charen KH, Sherman SL (2008) Fragile X-associated primary ovarian insufficiency: evidence for additional genetic contributions to severity. Genet Epidemiol 32(6):553–559
Hunter JE, Rohr JK, Sherman SL (2010) Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet 77(4):374–381
Jacobsen BK, Heuch I, Kvale G (2003) Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol 157(10):923–929
Janicki SC, Schupf N (2010) Hormonal influences on cognition and risk for Alzheimer’s disease. Curr Neurol Neurosci Rep 10(5):359–366
Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, Paraskevaidis EA, Sideris DA, Tsatsoulis A, Chrousos GP, Michalis LK (2004) Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab 89(8):3907–3913
Kallinen J, Korhonen K, Kortelainen S, Heinonen S, Ryynanen M (2000) Pregnancy outcome in carriers of fragile X. BJOG 107(8):969–972
Karimov CB, Moragianni VA, Cronister A, Srouji S, Petrozza J, Racowsky C, Ginsburg E, Thornton KL, Welt CK (2011) Increased frequency of occult fragile X-associated primary ovarian insufficiency in infertile women with evidence of impaired ovarian function. Human reproduction. Oxford, England
Kenna HA, Tartter M, Hall SS, Lightbody AA, Nguyen Q, De Los Angeles CP, Reiss AL, Rasgon NL (2013) High rates of comorbid depressive and anxiety disorders among women with premutation of the FMR1 gene. Am J Med Genet B Neuropsychiatr Genet 162B(8):872–878
Kenneson A, Zhang F, Hagedorn CH, Warren ST (2001) Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet 10(14):1449–1454
Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM (1997) Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol 89(5 Pt 1):777–779
Lambalk CB, De Koning CH, Braat DD (1998). The endocrinology of dizygotic twinning in the human. MolCell Endocrinol 145(1-2):97–102
Lomniczi A, Garcia-Rudaz C, Ramakrishnan R, Wilmot B, Khouangsathiene S, Ferguson B, Dissen GA, Ojeda SR (2012) A single-nucleotide polymorphism in the EAP1 gene is associated with amenorrhea/oligomenorrhea in nonhuman primates. Endocrinology 153(1):339–349
Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N (2003) Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod 18(1):199–206
Machado-Ferreira MC, Costa-Lima MA, Boy RT, Esteves GS, Pimentel MM (2004) Premature ovarian failure and FRAXA premutation: positive correlation in a Brazilian survey. Am J Med Genet A 126(3):237–240
Mailick MR, Hong J, Greenberg J, Smith L, Sherman S (2014) Curvilinear association of CGG repeats and age at menopause in women with FMR1 premutation expansions. Am J Med Genet B Neuropsychiatr Genet 165B(8):705–711
Mallolas J, Duran M, Sanchez A, Jimenez D, Castellvi-Bel S, Rife M, Mila M (2001) Implications of the FMR1 gene in menopause: study of 147 Spanish women. Menopause 8(2):106–110
Mateu-Huertas E, Rodriguez-Revenga L, Alvarez-Mora MI, Madrigal I, Willemsen R, Mila M, Marti E, Estivill X (2014) Blood expression profiles of fragile X premutation carriers identify candidate genes involved in neurodegenerative and infertility phenotypes. Neurobiol Dis 65:43–54
Mondul AM, Rodriguez C, Jacobs EJ, Calle EE (2005) Age at natural menopause and cause-specific mortality. Am J Epidemiol 162(11):1089–1097
Murray A (2000) Premature ovarian failure and the FMR1 gene. Semin Reprod Med 18(1):59–66
Murray A, Webb J, Macswiney F, Shipley EL, Morton NE, Conway GS (1999) Serum concentrations of follicle stimulating hormone may predict premature ovarian failure in FRAXA premutation women. Hum Reprod 14(5):1217–1218
Murray A, Ennis S, Macswiney F, Webb J, Morton NE (2000) Reproductive and menstrual history of females with fragile X expansions. Eur J Hum Genet 8(4):247–252
Murray A, Schoemaker MJ, Bennett CE, Ennis S, Macpherson JN, Jones M, Morris DH, Orr N, Ashworth A, Jacobs PA, Swerdlow AJ (2014) Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genet Med 16(1):19–24
Nelson LM (2009) Clinical practice. Primary ovarian insufficiency. N Engl J Med 360(6):606–614
Nelson LM, Anasti JN, Kimzey LM, Defensor RA, Lipetz KJ, White BJ, Shawker TH, Merino MJ (1994) Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab 79(5):1470–1475
Nelson LM, Covington SN, Rebar RW (2005) An update: spontaneous premature ovarian failure is not an early menopause. Fertil Steril 83(5):1327–1332
North American Menopause Society (2006) The role of calcium in peri- and postmenopausal women: 2006 position statement of the North American Menopause Society. Menopause 13(6):862–877, quiz 878-80
Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P (2010) Age of menopause and impact of climacteric symptoms by geographical region. Climacteric 13(5):419–428
Peprah E, He W, Allen E, Oliver T, Boyne A, Sherman SL (2010) Examination of FMR1 transcript and protein levels among 74 premutation carriers. J Hum Genet 55(1):66–68
Perry JR, Corre T, Esko T, Chasman DI, Fischer K, Franceschini N, He C, Kutalik Z, Mangino M, Rose LM, Vernon Smith A, Stolk L, Sulem P, Weedon MN, Zhuang WV, Arnold A, Ashworth A, Bergmann S, Buring JE, Burri A, Chen C, Cornelis MC, Couper DJ, Goodarzi MO, Gudnason V, Harris T, Hofman A, Jones M, Kraft P, Launer L, Laven JS, Li G, Mcknight B, Masciullo C, Milani L, Orr N, Psaty BM, Ridker PM, Rivadeneira F, Sala C, Salumets A, Schoemaker M, Traglia M, Waeber G, Chanock SJ, Demerath EW, Garcia M, Hankinson SE, Hu FB, Hunter DJ, Lunetta KL, Metspalu A, Montgomery GW, Murabito JM, Newman AB, Ong KK, Spector TD, Stefansson K, Swerdlow AJ, Thorsteinsdottir U, Van Dam RM, Uitterlinden AG, Visser JA, Vollenweider P, Toniolo D, Murray A (2013) A genome-wide association study of early menopause and the combined impact of identified variants. Hum Mol Genet 22(7):1465–1472
Popat VB, Calis KA, Kalantaridou SN, Vanderhoof VH, Koziol D, Troendle JF, Reynolds JC, Nelson LM (2014) Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J Clin Endocrinol Metab 99(9):3418–3426
Practice Committee of the American Society for Reproductive Medicine (2004) Current evaluation of amenorrhea. Fertil Steril 82(Suppl 1):S33–S39
Pu D, Xing Y, Gao Y, Gu L, Wu J (2014) Gene variation and premature ovarian failure: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 182:226–237
Qin Y, Sun M, You L, Wei D, Sun J, Liang X, Zhang B, Jiang H, Xu J, Chen ZJ (2012) ESR1, HK3 and BRSK1 gene variants are associated with both age at natural menopause and premature ovarian failure. Orphanet J Rare Dis 7:5
Rebar RW (2009) Premature ovarian failure. Obstet Gynecol 113(6):1355–1363
Richardson SJ, Senikas V, Nelson JF (1987) Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab 65(6):1231–1237
Rodriguez-Revenga L, Madrigal I, Badenas C, Xuncla M, Jimenez L, Mila M (2009a) Premature ovarian failure and fragile X female premutation carriers: no evidence for a skewed X-chromosome inactivation pattern. Menopause 16(5):944–949
Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xuncla M, Badenas C, Kulisevsky J, Gomez B, Mila M (2009b) Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet 17(10):1359–1362
Rohr J, Allen EG, Charen K, Giles J, He W, Dominguez C, Sherman SL (2008) Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod 23(5):1220–1225
Santoro N (2003). Mechanisms of premature ovarian failure. AnnEndocrinol (Paris) 64(2):87–92
Sapre S, Thakur R (2014) Lifestyle and dietary factors determine age at natural menopause. J Midlife Health 5(1):3–5
Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD (2014) Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol 43(5):1542–1562
Schwartz CE, Dean J, Howard-Peebles PN, Bugge M, Mikkelsen M, Tommerup N, Hull C, Hagerman R, Holden JJ, Stevenson RE (1994) Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet 51(4):400–402
Seifer DB, Maclaughlin DT, Christian BP, Feng B, Shelden RM (2002) Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril 77(3):468–471
Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, Mandel D (2012) Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am J Med Genet B Neuropsychiatr Genet 159B(5):589–597
Shen C, Delahanty RJ, Gao YT, Lu W, Xiang YB, Zheng Y, Cai Q, Zheng W, Shu XO, Long J (2013) Evaluating GWAS-identified SNPs for age at natural menopause among chinese women. PLoS One 8(3):e58766
Sherman SL (2000) Seminars in genetics: premature ovarian failure in the fragile X syndrome. Am J Med Genet 97:189–195
Sherman SL, Meadows KL, Ashley AE (1996) Examination of factors that influence the expansion of the fragile X mutation in a sample of conceptuses from known carrier females. Am J Med Genet 64(2):256–260
Sherman SL, Taylor K, Allen EG (2007) FMR1 premutation: a leading cause of inherited ovarian dysfunction. In: Arrieta I, Penagarikano O, Telez M (eds) Fragile sites: new discoveries and changing perspectives. Nova Science, Hauppauge
Sherman SL, Allen EG, Bean LJH (2013) Maternal age and oocyte aneuploidy: lessons learned from trisomy 21. In: Schlegel PN, Fauser BCJM, Carrell DT, Racowsky C (eds) Biennial review of infertility. Springer Science-Business Media, New York, pp 69–85
Slade P, O’neill C, Simpson AJ, Lashen H (2007) The relationship between perceived stigma, disclosure patterns, support and distress in new attendees at an infertility clinic. Hum Reprod 22(8):2309–2317
Smith LE, Seltzer MM, Greenberg JS (2012) Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 42(9):1836–1846
Spath MA, Nillesen WN, Smits AP, Feuth TB, Braat DD, Van Kessel AG, Yntema HG (2010) X chromosome inactivation does not define the development of premature ovarian failure in fragile X premutation carriers. Am J Med Genet A 152A(2):387–393
Spath MA, Feuth TB, Allen EG, Smits AP, Yntema HG, Van Kessel AG, Braat DD, Sherman SL, Thomas CM (2011a) Intra-individual stability over time of standardized anti-Mullerian hormone in FMR1 premutation carriers. Hum Reprod 26(8):2185–2191
Spath MA, Feuth TB, Smits AP, Yntema HG, Braat DD, Thomas CM, Van Kessel AG, Sherman SL, Allen EG (2011b) Predictors and risk model development for menopausal age in fragile X premutation carriers. Genet Med 13(7):643–650
Sterling EW, Nelson LM (2011) From victim to survivor to thriver: helping women with primary ovarian insufficiency integrate recovery, self-management, and wellness. Semin Reprod Med 29(4):353–361
Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, Barbalic M, Broer L, Byrne EM, Ernst F, Esko T, Franceschini N, Gudbjartsson DF, Hottenga JJ, Kraft P, Mcardle PF, Porcu E, Shin SY, Smith AV, Van Wingerden S, Zhai G, Zhuang WV, Albrecht E, Alizadeh BZ, Aspelund T, Bandinelli S, Lauc LB, Beckmann JS, Boban M, Boerwinkle E, Broekmans FJ, Burri A, Campbell H, Chanock SJ, Chen C, Cornelis MC, Corre T, Coviello AD, D’adamo P, Davies G, De Faire U, De Geus EJ, Deary IJ, Dedoussis GV, Deloukas P, Ebrahim S, Eiriksdottir G, Emilsson V, Eriksson JG, Fauser BC, Ferreli L, Ferrucci L, Fischer K, Folsom AR, Garcia ME, Gasparini P, Gieger C, Glazer N, Grobbee DE, Hall P, Haller T, Hankinson SE, Hass M, Hayward C, Heath AC, Hofman A, Ingelsson E, Janssens AC, Johnson AD, Karasik D, Kardia SL, Keyzer J, Kiel DP, Kolcic I, Kutalik Z, Lahti J, Lai S, Laisk T, Laven JS, Lawlor DA, Liu J, Lopez LM, Louwers YV, Magnusson PK, Marongiu M, Martin NG, Klaric IM, Masciullo C, Mcknight B, Medland SE, Melzer D, Mooser V, Navarro P, Newman AB, Nyholt DR, Onland-Moret NC, Palotie A, Pare G, Parker AN, Pedersen NL, Peeters PH, Pistis G, Plump AS, Polasek O, Pop VJ, Psaty BM, Raikkonen K, Rehnberg E, Rotter JI, Rudan I, Sala C, Salumets A, Scuteri A, Singleton A, Smith JA, Snieder H, Soranzo N, Stacey SN, Starr JM, Stathopoulou MG, Stirrups K, Stolk RP, Styrkarsdottir U, Sun YV, Tenesa A, Thorand B, Toniolo D, Tryggvadottir L, Tsui K, Ulivi S, Van Dam RM, Van Der Schouw YT, Van Gils CH, Van Nierop P, Vink JM, Visscher PM, Voorhuis M, Waeber G, Wallaschofski H, Wichmann HE, Widen E, Wijnands-Van Gent CJ, Willemsen G, Wilson JF, Wolffenbuttel BH, Wright AF, Yerges-Armstrong LM, Zemunik T, Zgaga L, Zillikens MC, Zygmunt M, Study TL, Arnold AM, Boomsma DI, Buring JE, Crisponi L, Demerath EW, Gudnason V, Harris TB, Hu FB, Hunter DJ, Launer LJ, Metspalu A, Montgomery GW, Oostra BA, Ridker PM, Sanna S, Schlessinger D, Spector TD, Stefansson K, Streeten EA, Thorsteinsdottir U, Uda M, Uitterlinden AG, Van Duijn CM, Volzke H, Murray A, Murabito JM, Visser JA, Lunetta KL (2012) Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet 44(3):260–268
Streuli I, Fraisse T, Ibecheole V, Moix I, Morris MA, De ZD (2009) Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil Steril 92(2):464–470
Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL (2005) Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod 20(2):402–412
Sullivan SD, Welt C, Sherman S (2011) FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med 29(4):299–307
Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ (2007) Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 13(4):555–562
Tejada MI, Garcia-Alegria E, Bilbao A, Martinez-Bouzas C, Beristain E, Poch M, Ramos-Arroyo MA, Lopez B, Fernandez Carvajal I, Ribate MP, Ramos F (2008) Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause 15(5):945–949
Tosh D, Rao KL, Rani HS, Deenadayal DA, Murty US, Grover P (2014) Association between fragile X premutation and premature ovarian failure: a case-control study and meta-analysis. Arch Gynecol Obstet 289(6):1255–1262
Treloar AE, Boynton RE, Behn BG, Brown BW (1967) Variation of the human menstrual cycle through reproductive life. Int J Fertil 12(1 Pt 2):77–126
Tremellen KP, Kolo M, Gilmore A, Lekamge DN (2005) Anti-mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol 45(1):20–24
Turner G, Robinson H, Wake S, Martin N (1994) Dizygous twinning and premature menopause in fragile X syndrome [letter] [see comments]. Lancet 344(8935):1500
Uzielli ML, Guarducci S, Lapi E, Cecconi A, Ricci U, Ricotti G, Biondi C, Scarselli B, Vieri F, Scarnato P, Gori F, Sereni A (1999a) Premature ovarian failure (POF) and fragile X premutation females: from POF to to fragile X carrier identification, from fragile X carrier diagnosis to POF association data. Am J Med Genet 84(3):300–303
Uzielli ML, Guarducci S, Lapi E, Cecconi A, Ricci U, Ricotti G, Biondi C, Scarselli B, Vieri F, Scarnato P, Gori F, Sereni A (1999b) Premature ovarian failure (POF) and fragile X premutation females: from POF to to fragile X carrier identification, from fragile X carrier diagnosis to POF association data. Am J Med Genet 84(3):300–303
Van Der Stege JG, Groen H, Van Zadelhoff SJ, Lambalk CB, Braat DD, Van Kasteren YM, Van Santbrink EJ, Apperloo MJ, Weijmar Schultz WC, Hoek A (2008) Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause 15(1):23–31
Van Kasteren YM, Schoemaker J (1999) Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update 5(5):483–492
Van Rooij IA, Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, De Jong FH, Themmen AP, Te Velde ER (2004) Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause 11(6 Pt 1):601–606
Vianna-Morgante AM (1999) Twinning and premature ovarian failure in premutation fragile X carriers. Am J Med Genet 83(4):326
Vianna-Morgante AM, Costa SS (2000) Premature ovarian failure is associated with maternally and paternally inherited premutation in Brazilian families with fragile X [see comments] [letter]. Am J Hum Genet 67:254–255
Vianna-Morgante AM, Costa SS, De C.M. Pavanello R, Otto PA, Mingroni-Netto RC (1999) Premature ovarian failure (POF) in Brazilian fragile X carriers. Genet Mol Biol 22(4):471–474
Vollman RF (1977) The menstrual cycle
Welt CK (2008) Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 68(4):499–509
Welt CK, Smith PC, Taylor AE (2004) Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab 89(9):4569–4574
Wheeler AC, Raspa M, Green A, Bishop E, Bann C, Edwards A, Bailey DB Jr (2014) Health and reproductive experiences of women with an FMR1 premutation with and without fragile X premature ovarian insufficiency. Front Genet 5:300
Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, Nguyen DV, Faradz SM, Van De Water J, Cook K, Hamlin A, Mu Y, Hagerman PJ, Hagerman RJ (2012) Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A 158A(10):2473–2481
Ye Y, Lan X, Cong J, Li N, Wu Y, Zhang M, Liu J, Cui Y, Wu BL, An Y, Wu J (2014) Analysis of CGG repeats in FMR1 in Chinese women with idiopathic premature ovarian failure. Reprod Biomed Online 29(3):382–387
Acknowledgements
This work was supported in part by an award (NS091859) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Neurological Disorders and Stroke (NINDS) (S.L.S., E.G.A., and J.S.) and by the Intramural Research Program, Eunice Kennedy Shriver NICHD, National Institutes of Health (L.M.N.)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sherman, S.L., Allen, E.G., Spencer, J.B., Nelson, L.M. (2016). Clinical Manifestation and Management of FXPOI. In: Tassone, F., Hall, D. (eds) FXTAS, FXPOI, and Other Premutation Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-33898-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-33898-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33896-5
Online ISBN: 978-3-319-33898-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)