Abstract
A statistical experimental design using response surface methodology (RSM) was carried out to optimize the mixotrophic growth using glutamic acid, tri-sodium citrate, succinic acid, sodium aspartate, and sodium pyruvate to improve the production of fucoxanthin. First, a fractional factorial design as a screening test was done with the above-mentioned intermediates. Then, glutamic acid, sodium aspartate, and sodium pyruvate were chosen according to their statistically significant (P<0.05) and positive effects on fucoxanthin production. The three selected metabolic intermediates were optimized employing RSM to obtain a high level of fucoxanthin. The data were adjusted with a second-order polynomial equation to determine the relationship between fucoxanthin production and three optimized metabolic intermediates. Using multiple regression techniques and to attain the high fucoxanthin productivity (22.4 mg L−1 day−1), the optimum amount of the metabolic intermediates were found as follows: sodium aspartate, 7.5 mM; sodium pyruvate, 7.5 mM; glutamic acid, 3.29 mM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fucoxanthin is an orange-colored pigment that contributes more than 10% of the total production of carotenoids in nature. This xanthophyll has several properties, e.g., anti-cancer, antioxidant, anti-angiogenic, anti-diabetic, anti-obese, anti-inflammatory, anti-malarial, and photo-protection (Eilers et al. 2016; Beuzenberg et al. 2017; Mohamadnia et al. 2021). Owing to mentioned effects, fucoxanthin has potential applications in various industries such as medicine, cosmetics, pharmaceuticals, and food (Mohamadnia et al. 2020). The production of this high-value metabolite from its natural resources has received much attention because of difficult direct chemical synthesis (Ausich 1997). Furthermore, due to the demand of the carotenoid market, it is essential to enhance the ability to produce these valuable materials by their producing host (de Miguel et al. 2001; Zhang and Lee 2001; Pelah et al. 2004). Manipulation of the biosynthetic pathway of carotenoid-producing microorganisms is employed by the extra addition of some metabolic intermediates in many studies (Doerfling 1971; Bjork 1969; Choudhari et al. 2008). Fucoxanthin is a primary and growth-related metabolite that functions as the light-harvesting antenna in fucoxanthin-chlorophyll- protein (FCP) complex the chloroplast of the algae (Guler et al. 2019). Among algae, microalgae with higher biomass productivity, easier cultivation, and more fucoxanthin accumulation are preferred over macroalgae. Tisochrysis lutea is the best microalgal candidate because of its high fucoxanthin content (79.40 ± 0.95 mg g−1 DW) and growth rate (0.592 ± 0.005 day−1) (Mohamadnia et al. 2020a) in comparison to other microalgal sources. Nevertheless, there is little information about mixotrophic growth of algae using the metabolic intermediates and carotenoid production, especially fucoxanthin. According to available knowledge about the carotenogenic microbial strains and a few algae, exploiting various inducers led to a high amount of carotenoid per cell. Several stimulators such as pyruvic acid, acetate, and glycerol have been tudied for their motivating effects on Chromochloris zofingiensis and Haematococcus pluvialis for astaxanthin production (Kobayashi et al. 1991; Zhang et al. 2020; Chen et al. 2021). Of all the stimuli available in this field, tricarboxylic acid (TCA) cycle intermediates and precursors have been used to produce carotenoids from carotenogenic organisms (Harker et al. 1995).
Design of experiments (DOE) methodology is applied in several fields such as food, feed, agriculture, cosmetic, and pharmaceutical industries (Xiong et al. 2005). Generally, culture medium optimization is carried out through the one-factor-at-a-time method, in which one factor is changed, and the others are kept stable. In this approach, the probable interactions between parameters might be ignored while they are vital. The statistical methodology is a more common and efficient procedure in experimental works due to its whole controlling variables at the same time alongside the determination of their interaction (Nasrabadi and Razavi 2010). Full or fractional factorial designs, central composite design (CCD), response surface methodology (RSM), etc. are examples of experimental design methods that estimate the essential variables and the interactions of the parameters. Fractional factorial designs screen between factors and determine the practical factors and interactions (Davies 1993). In addition, response surface methodology (RSM) is a practical approach that can be applied to construct the model, design and establish the experiments, and optimize meaningful factors and concentrations. Responses predict by the numerical equations (Myers et al. 2002). There is little information about using experimental design to optimize the culture medium of fucoxanthin-producing algae even though it has been applied to screening and optimizing the principal factors in optimizing carotenoid production by algae (Harker et al. 1995; Cifuentes et al. 2003).
The first goal of the present study was to investigate the influence of metabolic intermediates addition on the enhancement of the production of fucoxanthin from T. lutea in f/2 medium that was enriched from our previous experiments (Mohamadnia et al. 2021). Second, screening the main vital factors which can be affecting on the fucoxanthin production by the factorial design from those which have no effect. Finally, the metabolic intermediate concentration was optimized using a central composite design for the maximum photosynthetic production of fucoxanthin.
Materials and methods
Microalga and culture medium preparation
Tisochrysis lutea CCAP 927/14 was from the Culture Collection of Algae and Protozoa (CCAP, Oban, Scotland). Tisopchrysis lutea was cultivated in 1000-mL flasks (500-mL medium volume) at 24 ± 1 °C and pH = 7.9 ± 0.1. The flasks were sterilized for 20 min in an autoclave and then inoculated with 100 mL of T. lutea (X0 = 1.21 × 107 cells mL–1). Each flask contained 500 mL with f/2 medium prepared with autoclaved seawater (36.27 g L–1) and was enriched with sterilized starch 3.9 g L−1 and nitrate 0.162 g L−1 according to our previous findings (Mohamadnia et al. 2021). Metabolic intermediates were added to the medium, as shown in Table 1 (screening tests), and then for the optimization tests presented in Table 3. Light was supplied by 60-W fluorescent lamps at 60 µmol photons m−2 s−1 irradiance with (16:8) light:dark conditions in the phytotron. An air pump performed aeration at 5 L min−1 after passing through 0.45-μm filters.
Growth profile
Algal growth was assessed in two ways: cell number using a hemocytometer after Lugol dying and cell dried weight at the end of cultivation. Each sample was enumerated in three replicates, and the mean was used as the algal density for each replicate. Biomass productivity of microalgae was determined by sampling 5 mL of each flask. After centrifugation at 2800 × g for 5 min, the remaining biomass was washed with isotonic ammonium formate to remove the excess salt and after filtration through a pre-weighed 2.5-μm GF/C filter (Whatman) dried at 75 °C for 24 h until achieving a constant weight. Finally, biomass productivity was obtained by Eq. (1) (Ishika et al. 2019).
where w0 and wf are the biomass concentration (mg L−1) after inoculation and the end of the cultivation (ttot = 6 days), respectively.
The specific growth rate (µ) was evaluated during the exponential phase according to Eq. (2).
where X2 and X1 are the cell density (cells mL−1) at time t2 and t1 (day), respectively.
Pigment determination
The sample was harvested by centrifugation at 5000 × g for 5 min at 4°Cand the pellet was washed with distilled water and freeze-dried at − 80 °C for 48 h. The extraction process was carried out with ethanol (Markou and Nerantzis 2013; Xia et al. 2013), without any cell disruption. Tisochrysis lutea has no cell wall, which leads to a more straightforward downstream process than the other algae. The extraction process was done in triplicate at ambient temperature under dim light to eliminate pigment oxidation. Distilled water and HPLC-grade solvents like methanol, acetonitrile, ethanol, and hexane were purchased from Duksan Pure Chemical Company (South Korea).
Jeffrey and Humphrey’s protocol (Jeffrey and Humphrey 1975) was used to estimate the chlorophyll-a content. The absorbance was measured at 664 nm and 647 nm.
For total carotenoid extraction, freeze-dried microalgae and 90% acetone were mixed. Total carotenoids in the acetone extract were quantified at 452 nm by a microplate reader through Eq. (3) (Mohamadnia et al. 2021):
where C is the total carotenoids (mg L−1), A452 is the absorbance at 452 nm, Ve is the volume of extract (mL), and Vt is the volume of culture sample (mL).
For fucoxanthin evaluation, 100 mg freeze-dried algae and 4 mL ethanol were mixed and then vortexed for almost 5 min. This process was repeated until the cells were colorless. Then, all supernatants were collected and filtered through a 0.45-µm cellulose acetate filter and used for HPLC analysis (Kim et al. 2012).
The samples were analyzed by an HPLC system equipped with a PDA Detector 2800 and a Pump 1000. The samples (20 µL) were injected into a Eurospher 100 RP & C18 column (5 μm, 250 mm \(\times\) 4.6 mm) (Knauer, Germany). Methanol/water at a ratio of (95:5 v/v) was used as an isocratic mobile phase, and fucoxanthin was detected at 450 nm. The retention times of fucoxanthin (at flow rate of 1 mL min−1) were 7.5 min. The standard fucoxanthin solution (Sigma-Aldrich, USA) was employed for quantification.
Statistical analysis
The statistical methodology was carried out with screening tests with the help of fractional factorial design (2n −1, n = 5), and the main factors were optimized with response surface methodology. In the experimental design, the factors Ai were coded as ai according to Eq. (4).
where Ai is the actual value of the ith factor, A0 is the factor’s actual value of Ai at the center point, ΔAi is the step change size, and ai is the coded value of the ith factor. All five metabolic intermediates (n = 5) were screened through a fractional factorial design (25 −1) to estimate their effects on chlorophyll-a, total carotenoid, and fucoxanthin production. The experimental design consists of 16 experimental runs and four center points, as shown in Table 2. In addition, three coded variables and their actual values for screening trials are exhibited in Table 1.
Response surface methodology was employed for optimization of the obtained main factors according to Table 4. The real and coded values (− 1.682, − 1, 0, + 1, + 1.682) of significant variables are manifested in Table 3. Finally, the experimental results were correlated to a second-order polynomial equation by applying the multiple regression method. Hence, optimum responses were inferred according to Eq. (5).
where Yn is the response function; βn0 is the model intercept; βni, βnii, and βnij show the coefficients of the linear, quadratic, and interactive terms, respectively; and ai, aii, and aiaj represent the linear, quadratic, and interactive terms of the coded independent factors, respectively. The analysis of variance table was composed, and all term significances were evaluated with the help of F-value at a probability (P < 0.05) and coefficient of determination (R2). Three-dimensional response surface plots were achieved when one response was kept constant at the optimum point against two independent factors.
Results
Screening the main factors
In the present work, the fractional factorial design (25 −1) was applied to detect the influence of five metabolic intermediates (sodium aspartate, glutamic acid, sodium pyruvate, tri-sodium citrate, succinic acid) on total carotenoid, fucoxanthin, and chlorophyll-a production in batch trials. Table 2 shows independent factors and the responses as a total carotenoid, fucoxanthin, and chlorophyll-a production for designed trials according to fractional factorial design. Supplementary Figs. S1, S2, and S3 present the Pareto diagram and the standard probability plot for total carotenoid, fucoxanthin, and chlorophyll-a production, respectively. Among tested metabolic intermediates, only sodium aspartate, glutamic acid, and sodium pyruvate significantly impact the production of total carotenoid, fucoxanthin, and chlorophyll by T. lutea with P˂0.05. Succinic acid and tri-sodium citrate had a negative effect. Hence, just sodium aspartate, glutamic acid, and sodium pyruvate were chosen for further optimization studies (Fig. 1).
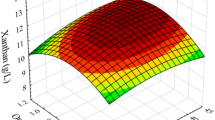
Contour plots (a, c, e) and surface plots (b, d, f) of the model equation fitted to the data for total carotenoid production. a, b Interaction of a sodium aspartate and glutamic acid concentration. c, d Interaction of sodium aspartate and sodium pyruvate concentration. e, f Interaction of glutamic acid and sodium pyruvate concentration
Optimization of the production of fucoxanthin
The experimental design for optimization tests and the obtained responses as the pigment production and growth are shown in Tables 4 and 5, respectively. In optimization trials, the succinic acid and tri-sodium citrate amounts were constant on their optimum value when the maximum fucoxanthin production was obtained, according to Table 2 and Fig. 2. The experimental outcomes achieved by RSM were correlated with a second-order polynomial equation (Eq. (5)) that resulted a second-order response surface model in the form of analysis of variance (ANOVA) (Tables S1–S3). Finally, the polynomial model for the production of total carotenoid, fucoxanthin, and chlorophyll-a based on three main parameters is described as Eqs. (6), (7), and (8), respectively.
where Ai are the coded independent factors (A1 = sodium aspartate, A2 = glutamic acid, and A3 = sodium pyruvate) (Table 4). Contour plots show the influence of the three main factors (A1 = sodium aspartate, A2 = glutamic acid, and A3 = sodium pyruvate) on dependent factors (total carotenoid, fucoxanthin, and chlorophyll-a production). In Fig. 2a and b, fucoxanthin production improved by increasing the concentration of sodium aspartate and glutamic acid to 7.5 and 3.29 mM, respectively. When the amount of sodium aspartate and glutamic acid was kept constant, fucoxanthin production decreased with an increasing level of sodium aspartate and glutamic acid above 7.5 and 3.29 mM, respectively. Figure 2c and d present the interaction between sodium aspartate and sodium pyruvate concentration on fucoxanthin production. In this plot, the content of fucoxanthin was enhanced by increasing the amount of both metabolic intermediates concentration (sodium aspartate and sodium pyruvate) up to 7.5 mM. When the sodium aspartate and sodium pyruvate amount go beyond 7.5 mM, fucoxanthin biosynthesis will decrease.
Contour plots (a, c, e) and surface plots (b, d, f) of the model equation fitted to the data for fucoxanthin production. a, b Interaction of a sodium aspartate and glutamic acid concentration. c, d Interaction of sodium aspartate and sodium pyruvate concentration. e, f Interaction of glutamic acid and sodium pyruvate concentration
The interaction of sodium pyruvate and glutamic acid is shown in Fig. 2e and f. When the mentioned parameters amount increased up to 7.5 and 3.29 mM, respectively, the biosynthesis of fucoxanthin increased, while passing from these concentrations diminished its amount (Fig. 3). This pattern was also observed for total carotenoid and chlorophyll-a production in Figs. 1 and 3, respectively. The obtained responses from Table S1 and Eq. (6) for total carotenoid production resulted in the significant interaction between glutamic acid and sodium pyruvate (P = 0.0207). From Table S2 and Eq. (7) for fucoxanthin production, it can be inferred that the interaction between all three variables is not significant (P˃0.05). In addition, the obtained results from Table S3 and Eq. (8) for the production of chlorophyll-a resulted the significant interaction between glutamic acid and sodium pyruvate (P = 0.0073). Besides, the growth of T. lutea was investigated according to cell density and biomass productivity (Table 5).
Contour plots (a, c, e) and surface plots (b, d, f) of the model equation fitted to the data for chlorophyll production. a, b Interaction of a sodium aspartate and glutamic acid concentration. c, d Interaction of sodium aspartate and sodium pyruvate concentration. e, f Interaction of glutamic acid and sodium pyruvate concentration
Discussion
Optimization of the production of fucoxanthin and the growth of Tisochrysis lutea
The effect of TCA metabolic intermediates or precursors, e.g., aspartate, pyruvate, citrate, glutamate, and succinate as nitrogen and carbon sources on algal species has rarely investigated in literature (Chen et al. 2009).
The screening tests were applied to distinguish the primary and influential factors in the biosynthetic pathway of fucoxanthin and sodium aspartate, glutamic acid, and sodium pyruvate were selected as the main effective compounds. Next, to improve the production of fucoxanthin, response surface methodology (RSM) was applied to optimize the main effective factor amount in a batch experiment. The production of total carotenoid, fucoxanthin, and chlorophyll-a was measured at the end of each trial when T. lutea cells reached the stationary phase.
As mentioned before, fucoxanthin plays as the light-harvesting antenna in the FCP complex and accomplishes cellular function as a primary metabolite. Therefore, growth and pigments, especially fucoxanthin, are directly related (Kim et al. 2012; Sahin et al. 2019). Equation (6) was solved, and the optimum concentration for the maximum growth of T. lutea and fucoxanthin production were a1 = 0.18, a2 = –1, and a3 = 0.084 equal to the actual value of sodium aspartate = 7.95 mM, glutamic acid = 5 mM, and sodium pyruvate = 7.71 mM, respectively. By applying these concentrations, the model’s predicted response for the maximum content of fucoxanthin was 81.12 mg g–1 DW. Succinate, α-ketoglutarate, and oxaloacetate had a greater influence on carotenoid production of Dietzia natronolimnaea HS-1 (Nasrabadi and Razavi 2010). In another study, citrate, glutamate, malate, and succinate had more stimulatory impressions on carotenoid pigment production in the bacterium Cellulosimicrobium strain AZ (Bhattacharjya et al. 2020). The impact of TCA intermediates on the promotion of carotenoid biosynthesis varies among various species (Zhang et al. 2020; Chen et al. 2021). Chen et al. (2021) indicated that pyruvic acid enhanced the mixotrophic growth of Chromochloris zofingiensis and improved the contents of astaxanthin and lipids up to 10.7 mg g−1 and 66.1% dry weight. In another study, Liu et al. (2013) sing the low-cost carbon source cane molasses observed that molasses stimulated astaxanthin production may be attributed to the triggered expression of carotenogenic genes. These genes are responsible for conversion of β-carotene to astaxanthin through ketolation and hydroxylation (Liu et al. 2013). In fact, various patterns of one species to another for nutrient consumption and algal metabolism may inflence cell growth and fucoxanthin production and that is why T. lutea has relatively better growth and produced more fucoxanthin in the medium enriched with sodium aspartate, glutamic acid, and sodium pyruvate.
Externally adding sodium aspartate, glutamic acid, and sodium pyruvate to the medium of the T. lutea provides extra carbon and energy for cell propagation and growth (Alkhamis and Qin 2016). Moreover, sodium aspartate and glutamic acid contain nitrogen, an essential element for microalgal reproduction and growth and can lead to overproduction of chlorophyll a, carotenoid, and fucoxanthin (Sahin et al. 2019).
Validation test for the optimal conditions and model
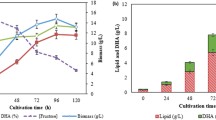
Verifying the optimal conditions and quadratic models was accomplished through three replicates of batch experiments. The highest amount of fucoxanthin (81.7 ± 0.75 mg g–1 DW) was achieved in confirmatory tests on day fourth. This value is close to the predicted response obtained from the quadratic model (81.12 mg g–1 DW). Hence, response surface methodology is a suitable statistic procedure to predict the experimental results. Figure 4 demonstrates the cell density, total carotenoid, fucoxanthin, and chlorophyll-a production achieved under optimal conditions. The maximum cell number (5.44 × 108 ± 0.07) cell mL–1 and fucoxanthin (81.7 ± 0.75 mg g–1 DW) production were achieved on day 4th of cultivation. This was by the other carotenogenic strains such as D. natronolimnaea HS-1 (Nasrabadi and Razavi 2010), Chlorococcum sp. (Zhang and Lee 2001), Chlorella zofingiensis (Li et al. 2006), Chlorella pyrenoidosa (Czycan 1964), and Sporobolomyces ruberrimus H110 (Razavi and Marc 2006). The presence of some metabolic intermediates in the biosynthetic pathway of fucoxanthin adjusts and upregulates some enzymes and reactions that increase fucoxanthin production. For example, key enzymes such as thiolase and acetyl-CoA-carboxylase that participate in the tricarboxylic acid (TCA) cycle are affected by metabolic intermediates; therefore, the carotenoid biosynthesis is promoted. They form the isoprene units and carbon skeleton of the carotenoid biosynthetic pathway through aerobic respiration (Sandmann 1994). Another possible reason is that the intermediates increase the acetyl-CoA pool; thus, the isoprene units (ip) are constructed and carotenoid biosynthesis is improved (Alcantara and Sanchez 1999).
Some studies have employed TCA cycle intermediates to enhance the production of carotenoids (Bjork 1969; Doerfling 1971; Flores-Cotera et al. 2001). Our results in the present study were also confirmed the stimulatory effect of metabolic intermediates on the production of carotenoids, particularly fucoxanthin. Similarly Alcantara and Sanchez (1999) report that oxaloacetate influences zeaxanthin production in Flavobacterium sp. Furthermore, the β-carotene biosynthesis was enhanced by α-ketoglutarate, oxaloacetate, and pyruvate (Bjork 1969). Nefelova et al. also reported that α-ketoglutarate, oxaloacetate, fumarate, citrate, and malate increased the carotenoid biosynthesis in Actinomyces chrysomallus (Nefelova et al. 1978).
Conclusions
In the present study, response surface methodology (RSM) was used as a statistical tool to optimize the concentration of main factors in the biosynthetic pathway of fucoxanthin from T. lutea. Among five metabolic intermediates screened by fractional factorial design, sodium aspartate, glutamic acid, and sodium pyruvate were selected as the effective parameters (P˂ 0.05) in the growth of T. lutea and the production of fucoxanthin. Afterward, response surface methodology (RSM) was employed to find the optimum concentration of effective variables to enhance fucoxanthin production. Three quadratic polynomial equations were constructed to determine the relationship between three main factors and total carotenoid, fucoxanthin, and chlorophyll-a production. Finally, the optimum amount of sodium aspartate, glutamic acid, and sodium pyruvate was assessed as 7.5, 3.29, and 7.5 mM, respectively. Under this condition, the maximum cell number and fucoxanthin productions were 5.44 × 108 ± 0.07 cells mL–1 and 81.7 ± 0.75 mg g–1 DW, respectively.
Availability of data and material
The data supporting the results of this study will be made available by the corresponding author upon reasonable request.
References
Alcantara S, Sanchez S (1999) Influence of carbon and nitrogen sources on Flavobacterium growth and zeaxanthin biosynthesis. J Ind Microbiol Biotechnol 23:697–700
Alkhamis Y, Qin JG (2016) Comparison of pigment and proximate compositions of Tisochrysis lutea in phototrophic and mixotrophic cultures. J Appl Phycol 28:35–42
Ausich RL (1997) Commercial opportunities for carotenoid production by biotechnology. Pure Appl Chem 69:2169–2174
Beuzenberg V, Goodwin EO, Puddick J, Romanazzi D, Adams SL, Packer MA (2017) Optimising conditions for growth and xanthophyll production in continuous culture of Tisochrysis lutea using photobioreactor arrays and central composite design experiments. N Z J Bot 55:64–78
Bhattacharjya R, Marella TK, Tiwari A, Saxena A, Singh PK, Mishra B (2020) Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Bioresour Technol 318:124073
Bjork L (1969) Stimulation of β-carotene synthesis in Blakeslea trispora by pyruvate and intermediates of tricarboxylic acid (TCA) cycle. Acta Chem Scand 23:2908–2909
Chen T, Dong Wei Gu, Chen YW, Chen F (2009) Employment of organic acids to enhance astaxanthin formation in heterotrophic Chlorella zofingiensis. J Food Process Preserv 33:271–284
Chen J-H, Wei D, Lim P-E, Xie J, Chen WN (2021) Screening and effect evaluation of chemical inducers for enhancing astaxanthin and lipid production in mixotrophic Chromochloris zofingiensis. J Appl Phycol 34:159–176
Choudhari SM, Ananthanarayan L, Singhal RS (2008) Use of metabolic stimulators and inhibitors for enhanced production of β-carotene and lycopene by Blakeslea trispora NRRL 2895 and 2896. Bioresour Technol 99:3166–3173
Cifuentes AS, Gonzalez MA, Vargas S, Hoeneisen M, Gonzalez N (2003) Optimization of biomass, total carotenoids and astaxanthin production in Haematococcus pluvialis Flotow strain Steptoe (Nevada, USA) under laboratory conditions. Biol Res 36:343–357
Czycan FC (1964) Canthaxanthin as secondary carotenoid in some green algae. Experientia 20:573–574
Davies KW (1993) Design of experiments for predictive microbial modeling. J Ind Microbiol 12:295–300
de Miguel T, Sieiro C, Poza M, Villa TG (2001) Analysis of canthaxanthin and related pigments from Gordonia jacobaea mutants. J Agric Food Chem 49:1200–1202
Doerfling P (1971) Significance of citric acid and other acids of the tricarboxylic acid cycle for growth and pigment formation in the unicellular alga Poteriochromonas stipitata. Z Allg Mikrobiol 11:161–165
Eilers U, Bikoulis A, Breitenbach J, Büchel C, Sandmann G (2016) Limitations in the biosynthesis of fucoxanthin as targets for genetic engineering in Phaeodactylum tricornutum. J Appl Phycol 28:123–129
Flores-Cotera L, Martin R, Sanchez S (2001) Citrate, a possible precursor of astaxanthin in Phaffia rhodozyma: influence of varying levels of ammonium, phosphate and citrate in a chemically defined medium. Appl Microbiol Biotechnol 55:341–347
Guler BA, Deniz I, Demirel Z, Oncel SS, Imamoglu E (2019) Transition from start-up to scale-up for fucoxanthin production in flat plate photobioreactor. J Appl Phycol 31:1525–1533
Harker M, Tsavalos AJ, Young AJ (1995) Use of response surface methodology to optimise carotenogenesis in the microalga Haematococcus pluvialis. J Appl Phycol 7:399–406
Ishika T, Laird DW, Bahri PA, Moheimani NR (2019) Co-cultivation and stepwise cultivation of Chaetoceros muelleri and Amphora sp. for fucoxanthin production under gradual salinity increase. J Appl Phycol 31:1535–1544
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Kim SM, Kang S-W, Kwon O-N, Chung D, Pan C-H (2012) Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: characterization of extraction for commercial application. J Korean Soc Appl Biol Chem 55:477–483
Kobayashi M, Kakizono T, Nagai S (1991) Astaxanthin production by a green alga, Haematococcus pluvialis accompanied with morphological changes in acetate media. J Ferment Bioeng 71:335–339
Li H-B, Fan K-W, Chen F (2006) Isolation and purification of canthaxanthin from the microalga Chlorella zofingiensis by high-speed counter-current chromatography. J Sep Sci 29:699–703
Liu J, Sun Z, Zhong Y, Gerken H, Huang J, Chen F (2013) Utilization of cane molasses towards cost-saving astaxanthin production by a Chlorella zofingiensis mutant. J Appl Phycol 25:1447–1456
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542
Mohamadnia S, Tavakoli O, Faramarzi MA, Shamsollahi Z (2020b) Production of fucoxanthin by the microalga Tisochrysis lutea: a review of recent developments. Aquaculture 516:734637
Mohamadnia S, Tavakoli O, Faramarzi MA (2021) Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture 533:736074
Myers RH, Montgomery DC, Anderson-Cook CM (2002) Response surface methodology: process and product optimization using designed experiments. Wiley, NY
Nasrabadi MRN, Razavi SH (2010) Use of response surface methodology in a fed-batch process for optimization of tricarboxylic acid cycle intermediates to achieve high levels of canthaxanthin from Dietzia natronolimnaea HS-1. J Biosci Bioeng 109:361–368
Nefelova MV, Sverdlova AN, Alekseeva LN (1978) Effect of organic acids on the biosynthesis of carotenes by an Actinomyces chrysomallus strain. Mikrobiologiia 47:208–211
Pelah D, Sintov A, Cohen E (2004) The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J Microbiol Biotechnol 20:483–486
Razavi SH, Marc I (2006) Effect of temperature and pH on the growth kinetics and carotenoid production by Sporobolomyces ruberrimus H110 using technical glycerol as carbon source. Iran J Chem Chem Eng 25:59–64
Sahin MS, Khazi MI, Demirel Z, Dalay MC (2019) Variation in growth, fucoxanthin, fatty acids profile and lipid content of marine diatoms Nitzschia sp. and Nanofrustulum shiloi in response to nitrogen and iron. Biocatal Agric Biotechnol 17:390–398
Sandmann G (1994) Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem 223:7–24
Xia S, Ke W, Wan L, Li A, Qiang Hu, Zhang C (2013) Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar Drugs 11:2667–2681
Xiong C, Shouwen C, Ming S, Ziniu Yu (2005) Medium optimization by response surface methodology for poly-γ-glutamic acid production using dairy manure as the basis of a solid substrate. Appl Microbiol Biotechnol 69:390–396
Zhang D-H, Lee Y-K (2001) Two-step process for ketocarotenoid production by a green alga, Chlorococcum sp. strain MA-1. Appl Microbiol Biotechnol 55:537–540
Zhang L, Zhang C, Liu J, Yang N (2020) A strategy for stimulating astaxanthin and lipid production in Haematococcus pluvialis by exogenous glycerol application under low light. Algal Res 46:101779
Author information
Authors and Affiliations
Contributions
Sonia Mohamadnia designed the study and experiments. Sonia Mohamadnia performed the experiments and data analysis. Sonia Mohamadnia drafted the manuscript. Omid Tavakoli and Mohammad Ali Faramarzi reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohamadnia, S., Tavakoli, O. & Faramarzi, M.A. Optimization of metabolic intermediates to enhance the production of fucoxanthin from Tisochrysis lutea. J Appl Phycol 34, 1269–1279 (2022). https://doi.org/10.1007/s10811-022-02717-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02717-y