Abstract
Objective
To determine whether concentrations of oxidative stress markers of follicular fluid and serum are different in GnRH agonist protocol from GnRH antagonist protocol.
Material and method
This was a cross-sectional study. Eighty-four women undergoing controlled ovarian stimulation with either GnRH agonist (n = 39) or GnRH antagonist protocols (n = 45) for IVF/ICSI treatment were assigned by a physician. Blood was obtained at the time of oocyte retrieval, and follicular fluid (FF) from the mature follicles of each ovary was centrifuged and frozen until analysis. Malondialdehyde (MDA), nitric oxide (NO), protein carbonyl (PC), hydroxyl proline (OH-P), sodium oxide dismutase (SOD), reduced glutathione (GSH), glutathione peroxidase (GSH-Px), adenosine deaminase (ADA) and xanthine oxidase (XO) were assessed in the serum and follicular fluid of each participants.
Results
The mean serum concentrations of GSH-Px, GSH and MDA were lower in the GnRH antagonist group compared to GnRH agonist group, but mean serum SOD was higher in the GnRH antagonist group. The mean follicular SOD, ADA and NO were higher in GnRH antagonist group than GnRH agonist group. The IVF/ICSI outcomes were similar in both groups.
Conclusion(s)
GnRH antagonist protocol is associated with increased oxidative stress. The relation of GnRH analogues with oxidative stress and its implication in follicular growth needs to be addressed in further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in assisted reproduction technology, poor oocyte quality remains a subtle problem for female infertility. Numerous animal and human studies have demonstrated that reactive oxygen species (ROS) were produced in ovaries, fallopian tubes and embryos [1–3]. Evidence from recent studies has suggested that ROS plays an essential role in oocyte maturation, formation of corpus luteum and luteolysis, furthermore, ROS is unavoidably associated with adverse reproductive outcome in both female and male [1, 4–7]. The impact of follicular fluid oxidative stress on oocyte maturation, fertilization and implantation of embryo have received considerable attention for therapeutic measures of ovulation induction for in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) during last few years. The study assessing the relationship of oxidative stress with oocyte quality in women who underwent ovulation induction for IVF/ICSI suggested that oxidative stress might result in poor oocyte quality [8].
Adenosine deaminase (ADA) is a catalyzing enzyme that may contribute to regulation of menstrual cycle. The study on evaluation of serum ADA during menstrual cycle has detected the activities of ADA and its isoenzymes were lowest during follicular phase [9]. Further, the authors suggested that low ADA activity in the follicular phase may be a regulatory mechanism to increase adenosine availability, which can lead to two important processes including vasodilation and rise vascular permeability for ovulation [9]. Sodium oxide dismutase (SOD) is an enzymatic antioxidant that converts the pro-oxidant superoxide into hydrogen peroxide. Recent study conducted by Combelles et al [10] has demonstrated the highest SOD activity in small follicles in comparison to medium and large follicles, and suggested that SOD plays a role in follicular development. The other study evaluating role of nitric oxide (NO) showed that in vitro inhibition NO system suppressed the ovulatory process [11]. Importantly, Lee et al [12] has found NO has an inducing effect on SOD mRNA and protein in cultured bovine luteal endothelial cells of corpus luteum. Therefore, we hypothesized that the ADA, SOD and NO may play a crucial role in follicular microenvironment during maturation, and may alter respect to stimulation protocols.
Gonadotropin-releasing hormone antagonists (GnRH–ant) have emerged as an alternative treatment protocol for controlled ovarian hyperstimulation (COH) in women undergoing for IVF/ICSI. GnRH-ant has several advantages over the GnRH agonist (GnRH-a) such as a dramatic reduction in the duration of treatment and the amount of gonadotropin used for stimulation in comparison with agonist protocol [13, 14]. Other potential benefits include the lower risk of ovarian hyperstimulation syndrome [15]. However, its influence on IVF outcome and pregnancy rate, in comparison to GnRH-a protocol is controversial [16–20]. It is, therefore, important to understand the impact of regimes used for COH on IVF outcome as it may help physicians in predicting the chance of women becoming pregnant after IVF. Thus, the objective of current study were to compare the serum and follicular fluid levels of free radicals including lipid peroxidation (malondialdehyde – MDA), NO, protein carbonyl (PC), hydroxyl proline (OH-P), and antioxidant enzymes such as SOD, GSH-Px, ADA, xanthine oxidase (XO) and GSH between women undergoing COH protocol for IVF/ICSI with GnRH-a and GnRH-ant.
Materials and methods
Study subjects
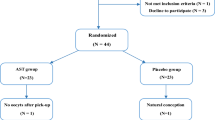
This prospective clinical study was conducted at the Obstetrics and Gynecology clinics of Hospital of Firat University and Inonu University, Schools of Medicine from February 2010 through May 2011. We recruited a cohort of 211 consecutive infertile women who admitted for fertility treatment with COH-IVF/ICSI. Follow chart of participants is presented in Fig. 1. Inclusion criteria for this study were regular menstrual cycle ranging between 25–32 days, presence of both ovaries, and diagnosis of infertility with male factor, tubal occlusion, unexplained infertility and polycystic ovaries. The protocols of patients were determined by a physician to either GnRH-a or GnRH-ant. Follicular fluid (FF) and serum samples were collected during oocyte retrieval from 84 women who undergoing COH for IVF/ICSI with either GnRH-a or GnRH-ant protocols. After centrifugation using spectrophotometric methods, samples were shock-frozen at -80 °C before analyses. Informed consents were obtained from all candidates after the approval of study by the local Investigation and Ethics Committee. Women with stage III and IV endometriosis (n = 39), polycystic ovary syndromes (n = 27), and endometrial pathology (n = 4), FF contaminated with blood during oocyte retrieval (n = 16) and any medical disease or medication (n = 12) were excluded.
Controlled ovarian hyperstimulation protocols
Patients in the GnRH-a protocol (n = 39) were received leuprolide acetate (Lucrin®, Abbott, France) at 0.5 mg daily dose starting in the mid-luteal phase of the previous cycle. After suppression of pituitary which was confirmed by serum estradiol level <70 pg/ml and transvaginal ultrasound scan showing no ovarian cysts >10 mm, recombinant FSH (Gonal–F®, Serono, Switzerland) were commenced at a dose of 225 – 450 IU/day regarding to age, ovarian reserve, and previous induction cycles until the leading follicle reached a mean diameter of 20 mm or two follicles or more reached a diameter of 18 mm. In the GnRH-ant protocol (n = 45), the same gonadotropins with the same doses as mentioned above were administered on the second day of menstrual cycle after transvaginal ultrasound examination. GnRH-ant was added daily subsequent to the leading follicle reached a diameter of 14 mm and carried on until and including the day of HCG injection.
In both stimulation protocols, sequential transvaginal ultrasonography and serum estradiol concentrations were performed to monitor ovarian response. Once consistent rise in serum estradiol concentrations was related with presence of two or more follicle of >18 mm in diameter, recombinant human chorionic gonadotropin 250 μg (r-hCG) (Ovitrelle®, Serono, Switzerland) was administered subcutaneously 36 h before transvaginal oocyte pick-up. ICSI was the usual method of fertilization. Two to five days after oocyte retrieval, the embryos were transferred into uterine cavity under ultrasound guidance. The luteal phase was supported progesterone vaginally (Crinone®, Serono, Switzerland) initiated on the day of oocyte pick-up and continued until the 12th week of gestation in cases where a pregnancy was achieved.
Biochemical analysis
In order to evaluate the prooxidant–antioxidant balance, the free radicals production were determined by measuring of lipid peroxidation (MDA), NO, PC, OH-proline levels; activity of some enzymatic antioxidants (SOD, GSH-Px, ADA, XO) and GSH levels. Assays were conducted blind to clinical information. The biochemist was blinded to the samples.
The follicular fluid and serum were extracted with an equal volume of an ethanol/chloroform mixture (5:3, volume per volume [v/v]). After centrifugation at 5000 × g for 30 min, the clear upper layer (the ethanol phase) was collected and used in the SOD activity assay. All preparation procedures were performed at 4 °C. Total (Cu–Zn and Mn) SOD (EC 1.15.1.1) activity was determined according to the method of Sun [21]. GSH-Px activity was evaluated by the method of Paglia and Valentine [22] and results were expressed as units per liter (U/L). Serum adenosine deaminase (ADA) activity was estimated spectrophotometrically by the method of Giusti [23], which is based on the indirect measurements of the formation ammonia, produced when ADA acts in excess of adenosine. Plasma XO (EC 1.2.3.2) activity was assayed spectrophotometrically by the formation of uric acid from xanthine through the increase in absorbance at 293 nm [24]. OH-proline levels were determined spectrophotometrically with Woessner's method [25]. Serial dilutions of commercial pure OH-proline (Sigma) were used as standard. Malondialdehyde level was determined by a method of Esterbauer and Cheeseman [26] based on the reaction with thiobarbituric acid (TBA) at 90–100 °C. The sample was mixed with two volumes of cold 10 % (w/v) trichloroacetic acid to precipitate protein. After centrifugation of the precipitate, an aliquot of the supernatant was reacted with an equal volume of 0.67 % (w/v) TBA and then, the absorbance was read at 532 nm. The results were expressed according to a standard graphic that was prepared from a standard solution (1, 1, 3, 3-tetramethoxypropane).
Protein carbonyl content was determined spectrophotometrically at 360 nm by the method based on the reaction of the carbonyl group with 2,4-dinitropheniylhydrazine to form 2,4-dinitrophenylhydrazone [27]. The calculation of carbonyl content was done using a molar absorption coefficient of 22,000. GSH content was determined according to Ellman [28]. GSH reacts with 5,5′-dithiobis-2-nitrobenzoic acid, and the absorbance spectra of the product have a maximum absorbance at 410 nm. The results were expressed as micromol/L.
The method for plasma nitrite and nitrate levels was based on the Griess reaction [29]. Total nitrite (nitrite + nitrate) was measured by spectrophotometry at 545 nm after conversion of nitrate to nitrite by copperized cadmium granules. A standard curve was established with a set of serial dilutions (10− 8 – 10− 3 mol l − 1) of sodium nitrite. Using the peak area from nitrite standards did linear regression. The resulting equation was used to calculate the unknown sample concentrations.
Outcome measures
The peak plasma estradiol concentration was determined and the endometrial thickness was measured with transvaginal ultrasonography, on the day of HCG. The maturation rate was calculated as the ratio of mature oocyte number to the total number of oocytes retrieved. The fertilization rate was calculated as the ratio of the number of fertilized oocytes to the number of mature oocytes. The implantation rate was calculated as the ratio of the number of gestational sacs to the number of embryos transferred. When serum beta HCG value was >10 mIU/ml 12 days after embryo transfer procedure, pregnancy was determined. Clinical pregnancy was confirmed when the fetal heartbeat was demonstrated on the 7th gestational week. Live birth rate is defined as live birth per pregnancy achieved.
Statistical analyses
Comparison between two protocols was done by using Student’s t-test and Mann–Whitney U test for continuous variables and Pearson chi-squared test for categorical variables, where applicable. Results are expressed as mean and standard deviation (SD) or percentage and number, as appropriate.
The distribution of data was analyzed for Gaussian normality by using Kolmogorov–Smirnov test. Serum SOD, GSH, MDA, follicular SOD, ADA, NO, GSH and OH-proline required log (ln) transformation to achieve Gaussian normality (P = 0.0001, 0.002, 0.011, 0.001, 0.012, 0.001, 0.026 and 0.001, respectively). The age, BMI and D-3 FSH were compared with the One-Way ANOVA, however, the mean age was significantly different in the groups. Therefore, the numerical data were analyzed by using Univariate Analysis of Variance, taking the age as covariate. A probability of <0.05 was considered to be significant.
Results
The demographic features and clinical data for both groups are presented in Table 1. In the GnRH-a group, the mean age and serum FSH levels were significantly low compared to the GnRH-ant group (30.92 ± 5.07 vs 33.49 ± 5.76 and 5.63 ± 2.56 vs 7.57 ± 3.82, P = 0.035 and P = 0.009, respectively). The mean total and mature oocyte number as well as implantation, biochemical and clinical pregnancy and live birth rate per cycle were similar in the groups (Table 2). However, the mean number of metaphase (MII) oocytes and embryos at 2PN were high in GnRH-a when compared to GnRH-ant groups (8.77 ± 5.79 vs 6.02 ± 4.31 and 12.64 ± 7.14vs 8.36 ± 5.88, P = 0.016 and P = 0.004, respectively)
Both groups received similar amount of gonadotropin doses to achieve satisfactory follicular maturation (2821.15 ± 1130.13 vs 2930.5 ± 11.86.5 IU, P = 0.47). There were 13 of 39 patients in the agonist cycles and 15 of 45 patients with live births in the antagonist cycles (33.3 % and 33.3 %, respectively, P = 1.00).
In the GnRH-a group, compared to the GnRH-ant group, there was significantly lower mean serum SOD (6.62 ± 2.21 U/l vs 8.24 ± 2.05 U/l, P = 0.001) whereas there were significantly higher mean serum GSH-Px (157.51 ± 40.06 vs 140.17 ± 38.88 U/l, P = 0.048), GSH (18.63 ± 14.70 vs 11.66 ± 6.09 micromole/l, P = 0.001) and MDA (0.90 ± 0.42 vs 0.68 ± 0.41 micromole/l, p = 0.003). The serum concentrations of remaining free radicals and antioxidant enzyme activities were similar in both groups (Table 3).
In the GnRH-a group, the mean concentrations of FF SOD (11.75 ± 1.76 vs 13.78 ± 2.16 U/l, P < 0.001), ADA (61.41 ± 37.23 vs 84.04 ± 41.01 U/l, P = 0.021) and NO (18.77 ± 13.42 vs 42.50 ± 34.80 micromole/l, P < 0.001) were significantly lower than the GnRH-ant group. The mean FF concentration of SOD was significantly correlated with the mean FF concentration of NO (r = 0.46, P < 0.0001). However, there was significantly higher mean FF OH-proline (33.69 ± 8.34 vs 29.83 ± 9.83 mg/l, P < 0.001) in the GnRH-a protocol group. There were no significant differences in concentrations of GSH-Px, XO, GSH, MDA and PC between the outcome groups (Table 3). There was no correlation between serum estradiol and serum antioxidant concentrations either in the GnRH-a or GnRH-ant groups.
The mean serum SOD levels are strongly correlated with the mean follicular fluid SOD levels (r = 0.527, P < 0.0001) and also the mean serum GSH-Px levels are inversely correlated with the mean follicular fluid GSH-Px levels (r = -0.269, P = 0.013). The remaining mean serum levels of antioxidants enzymes and free radicals are not correlated with the mean follicular fluid levels of those. Neither serum nor follicular antioxidant enzymes activities and free radicals are correlated with the demographic features and/or cycle characteristics in the entire groups. Besides, the mean concentration of follicular MDA level is correlated with the rate of MII oocytes in the whole groups (r = 0.237, P = 0.031).
In the GnRH-a group, the concentrations of serum PC and OH-proline were significantly high in women who achieved clinically pregnancy when compared to those who did not (19.96 ± 10.82 vs 25.24 ± 8.56, P = 0.028 and 32.59 ± 2.76 vs 35.92 ± 2.13, P = 0.006, respectively). In GnRH-ant group, the mean concentrations of serum MDA and XO were lower in patients who became clinically pregnant than those who did not (0.52 ± 0.15 vs 0.79 ± 0.48 micromole/l P = 0.037 and 0.10 ± 0.033 vs 0.14 ± 0.067 U/ml, P = 0.031, respectively). Further, the concentrations of ADA and PC in follicular fluid were higher in women who achieved clinically pregnant than those who failed to become pregnant (102.18 ± 41.26 vs 73.04 ± 37.41 U/l, P = 0.022 and 10.81 ± 5.36 vs 7.53 ± 5.09, P = 0.036 micromole/dl, respectively) in GnRH-ant group. There were no significant differences between serum or FF oxidative stress markers with live-birth rate either in GnRH-a (P > 0.05) or GnRH-ant group (P > 0.05).
Discussion
The results showed that the mean maturation, fertilization, implantation, clinical pregnancy and live birth rates were similar in the GnRH-a and GnRH-ant protocols. However, embryo at 2PN and MII oocyte rates were higher in the GnRH-a group than the GnRH-ant group. Although the debate on its efficacy in IVF cycles remains controversial, the GnRH-ant has been considerably used for the COH in the recent years. A previous meta-analysis including five prospective randomized trials comparing GnRH-a with GnRH-ant protocols for ovulation induction demonstrated similar pregnancy and implantation rates per treatment in both protocols, consistent with the findings of this study [30]. Consequently, several randomized studies and a latter meta-analysis also found no significant difference in the rate of clinical pregnancy [15, 17, 18, 31, 32]. The results of this study were in accordance with latter findings.
Although some experimental studies have suggested that GnRH-ant protocol may impair early folliculogenesis, the study on humans did not find any adverse effects of GnRH-ant on the growth of follicles [33–35]. Combelles et al [10] demonstrated fluctuated SOD activities during development stages of folliculogenesis, and the lowest SOD activity was detected in the large follicles. Thus, the authors concluded that lower SOD activity in follicular fluid reflects follicles containing qualified oocytes. The other study on women who underwent ovarian stimulation for IVF found that the follicular and serum activity of SOD is negatively correlated with the fertilization rate of oocytes [5]. Thus, we hypothesized that the follicular and serum SOD activity may correspond to follicular microenvironments, which may be altered by the different stimulation protocols. This study indicated that serum and follicular concentrations of SOD were significantly higher in the GnRH-ant group than the GnRH-a group. Taken together, it may be suggested that the follicular development or microenvironment is negatively affected by GnRH-ant, regarding to the high levels of SOD activity in women underwent GnRH-ant protocols.
Glutathione and glutathione peroxidase are both antioxidants that scavenge the free radicals and lipid peroxides to sustain the intracellular homeostasis [36]. The experimental studies have detected that the mean concentration of GSH was higher in mature oocytes than immature oocytes [37]. Among individuals undergoing IVF, the mean GSH-Px activity in follicular fluid yielding oocytes that were subsequently fertilized was higher when compared to that of follicles with the oocytes failed to fertilize [38]. In this study, the mean serum GSH and GSH-Px concentrations were significantly higher in the GnRH-a group than the GnRH-ant group. The follicular development may be dependent on the stimulation protocols. However, the mean follicular concentrations of these antioxidants were similar in the groups. The explanation for this discrepancy may be the gonadotropins have stimulatory effect on GSH, which is present in granulosa cells of various stages of follicles [39, 40]. In the GnRH-a group, the number of total follicles and mature oocytes retrieved was higher. Therefore, the mean serum concentration of antioxidant may be raised from a greater cohort of growing follicles at various stages of development.
In this study, the serum concentration of SOD activity was low while GSH and GSH-Px activity was high in the GnRH-a group in comparison to the GnRH-ant group. Likewise, it has previously been shown that the SOD activity was negatively correlated with GSH-Px activity [41]. This negative correlation between SOD with GSH-Px may be explained by H2O2, a by-product of SOD, scavenged by either catalase or GSH-Px [2, 42]. However, GSH-Px eliminates hydroxyperoxides as well [2].
The effect of reduced ADA activity on follicular development is not clear. There is evidence that vasodilation and high vascular permeability play a curial role during the ovulatory event in humans, and also ADA activity diminishes the amount of adenosine, deteriorating its vascular effect during follicular phase [9, 43]. However, a recent study evaluated follicular fluid adenosine concentration in women who undergoing ovarian stimulation indicated that there is no functional ADA in the follicular environment of mature oocyte [44]. Nevertheless, further assessment of oocyte growth showed that the higher levels of ADA activity were present in the small follicles, and the follicular fluid was rich in hypoxanthine that leads to conversion of adenosine to inosine, through a pathway. This study found the ADA activity in the follicular fluid of GnRH-ant group was significantly higher than the GnRH-a group. However, there was no difference in the concentration of follicular XO between the groups. In terms of biological significance to follicular maturation, this result could indicate that the GnRH-ant influence the follicular microenvironment. However, since there are no differences in the serum concentrations of ADA and XO activities between the groups and the follicular fluid was obtained from only one dominant follicle, an increase of this activity in GnRH-ant group may not truly reflect the follicular microenvironment.
Nitric oxide is a free radical gas, which has been shown to possess variety biological functions. However, previous studies have reported conflicting results in the involvement of NO in the follicular fluid environment, oocyte maturation and growth [45, 46]. Nevertheless, the impact of NO on oocyte maturation seems to be deleterious although its exact role is still unclear. Reduced levels of NO metabolites (nitrites and nitrates) in FF were determined in follicles containing mature oocytes [47]. A recent animal study evaluating the effect of different concentrations of L-NAME, a nitric oxide inhibitor, on meiotic resumption of sheep oocytes has shown that the addition of L-NAME to maturation medium significantly decreased the developing oocytes [48]. It has been suggested that NO system is associated with the maturation of oocytes [48]. In the current study, FF levels of NO were observed to be significantly lower in the GnRH-a group than the GnRH-ant group. Further, there was no correlation between FF NO levels and IVF/ICSI outcome in both groups, consistent with the previous study [49]. Since the measurement of NO and its derivatives is difficult, it is unlikely that this result indicates the follicular microenvironment in the gonadotropin protocols.
The serum and follicular fluid concentrations of MDA as an indicator for lipid peroxidation were also estimated. The serum level of MDA was significantly low in the GnRH-ant group when compared to the GnRH-a group, but FF level of this was similar in the groups. The animal study has shown that MDA concentrations did not differ between medium and large follicles; however, in the small follicles its concentration was lower at the follicular phase than the luteal phase [50]. The discrepancy of this result between FF and serum could be serum levels reflect entire follicles. The studies conducted to evaluate the relation of follicular fluid lipid peroxidation (LPO) with oocyte quality observed no correlation between LPO and oocyte maturation; hence, the FF levels of LPO were found to be higher in women who became pregnant than who did not [51, 52]. Similarly, the result of present study is consistent with the previous reports aforementioned. FF and serum MDA levels were not correlated with fertilization, implantation and clinical pregnancy rates in either GnRH-a or GnRH-ant groups.
Nevertheless, the present report has some limitations. First, it was an observational study; hence, confounding variables such as age that may influence the results could not be avoided during the recruitment of patients who were undergoing different GnRH analogues. However, we controlled the effect of age by taking the age as a covariate in the ANCOVA analysis. The follicular fluids were collected from only a dominant follicle, which may not reflect the microenvironments of other follicles in ovary. It would be suggested that FF specimens of a single follicle may not truly indicate granulosa cell production.
In summary, it appears that the impact of antioxidants and free radicals in relation to GnRH analogoues and oocyte maturation needs further assessment. Conflicting results are present the GnRH-ant affect the oocytes maturation and pregnancy rate. Our results suggest that high levels of SOD, ADA and NO in follicular fluid obtained from women undergoing GnRH-ant protocols tend to impair the follicular microenvironment for oocytes maturation. Future randomized studies on large sample of women undergoing COH for IVF to highlight the effect of GnRH analogues on oocyte maturation.
References
Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J Soc Gynecol Investig. 2001;8:S40–42.
Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–89.
Ishikawa M. Oxygen radicals-superoxide dismutase system and reproduction medicine. Nippon Sanka Fujinka Gakkai Zasshi. 1993;45:842–8.
Appasamy M, Jauniaux E, Serhal P, Al-Qahtani A, Groome NP, Muttukrishna S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil Steril. 2008;89:912–21.
Sabatini L, Wilson C, Lower A, Al-Shawaf T, Grudzinskas JG. Superoxide dismutase activity in human follicular fluid after controlled ovarian hyperstimulation in women undergoing in vitro fertilization. Fertil Steril. 1999;72:1027–34.
Sugino N. Reactive oxygen species in ovarian physiology. Reprod Med Biol. 2005;4:31–44.
Vural P, Akgul C, Yildirim A, Canbaz M. Antioxidant defence in recurrent abortion. Clin Chim Acta. 2000;295:169–77.
Tamate K, Sengoku K, Ishikawa M. The role of superoxide dismutase in the human ovary and fallopian tube. J Obstet Gynaecol. 1995;21:401–9.
Setarehbadi R, Hosseinipanah SM, Vatannejad A, Karimi M, Vaisi-raygani A, Tavilani H. Adenosine deaminase activity during menses, follicular and luteal phases of the menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 2011;155:233–4.
Combelles CM, Holick EA, Paolella LJ, Walker DC, Wu Q. Profiling of superoxide dismutase isoenzymes in compartments of the developing bovine antral follicles. Reproduction. 2010;139:871–81.
Yamauchi J, Miyazaki T, Iwasaki S, Kishi I, Kuroshima M, Tei C, Yoshimura Y. Effects of nitric oxide on ovulation and ovarian steroidogenesis and prostaglandin production in the rabbit. Endocrinology. 1997;138:3630–7.
Lee S, Acosta TJ, Nakagawa Y, Okuda K. Role of nitric oxide in the regulation of superoxide dismutase and prostaglandin F (2alpha) production in bovine luteal endothelial cells. J Reprod Dev. 2010;56:454–9.
Engel JB, Griesinger G, Schultze-Mosgau A, Felberbaum R, Diedrich K. GnRH agonists and antagonists in assisted reproduction: pregnancy rate. Reprod Biomed Online. 2006;13:84–7.
Pu D, Wu J, Liu J. Comparisons of GnRH antagonist versus GnRH agonist protocol in poor ovarian responders undergoing IVF. Hum Reprod. 2011;26:2742–9.
Al-Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, Abou-Setta AM. GnRH antagonists are safer than agonists: an update of a Cochrane review. Hum Reprod Update. 2011;17:435.
Akman MA, Erden HF, Tosun SB, Bayazit N, Aksoy E, Bahceci M. Comparison of agonistic flare-up-protocol and antagonistic multiple dose protocol in ovarian stimulation of poor responders: results of a prospective randomized trial. Hum Reprod. 2001;16:868–70.
Choi YS, Ku SY, Jee BC, Suh CS, Choi YM, Kim JG, Moon SY, Kim SH. Comparison of follicular fluid IGF-I, IGF-II, IGFBP-3, IGFBP-4 and PAPP-A concentrations and their ratios between GnRH agonist and GnRH antagonist protocols for controlled ovarian stimulation in IVF-embryo transfer patients. Hum Reprod. 2006;21:2015–21.
Devesa M, Martínez F, Coroleu B, Tur R, González C, Rodríguez I, Barri PN. Poor prognosis for ovarian response to stimulation: results of a randomised trial comparing the flare-up GnRH agonist protocol vs. the antagonist protocol. Gynecol Endocrinol. 2010;26:509–15.
Kahraman K, Berker B, Atabekoglu CS, Sonmezer M, Cetinkaya E, Aytac R, Satiroglu H. Microdose gonadotropin-releasing hormone agonist flare-up protocol versus multiple dose gonadotropin-releasing hormone antagonist protocol in poor responders undergoing intracytoplasmic sperm injection-embryo transfer cycle. Fertil Steril. 2009;91:2437–44.
Schmidt DW, Bremner T, Orris JJ, Maier DB, Benadiva CA, Nulsen JC. A randomized prospective study of microdose leuprolide versus ganirelix in in vitro fertilization cycles for poor responders. Fertil Steril. 2005;83:1568–71.
Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500.
Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69.
Giusti G. Adenosine deaminase. In: Bergmeyer MV, editor. Methods of enzymatic analysis. 2nd ed. New York: Academic; 1974. p. 1092–8.
Prajda N, Weber G. Malignant transformation-linked imbalance: decreased xanthine oxidase activity in hepatomas. FEBS Lett. 1975;59:245–9.
Woessner Jr JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7.
Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–21.
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7.
Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–3.
Baruffi RL, Mauri AL, Petersen CG, Felipe V, Martins AM, Cornicelli J, Cavagna M, Oliveira JB, Franco Jr JG. Recombinant LH supplementation to recombinant FSH during induced ovarian stimulation in the GnRH-antagonist protocol: a meta-analysis. Reprod Biomed Online. 2007;14:14–25.
Albano C, Felberbaum RE, Smitz J, Riethmuller-Winzen H, Engel J, Diedrich K, Devroey P. Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist and the LHRH-agonist buserelin. Hum Reprod. 2000;15:526–31.
Roulier R, Chabert-Orsini V, Sitri MC, Barry B, Terriou P. Depot GnRH agonist versus the single dose GnRH antagonist regimen (cetrorelix, 3 mg) in patients undergoing assisted reproduction treatment. Reprod Biomed Online. 2003;7:185–9.
de Jong D, Macklon NS, Eijkemans MJ, Mannaerts BM, Coelingh Bennink HJ, Fauser BC, Ganirelix Dose-Finding Study Group. Dynamics of the development of multiple follicles during ovarian stimulation for in vitro fertilization using recombinant follicle stimulating hormone (Puregon) and various doses of the gonadotropin-releasing hormone antagonist ganirelix (Orgalutran/Antagon). Fertil Steril. 2001;75:688–93.
Funston RN, Seidel Jr GE. Gonadotropin-releasing hormone increases cleavage rates of bovine oocytes fertilized in vitro. Biol Reprod. 1995;53:541–5.
Raga F, Casañ EM, Kruessel J, Wen Y, Bonilla-Musoles F, Polan ML. The role of gonadotropin-releasing hormone in murine preimplantation embryonic development. Endocrinology. 1999;140:3705–12.
Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28.
Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev. 2003;64:106–12.
Paszkowski T, Traub AI, Robinson SY, McMaster D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin Chim Acta. 1995;236:173–80.
Rahilly M, Carder PJ, al Nafussi A, Harrison DJ. Distribution of glutathione S transferase isoenzymes in human ovary. J Reprod Fertil. 1991;93:303–11.
Toft E, Becedas L, Soderstrom M, Lundqvist A, Depierre JW. Glutathione transferase isoenzyme patterns in the rat ovary. Chem Biol Interact. 1997;108:79–93.
Lasota B, Błaszczyk B, Stankiewicz T, Udała J, Szewczyk M, Matusiak-Bielska D. Superoxide dismutase and glutathione peroxidase activity in porcine follicular fluid in relation to follicle size, birth status of gilts, ovarian location and year season. Acta Scientiarum Polonorum—Zootechnica. 2009;8:19–30.
Comhair SA, Erzurum SC. The regulation and role of extracellular glutathione peroxidase. Antioxid Redox Signal. 2005;7:72–9.
Stouffer RL, Xu F, Duffy DM. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci. 2007;1:297–307.
Wen X, Perrett D, Jones N, Tozer AJ, Docherty SM, Iles RK. High follicular fluid adenosine levels may be pivotal in the metabolism and recycling of adenosine nucleotides in the human follicle. Metabolism. 2010;59:1145–55.
Anteby EY, Hurwitz A, Korach O, Revel A, Simon A, Finci-Yeheskel Z, Mayer M, Laufer N. Human follicular nitric oxide pathway: relationship to follicular size, oestradiol concentrations and ovarian blood flow. Hum Reprod. 1996;11:1947–51.
Sugino N, Takiguchi S, Ono M, Tamura H, Shimamura K, Nakamura Y, Tsuruta R, Sadamitsu D, Ueda T, Maekawa T, Kato H. Nitric oxide concentrations in the follicular fluid and apoptosis of granulosa cells in human follicles. Hum Reprod. 1996;11:2484–7.
Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 1998;4:3–24.
Amale MH, Shahne AZ, Abavisani A, Nasrollahi S. Effects of inhibiting nitric oxide synthase on cumulus expansion and nuclear maturation of sheep oocytes. Czech J Anim Sci. 2011;56:284–91.
Manau D, Balasch J, Jiménez W, Fábregues F, Civico S, Casamitjana R, Creus M, Vanrell JA. Follicular fluid concentrations of adrenomedullin, vascular endothelial growth factor and nitric oxide in IVF cycles: relationship to ovarian response. Hum Reprod. 2000;15:1295–9.
El-Shahat KH, Kandil M. Antioxidant capacity of follicular fluid in relation to follicular size and stage of estrous cycle in buffaloes. Theriogenology. 2012;77:1513–8.
Das S, Chattopadhyay R, Ghosh S, Ghosh S, Goswami SK, Chakravarty BN, Chaudhury K. Reactive oxygen species level in follicular fluid–embryo quality marker in IVF? Hum Reprod. 2006;21:2403–7.
Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, Rose BI. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973–6.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule GnRH antagonist may have negative effect on follicular environment. The oxidative stress markers may reflect the follicular environment and may be related to follicular development.
Rights and permissions
About this article
Cite this article
Celik, E., Celik, O., Kumbak, B. et al. A comparative study on oxidative and antioxidative markers of serum and follicular fluid in GnRH agonist and antagonist cycles. J Assist Reprod Genet 29, 1175–1183 (2012). https://doi.org/10.1007/s10815-012-9843-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9843-6