Abstract
Purpose
To investigate the correlation between the ooplasmic volume and the number of mitochondrial DNA (mtDNA) copies in embryos and how they may affect fecundity.
Method
Using real-time PCR, mtDNA quantification was analyzed in unfertilized oocytes and uncleaved embryos. The size of the ovum was also assessed by calculating the ooplasmic volume at the time of granulosa cell removal for IVF or ICSI. Quantification analysis of the mtDNA in blastomeres was performed by real-time PCR at the 7–8 cell stage of the cleaved embryos at 72 h after oocyte retrieval. We calculated the cytoplasmic volume of the blastomeres.
Result
Our studies showed a significantly lower mtDNA copy number in unfertilized oocytes and uncleaved embryos in women who were older than 40 years of age (p < 0.05). The larger ooplasmic volume was also associated with earlier and more rapid cleavage (p < 0.05). The ooplasmic volume was also significantly larger in the group achieving pregnancy. We found a significant positive correlation between blastomere volume and the number of mtDNA copies (r = 0.76, p < 0.01, from Pearson product–moment correlation coefficient).
Conclusions
We have shown that blastomere volume is directly proportional to the number of mtDNA copies. Therefore, larger cytoplasmic volume, with earlier cleavage speed, implies more mtDNA copies. Evaluation of mtDNA quantification and the measurement of ooplasmic and blastomere volume may be useful for selection of high quality embryo and pregnancy outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
How to achieve high pregnancy rates is the main challenge that we are facing today in assisted reproductive technology (ART). Gross embryo morphology [1, 2] is one of the most reliable markers of embryo viability. The correlation between blastomere uniformity and a positive outcome of either in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) procedures is well established. Other morphological features such as variation in zona thickness [3], the presence of multinucleated blastomeres [4], and the appearance of the cytoplasm, pronuclei, and polar bodies [5, 6] have been shown to affect the implantation rate.
In addition to the morphological assessment, cytoplasmic factors (intrinsic factors) also play an important role in oocyte maturation, fertilization and early development [7, 8]. Live births have been achieved after the transfer of anucleate donor oocyte cytoplasm into recipient eggs [9, 10]. Major subcellular organs in the cytoplasm is mitochondria, and mitochondria account for 23 % of the cytoplasm in the pre-implantation human embryo. Mitochondria are maternally inherited organelles that use oxidative phosphorylation to supply ATP to the cell. Unlike other organelles which are produced via transcription and translation of nuclear chromosomal DNA, the genetic information for mitochondria is contained within the organelle itself. Mitochondrial DNA (mtDNA) is a double-stranded circular DNA molecule of approximately 16.5 kb in all mammals in which it has been sequenced [11]. The mitochondrial genome is not transmitted in a pattern of Mendelian inheritance, rather it passes from one generation to the next by way of the oocyte cytoplasm. Therefore, an individual’s mtDNA is entirely derived from his or her mother as any paternal mtDNA is expelled from the cleaving pre-embryo at the two cell stage [12]. Usually all the mitochondrial chromosomes in a cell carry identical copies of mtDNA (homoplasmy) [13]. Due to the location within the mitochondrial matrix as well as the lack of histones and intervening sequences, mtDNA is particularly susceptible to the detrimental effects of reactive oxygen species [14, 15]. The mutation rate of mtDNA is almost 20 times greater than that of nuclear DNA [16]. MtDNA therefore accumulates both point mutations and deletions over time. The proportion of mutant mtDNA varies within different organs in a single individual and among individuals within the same family [17]. Single-cell studies and hybrid-cell studies both show that the proportion of mutant mtDNA must exceed a critical threshold level before a cell expresses a biochemical abnormality of the mitochondrial respiratory chain (the threshold effect) [18]. Individuals with mitochondrial disorders resulting from mtDNA mutations may harbor a mixture of mutant and wild-type mtDNA within each cell (heteroplasmy) [19, 20]. The expression of a mtDNA mutation is not proportional to its degree of heteroplasmy. It is necessary to have a high degree of heteroplasmy in order to observe the clinical signs of the pathology [21]. Regarding the number of mtDNA mutation, deletions increase with age [22].
In the context of fertility, an association exists between maternal age and the rate of mtDNA deletion in human oocytes and granulosa cells [14, 23, 24]. There have been studies that show the relationship between mtDNA deletion and the oocyte diameter in human [25],and the relationship between oocyte diameter and the developmental competence of oocytes [26, 27]. But to our knowledge, there has been no study that shows how the ooplasmic volume affects fertility and fecundity in human. Therefore, the importance of mtDNA content and the ooplasmic volume to human fertilization outcome and embryonic development needs to be clearly determined. We focused on the correlation between the ooplasmic volume and the number of mtDNA copies, and investigated the fertility and fecundity of human oocyte by quantitative analysis of the mtDNA and by the ooplasmic volume.
Materials and methods
Collection of oocytes, embryo and blastomeres
Human oocytes were donated with informed consent by 19 patients (31–44 years old) undergoing IVF at Keio University Hospital from August, 2005 to January, 2011. The research procedure was approved by the Research Ethics Committee of Keio University School of Medicine. Follicular growth was stimulated with FSH/hMG under the administration of GnRH agonist. Oocytes were collected after administration of hCG and fertilized under conventional IVF and ICSI.
We used unfertilized oocytes (with one or no polar body) and uncleaved embryos (with two polar bodies) (n = 29) (31–44 years old), which had failed to undergo cleavage by 44 h after oocyte retrieval. Under an inverted microscope the unfertilized oocytes and uncleaved embryos were rinsed in a drop of PBS, and transferred into individual PCR tubes containing cell lysis solution. We grouped subjects according to age <40 years old (from 31 to 39 years old, n = 15) and age ≥40 years old group (from 40 to 44 years old, n = 14); and we compared the numbers of mtDNA copies between the two groups.
We used degenerated oocytes (n = 9) (33–41 years old), which were classified as degenerated either before or after IVF or ICSI procedures. Degenerated oocytes were characterized by multiple abnormal morphologic aspects, such as darkened, vacuolated, and irregular ooplasm [28, 29] (Fig. 1a, b). Under an inverted microscope the degenerated oocytes were rinsed in a drop of PBS, and transferred into individual PCR tubes containing cell lysis solution.
Four cleaved embryos at the 7–8 cell stage were analyzed at 72 h after oocyte retrieval (Veeck’s classification Grade1,3). We used a vitrification kit (KIAZATO®) to thaw frozen cleaved embryos. Then, the embryos were irradiated by a non-contact 1.48 μm diode laser system (OCTAX Laser Shot®:MTG, Germany) to pierce the zona pellucida. Two or three short pulses (2.9 ms) were applied, and single blastomeres were aspirated with a biopsy pipette. The diameter of each blastomere was measured under an inverted microscope (OLYMPUS IX71 Cronus®, U.S.A.). The long axis (D) and minor axis (d) of each blastomere were measured under an inverted microscope. We calculated the blastomere volume using (πd2D÷6, π = 3.14) [30]. Under an inverted microscope the blastomeres were rinsed in a drop of PBS and transferred into individual PCR tubes containing cell lysis solution. Whole blastomeres from each embryo were examined.
Measurement of oocyte cytoplasmic volume
To assess the size of the ovum, the ooplasmic volume was calculated. Human oocytes were donated with informed consent by 48 patients (28–44 years old) undergoing IVF. Follicular growth was stimulated with FSH/hMG administered in conjunction with a GnRH agonist. Oocytes were collected after the administration of hCG and fertilized with conventional IVF or ICSI.
The diameter of each ooplasm cytoplasm was measured under an inverted microscope (OCTAX Laser Shot®:MTG, Germany) at the time of granulosa cell removal. The long axis (D) and minor axis (d) of each ooplasm were measured under an inverted microscope. We calculated the ooplasmic volume using (πd2D÷6, π = 3.14).
MtDNA quantification by real time PCR
Quantification of mtDNA was performed by real-time PCR. We used a cell line derived from a single human osteocarcinoma cell with a fixed 9100 mtDNA copy number (ATCC, 143B; CRL-8303) [31]. We diluted it with purified water, and manufactured dilute solutions of 100 × 104copy/μl, 50 × 104copy/μl, 25 × 104copy/μl, 10 × 104copy/μl, 5 × 104copy/μl, 1 × 104copy/μl, 5000 copy/μl, 1000copy/μl, and 500copy/μl.
Quantitative and qualitative analysis for mtDNA were performed by previous established protocol with real-time PCR analysis [32]. Real-time PCR primers and fluorescent probes (TaqMan® MGB probe; Applied Biosystems, USA) corresponding to sequences of ATPase6 gene were prepared. The forward primer (5′-CGAAACCATCAGCCTACTCATTCAA-3′) spanned from nt 8958 to nt 8982. The reverse primer (5′-CCTGCAGTAATGTTAGCGGTTAGG-3′) spanned from nt 9026 to nt 9003. The probe for wild sequences (8993T; CCAATAGCCC[T]GGCCGT) had “VIC” fluorochrome and the probe for mutant (8993G; AATAGCCC[G]GGCCGT) had the “FAM” fluorochrome. 8993 point is in the ATPase6 gene which is responsible for major energy production for embryo development. The T8993G point mutation was commonly found and is responsible for Leigh syndrome.
Each 25 μl PCR reaction was prepared with the following final concentrations: 12.5 μl TaqMan Universal PCR Master Mix, 5 μl distilled water, 1 μl × 2 TaqMan MGB Probe, 2.25 μl × 2 PCR primer, 1 μl specimen or 1 μl dilute solutions of 9100 mtDNA copy with distilled water or 1 μl TE buffer (negative control).
The reactions were performed with the following conditions: initial denaturation at 50°C for 2 min and 95°C for 10 min, and 35 cycles at 92°C for 15 s (denaturation), 60°C for 1 min (annealing and extension). The allelic discrimination assay using real-time PCR (ABI PRISM 7000) was used to measure each fluorescence signal.
Statistical analysis
Continuous data were compared using Student’s t-test or Welch’s t-test. p < 0.05 was considered statistically significant.
Results
The number of mtDNA copies in unfertilized oocytes and uncleaved embryos
The minimum mtDNA copy number found in unfertilized oocytes and uncleaved embryos was 277,059, and the maximum was 1045,000. Copy number was highly variable both within and between patients. Our studies have shown that the average number of mtDNA copies in unfertilized oocytes and uncleaved embryos was 697,176 ± 37,057. The average number of mtDNA copies was 784,307 ± 48,379 for age <40 years and 603,822 ± 46,153 for age ≥40 years. Our studies in unfertilized oocytes and uncleaved embryos in women who were older than 40 years of age showed significantly fewer mtDNA copies (p < 0.05) (Fig. 2).
The number of mtDNA copies in unfertilized oocytes and uncleaved embryos. Quantification of mtDNA was performed by real-time PCR. We grouped subjects according to age <40 years old (from 31 to 39 years old, n = 15) and age ≥40 years old group (from 40 to 44 years old, n = 14); and we compared the numbers of mtDNA copies between the two groups. The average number of mtDNA copies was 784,307 ± 48,379 for age <40 years and 603,822 ± 46,153 for age ≥40 years. Our studies in unfertilized oocytes and uncleaved embryos in women who were older than 40 years of age showed significantly fewer mtDNA copies (p < 0.05)
The number of mtDNA copies in degenerated oocytes
The average number of mtDNA copies for the nine degenerated oocytes was 330,513 ± 78,002. In this result, the number of mtDNA copies in degenerated oocytes showed a remarkable decrease in comparison with unfertilized oocytes and uncleaved embryos (p < 0.01) (Fig. 3).
The number of mtDNA copies in unfertilized oocytes and degenerate oocytes. Quantification of mtDNA was performed by real-time PCR. Our studies have shown that the average number of mtDNA copies in unfertilized oocytes and uncleaved embryos (from 31 to 44 years old, n = 29) was 697,176 ± 37,057. The average number of mtDNA copies for the nine degenerate oocytes (from 33 to 41 years old) was 330,513 ± 78,002. The number of mtDNA copies in degenerated oocytes showed a remarkable decrease in comparison with unfertilized oocytes and uncleaved embryos (p < 0.01)
The number of mtDNA copies in blastomeres
The average number of mtDNA copies in blastomeres from7 to 8 cell embryos at 72 h after oocyte retrieval was 673,722 ± 12,952. The number of mtDNA copies varied widely among individual blastomeres in an embryo. We evaluated the relationship between the volume and the number of mtDNA copies in isolated blastomeres from 7 to 8 cell embryos. We found a significant positive correlation between blastomere volume and the number of mtDNA copies (r = 0.76, p < 0.01, from Pearson product–moment correlation coefficient) (Fig. 4).
Blastomere volume and the number of mtDNA copies at 72 h after oocyte retrieval. Four cleaved embryos at the 7–8 cell stage were collected for study at 72 h after oocyte retrieval (Veeck’s classification Grade1,3). Each single blastomeres (n = 29) were aspirated with a biopsy pipette. The diameter of each blastomere was measured under an inverted microscope. The long axis (D) and minor axis (d) of each blastomere were measured under an inverted microscope. We calculated the blastomere volume using (πd2D÷6, π = 3.14). Quantification of mtDNA was performed by real-time PCR. The average number of mtDNA copies in blastomeres was 673,722 ± 12,952. We found a significant positive correlation between blastomere volume and the number of mtDNA copies (r = 0.76, p < 0.01, from Pearson product–moment correlation coefficient)
The T8993G point mutation was not detected in any unfertilized oocytes, degenerated oocytes or blastomeres in our studies (data not shown).
Ooplasmic volume
We measured the ooplasmic volume in order to evaluate the correlations with other factors. The speed of embryo cleavage and the ooplasmic volume were compared. In embryonic development, 3–4 cell embryos showed a normal (early) cleavage speed. At 44 h after oocyte retrieval, 348 embryos had reached the 3–4 cell stage and the remaining 326 had not. The ooplasmic volume was compared between these groups.
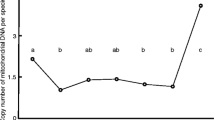
At 44 h after oocyte retrieval, the average ooplasmic volume of the 3–4 cell embryos was 615,222 ± 2,604 μm3 and that of those with fewer cells was 605,402 ± 3,145 μm3 (p < 0.05) (Fig. 5). This significant difference in volume implies that embryos that cleave at a faster rate have larger ooplasmic volume. Previous reports have also shown that in the early cleavage group there was a higher rate of pregnancy per transfer compared with the late cleavage group [33, 34], suggesting that early cleavage is a strong indicator of better embryo quality. We then adopted Veeck’s classification to further analyze 348 embryos which have reached the normal (early) cleavage speed at 44 h after oocyte retrieval. The Veeck’s classification and the average ooplasmic volume were as follows, respectively: Veeck 1 (n = 45), 626,774 ± 6,264 μm3; Veeck 2 (n = 64), 625,191 ± 5,844 μm3; Veeck 3 (n = 75), 611,083 ± 5,549 μm3; Veeck 4 (n = 164), 610,055 ± 3,938 μm3. When comparing Veeck 1 and Veeck 4, the ooplasmic volume was significantly different; the ooplasmic volume of Veeck1 was significantly larger than that of Veeck 4 (p < 0.05) (Fig. 6a). The comparison between Veeck 1 + 2 and Veeck 3 + 4 also showed a significant difference in the ooplasmic volume; the ooplasmic volume of Veeck 1 + 2 was significantly larger than that of Veeck 3 + 4 (p < 0.01) (Fig. 6b).
Comparison of ooplasmic volume at oocyte retrieval and the speed of embryo cleavage at 44 h after oocyte retrieval. In embryonic development, 3–4 cell embryos showed a normal (early) cleavage speed. At 44 h after oocyte retrieval, 348 embryos had reached the 3–4 cell stage and the remaining 326 had not. The ooplasmic volume was compared between these groups. At 44 h after oocyte retrieval, the average ooplasmic volume of the 3–4 cell embryos was 615,222 ± 2,604 μm3 and that of those with fewer cells was 605,402 ± 3,145 μm3 (p < 0.05)
a Comparison of ooplasmic volume at oocyte retrieval and morphological classification of embryo developed at 44 h after oocyte retrieval:Grade1 vs Grade4. There was a total of 348 embryos at 44 h after oocyte retrieval which reached the normal (early) embryonic development stage. The Veeck’s classification and the average ooplasmic volume were as follows, respectively: Veeck 1 (n = 45), 626,774 ± 6,264 μm3; Veeck 2 (n = 64), 625,191 ± 5,844 μm3; Veeck 3 (n = 75), 611,083 ± 5,549 μm3; Veeck 4 (n = 164), 610,055 ± 3,938 μm3. When comparing Veeck 1 and Veeck 4, the ooplasmic volume was significantly different; the ooplasmic volume of Veeck1 was significantly larger than that of Veeck 4 (p < 0.05). b Comparison of ooplasmic volume at oocyte retrieval and morphological classification of embryo developed at 44 h after oocyte retrieval: Grade1 + 2 vs Grade3 + 4. The comparison between Veeck 1 + 2 (n = 109), and Veeck 3 + 4 (n = 239) also showed a significant difference in the ooplasmic volume; the ooplasmic volume of Veeck 1 + 2 (625,845 ± 4,278 μm3) was significantly larger than that of Veeck 3 + 4 (610,377 ± 3,208 μm3) (p < 0.01)
The relationship between the rate of pregnancy and ooplasmic volume was analyzed. The number of embryos leading to pregnancy was 44, and the average ooplasmic volume was 625,019 ± 7,448 μm3. The pregnancies were achieved by either fresh or frozen embryos transfer with conventional IVF or ICSI. The number of embryos leading to pregnancy excluding miscarriage was 35, and the average ooplasmic volume was 631,911 ± 8,255 μm3. The number of non-pregnant embryos was 630, and the average ooplasmic volume was 609,456 ± 2,112 μm3. The number of non-pregnant embryos excluding frozen embryos that were not thawed was 549, and the average ooplasmic volume was 608,569 ± 2,246 μm3. The ooplasmic volume of the pregnant group was significantly larger (Table 1).
We also analyzed the association between age and ooplasmic volume. The probability of clinical pregnancy declines with age, with lower rates in women aged 35–39 in comparison to women aged 30–34 [35]. In the present study, the former age group (n = 209, volume 597,634 ± 3,731 μm3) showed significantly lower ooplasmic volume when compared to the latter age group (n = 146, volume 608,572 ± 3,997 μm3) (p ≤ 0.05) (Fig. 7).
Ooplasmic volume at oocyte retrieval between two different age groups: 30–34 vs 35–39. We analyzed the association between age and ooplasmic volume. The 35–39 year age group (n = 209, volume 597,634 ± 3,731 μm3) showed significantly lower ooplasmic volume when compared to the 30–34 year age group (n = 146, volume 608,572 ± 3,997 μm3) (p ≤ 0.05)
Discussion
It is commonly known that ovarian aging is associated with the impairment of specific functions of oocytes and granulosa cells, along with more global cellular dysfunction resulting from changes in gene and protein expression profiles, and energetic failure [36–41]. Embryo development in advanced reproductive age may be jeopardized by altered mitochondrial activity. Our studies showed significantly fewer mtDNA copies in unfertilized oocytes and uncleaved embryos from women who were older than 40 years of age. This suggests that the number of mtDNA copies decreases with aging. This low mtDNA copy number may result from the accumulation of mutations in the mtDNA or an inherent property of oocytes recruited later in the reproductive lifespan [42]. These observations allude to a relationship between mtDNA and oocyte quality. Our studies have shown that the average number of mtDNA copies in unfertilized oocytes and uncleaved embryos was 697,176 ± 37,057. Previous studies reported it as ranging average from 314,000 to 795,000 [23, 42–44]. Our results may be technically more accurate than those of previous studies, since our quantitation was based on a single standardized human cell with 9,100 mtDNA copies [31]. Previous study [23] has also reported that the number of mtDNA copies decreases with aging, which is the same as our study.
In a study performed in mouse, low mtDNA copy number did not significantly affect fertilization [45]. While others have shown that mitochondrial activity is crucial for the activation of development and for embryonic survival [46]. Mitochondrial dysfunction resulting from a variety of intrinsic and extrinsic influences, including genetic abnormalities, hypoxia and oxidative stress, can profoundly influence ATP generation in oocytes and early embryos, which in turn may result in aberrant chromosomal segregation or developmental arrest. In our studies, the number of mtDNA copies in degenerated oocytes showed a remarkable decrease in comparison with unfertilized oocytes (Fig. 2), suggesting that the extreme low mtDNA copy number present was insufficient to provide the necessary energetic reserves during follicular growth, thereby leading to oocyte degeneration.
While the low mtDNA copy does not negatively impact fertilization in the mouse, it does result in a dramatic reduction in post-implantation embryonic viability [45]. The post-implantation state is characterized by a high energy requirement, rendering the embryo particularly susceptible to deficiencies in mitochondrial function during this period. In the mouse, a minimal threshold for mtDNA copy number has been identified, below which normal post-implantation embryonic development does not occur. No minimum, however, has been defined for the human oocyte. The number of mtDNA copies could be a useful indicator of oocyte quality in experimental models. Although the method used to determine the number of mtDNA copies is retrospective analysis, identifying the standardizing thresholds for mtDNA number may prove useful in the evaluation of patients with IVF failures.
Regarding the ooplasmic volume, we found that higher cytoplasmic volume correlates to younger age and higher pregnancy rate. Higher ooplasmic volume was also frequently associated with better morphology, and earlier cleavage. It is likely therefore that ooplasmic volume affects fecundity. Larger oocyte diameter is a determinant factor for completion of meiosis and acquisition of full competence for embryo development in the goat [47]. It has been reported that a relationship between higher cytoplasmic volume and higher fertilization outcome in the pig [48]. There has been no study in humans correlating ooplasmic volume with fertility and fecundity. Oocyte diameter has been linked to developmental competence during the germinal vesicle (GV) stage in the ferret [26] and in human [27]. The configuration of the GV chromatin correlates with the developmental competence of oocytes. Chromatin condensation is related to the sequential achievement of meiotic competencies during oocyte growth and differentiation, and GV condensation corresponds to an increase in oocyte diameter. The relationship between ooplasmic volume and the optimization of nuclei is considered to be the next subject.
We also evaluated the relationship between the blastomere volume and the number of mtDNA copies in isolated blastomeres from 7 to 8 cell embryos. Blastomere volume was directly proportional to the number of mtDNA copies (r = 0.76, p < 0.01). This is in accordance with previous reports in the mouse [49]. Additionally, increased mitochondrial number correlates with cytoplasmic volume in cattle [50], implying that a sufficient supply of ATP is necessary for the development of proper blastomeres. In our study, the number of mtDNA copies in each blastomere from 7 to 8 cell-stage embryos was uneven. The inter-blastomere variation of mtDNA contents may occur due to intrinsic factors, or may be the result of external influences to which each individual embryo and blastomere are exposed [51, 52]. The asymmetric polar distribution of the mitochondria in mature oocytes may be retained through cleavage divisions, resulting in blastomeres with a varying mitochondrial load [46].
Conclusions
We conclude that higher fecundity is associated with an increased number of mtDNA copies in the embryo. The mitochondrion inherited maternally is an important organelle for reproduction, and mtDNA is a key determinant of its function. Evaluation of mtDNA quantification might be an underlying factor to explain the developed oocyte quality, and this may eventually lead to treatment. In the United Kingdom recently there is an ethical issues concerning reproductive-gene-therapy techniques that could prevent children from inheriting certain genetic diseases caused by faulty mitochondria. Mitochondrial therapy at the oocytic level is current burning topic to be discussed all over the world. Moreover so as it includes the issues about oocyte aging and capability for fecundity and we need to be prepared for dealing with this ethically sensitive matter. We found a significant positive correlation between blastomere volume and the number of mtDNA copies. So, low-invasive quantification of ooplasmic and blastomere volume is a novel factor, may be convenient and advantageous predictor for successful clinical outcome in selecting embryos to be transferred.
References
Hill GA, Freeman M, Bastias MC, Rogers BJ, Herbert CM 3rd, Osteen KG, Wentz AC (1989) The influence of oocyte maturity and embryo quality on pregnancy rate in a program for in vitro fertilization–embryo transfer. Fertil Steril 52(5):801–806
Shulman A, Ben Nun I, Ghetler Y, Kaneti H, Shilon M, Beyth Y (1993) Relationship between embryo morphology and implantation rate after in vitro fertilization treatment in conception cycles. Fertil Steril 60(1):123–126
Cohen J, Inge KL, Suzman M, Wiker SR, Wright G (1989) Videocinematography of fresh and cryopreserved embryos: a retrospective analysis of embryonic morphology and implantation. Fertil Steril 51(5):820–827
Pelinck MJ, De Vos M, Dekens M, Van der Elst J, De Sutter P, Dhont M (1998) Embryos cultured in vitro with multinucleated blastomeres have poor implantation potential in human in vitro fertilization and intracytoplasmic sperm injection. Hum Reprod 13(4):960–963
Wilding M, Di Matteo L, D’Andretti S, Montanaro N, Capobianco C, Dale B (2007) An oocyte score for use in assisted reproduction. J Assist Reprod Genet 24(8):350–358
Nagy ZP, Dozortsev D, Diamond M, Rienzi L, Ubaldi F, Abdelmassih R, Greco E (2003) Pronuclear morphology evaluation with subsequentincreases implantation rates. Fertil Steril 80(1):67–74
Muggleton-Harris A, Whittingham DG, Wilson L (1982) Cytoplasmic control of preimplantation development in vitro in the mouse. Nature 299(5882):460–462
Battaglia DE, Goodwin P, Klein NA, Soules MR (1996) Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 11(10):2217–2222
Huang CC, Cheng TC, Chan HH, Chang CC, Chen CI, Liu J, Lee MS (1999) Birth after the injection of sperm and the cytoplasm of tripronucleate zygotes into metaphase II oocytes in patients with repeated implantation failure after assisted fertilization procedures. Fertil Steril 72(4):702–706
Cohen J, Scott R, Schimmel T, Levron J, Willadsen S (1997) Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet 350(9072):186–187
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290(5806):457–465
Kaneda H, Hayashi J, Takahama S, Taya C, Lindahl KF, Yonekawa H (1995) Elimination of paternal mitochondrial DNA in intra specific crosses during early mouse embryogenesis. Proc Natl Acad Sci USA 92(10):4542–4546
Bitner-Glindzicz M (2002) Hereditary deafness and phenotyping in humans. Br Med Bull 63:73–94
Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S (1995) Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril 64(3):577–583
Jansen RP (2000) Germline passage of mitochondria: quantitative considerations and possible embryological sequelae. Hum Reprod 15(Suppl 2):112–128
Wallace DC, Ye JH, Neckelmann SN, Singh G, Webster KA, Greenberg BD (1987) Sequence analysis of cDNAs for the human and bovine ATP synthase beta subunit: mitochondrial DNA genes sustain seventeen times more mutations. Curr Genet 12(2):81–90
Macmillan C, Lach B, Shoubridge EA (1993) Variable distribution of mutant mitochondrial DNAs (tRNA(Leu[3243])) in tissues of symptomatic relatives with MELAS: the role of mitotic segregation. Neurology 43(8):1586–1590
Schon EA, Bonilla E, DiMauro S (1997) Mitochondrial DNA mutations and pathogenesis. J Bioenerg Biomembr 29(2):131–149
Holt IJ, Harding AE, Morgan-Hughes JA (1988) Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331(6158):717–719
Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA (1990) A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet 46(3):428–433
Mazat JP, Rossignol R, Malgat M, Rocher C, Faustin B, Letellier T (2001) What do mitochondrial diseases teach us about normal mitochondrial functions…that we already knew: threshold expression of mitochondrial defects. Biochim Biophys Acta 1504(1):20–30
Linnane AW, Marzuki S, Ozawa T, Tanaka M (1989) Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 1(8639):642–645
Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC (2005) Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol Hum Reprod 11(12):843–846
Seifer DB, DeJesus V, Hubbard K (2002) Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil Steril 78(5):1046–1048
Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC (2006) Mitochondrial DNA deletion in granulosa and cumulus oophorus cells. Fertil Steril 85(3):780–782
Sun X, Li Z, Yi Y, Ding W, Chen J, Engelhardt JF, Leno GH (2009) Chromatin configurations in the ferret germinal vesicle that reflect developmental competence for in vitro maturation. Reprod Domest Anim 44(2):320–325
Combelles CM, Cekleniak NA, Racowsky C, Albertini DF (2002) Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod 17(4):1006–1016
Raziel A, Schachter M, Strassburger D, Kasterstein E, Ron-El R, Friedler S (2006) In vivo maturation of oocytes by extending the interval between human chorionic gonadotropin administration and oocyte retrieval. Fertil Steril 86(3):583–587
Itskovitz J, Rubattu S, Rosenwaks Z, Liu HC, Sealey JE (1991) Relationship of follicular fluid prorenin to oocyte maturation, steroid levels, and outcome of in vitro fertilization. J Clin Endocrinol Metab 72:165–171
Yamamura Y, Tamano M, Iguchi T, Ohta Y (2001) Metallothionein expression and tumor growth in the transplantable pregnancy-independent mouse mammary tumor. J Vet Med Sci 63(6):687–689
Hofhaus G, Johns DR, Hurko O, Attardi G, Chomyn A (1996) Respiration and growth defects in transmitochondrial cell lines carrying the 11778 mutation associated with Leber’s hereditary optic neuropathy. J Biol Chem 271(22):13155–13161
Tajima H, Sueoka K, Moon SY, Nakabayashi A, Sakurai T, Murakoshi Y, Watanabe H, Iwata S, Hashiba T, Kato S, Goto Y, Yoshimura Y (2007) The development of novel quantification assay for mitochondrial DNA heteroplasmy aimed at preimplantation genetic diagnosis of Leigh encephalopathy. J Assist Reprod Genet 24(6):227–232
Fu J, Wang XJ, Wang YW, Sun J, Gemzell-Danielsson K, Sun XX (2009) The influence of early cleavage on embryo developmental potential and IVF/ICSI outcome. J Assist Reprod Genet 26(8):437–441
Bos-Mikich A, Mattos AL, Ferrari AN (2001) Early cleavage of human embryos: an effective method for predicting successful IVF/ICSI outcome. Hum Reprod 16(12):2658–2661
Dunson DB, Colombo B, Baird DD (2002) Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod 17(5):1399–1403
Van Blerkom J, Sinclair J, Davis P (1998) Mitochondrial transfer between oocytes: potential applications of mitochondrial donation and the issue of heteroplasmy. Hum Reprod 13(1O):2857–2868
Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R (2004) Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online 8(1):45–58
Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko M (2004) Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet 13(19):2263–2278
Thouas GA, Trounson AO, Jones GM (2005) Effect of female age on mouse oocyte developmental competence following mitochondrial injury. Biol Reprod 73(2):366–373
Steuerwald NM, Bermúdez MG, Wells D, Munné S, Cohen J (2007) Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online 14(6):700–708
Grøndahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R (2010) Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod 25(4):957–968
Steuerwald N, Barritt JA, Adler R, Malter H, Schimmel T, Cohen J, Brenner CA (2000) Quantification of mtDNA in single oocytes, polar bodies and subcellular components by real-time rapid cycle fluorescence monitored PCR. Zygote 8(3):209–215
Barritt JA, Kokot M, Cohen J, Steuerwald N, Brenner CA (2002) Quantification of human ooplasmic mitochondria. Reprod Biomed 4(3):243–247
Chen X, Prosser R, Simonetti S, Sadlock J, Jagiello G, Schon EA (1995) Rearranged mitochondrial genomes are present in human oocytes. Am J Hum Genet 57(2):239–247
Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA (2010) The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod 83(1):52–62
Dumollard R, Duchen M, Carroll J (2007) The role of mitochondrial function in the oocyte and embryo. Curr Top Dev Biol 77:21–49
Crozet N, Dahirel M, Gall L (2000) Meiotic competence of in vitro grown goat oocytes. J Reprod Fertil 118(2):367–373
El Shourbagy SH, Spikings EC, Freitas M, St John JC (2006) Mitochondria directly influence fertilisation outcome in the pig. Reproduction 131(2):233–245
Kameyama Y, Ohnishi H, Shimoi G, Hashizume R, Ito M, Smith LC (2010) Asymmetrical allocation of mitochondrial DNA to blastomeres during the first two cleavages in mouse embryos. Reprod Fertil Dev 22(8):1247–1253
Smith LC, Alcivar AA (1993) Cytoplasmic inheritance and its effects on development and performance. J Reprod Fertil Suppl 48:31–43
Van Blerkom J (2000) Intrafollicular influences on human oocyte developmental competence: perifollicular vascularity, oocyte metabolism and mitochondrial function. Hum Reprod 15(Suppl 2):173–188
Nayudu PL, Lopata A, Jones GM, Gook DA, Bourne HM, Sheather SJ, Brown TC, Johnston WI (1989) An analysis of human oocytes and follicles from stimulated cycles: oocyte morphology and associated follicular fluid characteristics. Hum Reprod 4(5):558–567
Acknowledgments
We appreciate our coworkers’ collaboration and advice on the study, especially Ms. Yoko Yasuda, Ms. Mariko Araga, Ms. Yoko Matumoto, and support from the mitochondrial disease working group of Ministry of Health, Labour and Welfare in Japan.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule In our studies, higher ooplasmic volume was frequently associated with fecundity. And we found a significant positive correlation between blastomere volume and the number of mtDNA copies.
Rights and permissions
About this article
Cite this article
Murakoshi, Y., Sueoka, K., Takahashi, K. et al. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet 30, 1367–1375 (2013). https://doi.org/10.1007/s10815-013-0062-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-013-0062-6