Abstract
Seaweed extracts contain many bio-elicitors that can greatly improve natural plant immunity, so stimulating plant immunity with these formulations is a sustainable approach. The red seaweed (Kappaphycus sp. and Eucheuma sp.)–derived biostimulants (LBS6 and LBD1) were tested for their ability to protect rice against fungal blast disease. Compared to non-primed plants, LBD-1 primed and challenged inoculated plants had substantially higher levels of defence-related enzymes such as phenylalanine ammonia lyase, chitinase, peroxidase, polyphenol oxidase and phenolic content. In primed and Magnaporthe oryzae isolate MG-01 challenge–inoculated plants, altered transcript levels of various defence genes such as OsPR-1, PAL-6, PR1-5 and PR-15 were observed. Disease tolerance to the blast pathogen was tested in glasshouse conditions using the foliar application or root dipping with various concentrations of LBD-1. Both approaches significantly decreased disease severity, but the combined spray and root dipping was more effective than either spray or root dipping alone. These findings indicate that priming rice plants with seaweed biostimulant induces resistance to blast fungus, most likely by inducing defence-related genes and enzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is a major staple food for almost half of the global population. India is the world’s second-largest rice-producing country in terms of both area and volume (Chatterjee et al. 2021). Different biotic stresses have a detrimental impact on the future rice yield. Rice blast disease, caused by the fungus Magnaporthe oryzae, is responsible for 10–30% annual yield losses alone (Kumar et al. 2021). Farmers are more likely to use fungicides in higher concentrations, putting natural biodiversity at risk, encouraging the emergence of newer strains and displaying the development of resistance. This also results in the rejection of export shipments due to pesticide residues in grains that exceed the defined qualitative and quantitative limits (Chatterjee et al. 2021). This illustrates why it is so important to focus on achieving optimal and long-term rice yields. The use of natural compounds such as seaweeds which act as elicitors of plant resistance is one of the methods for reducing pesticide use (Agnieszka 2020). These organic compounds, unlike pesticides, are biodegradable, non-toxic and non-polluting (Pal et al. 2015). Seaweeds are an important part of the aquatic and coastal ecosystems and have commercial importance in improving agricultural productivity (Ali et al. 2021). Some bioactive elicitors in different seaweed extracts induce pathogen-associated molecular patterns (PAMPs) because of their structural similarities to pathogen-derived molecules. This is accomplished by priming or eliciting the induced systemic resistance (ISR) and systemic acquired resistance (SAR) pathways’ defence responses (Shukla et al. 2021). The red and brown algae are used in most of the formulations. These often have resistance to various diseases because they contain many compounds that act as elicitor molecules against pathogens, such as laminarin, fucans, ulvans and carrageenans (Shukla et al. 2016). Research on red seaweed extracts and their formulated products for rice crop is limited in India, and no research has been done on the impact of these seaweeds on rice in terms of mediated disease resistance to blast disease. Hence, in the present study, we aimed to determine the biological effects of seaweed biostimulants (LBD1 and LBS6) obtained from red seaweed (Kappaphycus sp. and Eucheuma sp.) in conferring blast disease tolerance in rice.
Materials and methods
Sources of seaweed biostimulants
The red seaweed biostimulants (LBD-1 and LBS-6, 20% solid extract) used in the study were provided by Sea6Energy Pvt Ltd, Bengaluru, India. Briefly, the red seaweed biomass of Kappaphycus sp. and Eucheuma sp. was processed following two patented technologies (Nori et al. 2017; Girish et al. 2020) to obtain solid and liquid fractions. LBD-1 was prepared from the extracts of the solid fraction while LBS-6, by blending the extracts of liquid and solid fractions. The solid fractions are rich in bioactive sulphated galacto-oligosaccharides while liquid extracts are rich in natural minerals present in the seaweeds.
Plant and fungal material
Under greenhouse conditions, IR-64, a commonly grown susceptible rice variety, was grown. Magnaporthe oryzae isolate MG-01 was obtained from C-CAMP, National Centre for Biological Sciences (NCBS), Bengaluru. MG-01 was grown and sustained on oatmeal agar for two weeks in the dark at 28 °C for inoculation and then exposed to fluorescent lights for one week at room temperature for sporulation. With the aid of a haemocytometer, the spore concentration was set to 5 × 105 spores mL−1. The punch inoculation method described by Ono et al. (2001) was used for artificial inoculation.
Priming of rice seedlings
Three-week-old seedlings were selected and primed by foliar application of seaweed biostimulants as per the treatment details, viz., T1, MG-01 without priming; T2, priming of plants with LBD1 (2 mL L−1) and challenge inoculation with MG-01; T3, LBD1 (2 mL L−1); T4, LBS6 (1 mL L−1); and T5, water control. The inoculated plants were placed in the dark (incubator) at 25 °C for 24 h and then moved to the mist chamber with the relative humidity maintained at 90%. After the inoculation, leaves were monitored every day for the development of necrotic lesions. Leaf samples were collected at different intervals, viz., before priming, 24, 48, and 72 h post inoculation (hpi). All the samples were frozen in liquid nitrogen and stored at − 80 °C for estimation of different defence related enzymes and gene expression studies.
Defence-related enzymatic and biochemical assays
All the inoculated leaf samples collected at different intervals were used for enzymatic assays. Phenylalanine ammonia lyase (PAL) activity was assayed by Campos et al. (2004). The chitinase activity was determined as per the procedure given by Mathivana et al. (1965). The polyphenol oxidase (PPO) and peroxidase (POD) activity from the leaf samples were assayed by the method of Kumar and Khan (1982). The total phenol content was estimated as per the procedure given by Sadasivam and Manickam (1996).
Molecular changes induced by the seaweed biostimulants in response to blast disease
RNeasy plant mini kit (Qiagen, Germany) was used to extract total RNA from rice leaves, which were then converted to cDNA using the PrimeScript RT Reagent Kit (TaKaRa, Japan) according to the manufacturer’s instructions.
Quantitative real-time PCR analysis
The gene expression of resistance genes (R) OsPR1 (OsPR1# 012, OsPR1#022, OsPR1#074, OsPR1# 021, OsPR1#073 and OsPR1#121), PR1-5, PR-15 and PAL-6 was studied in the leaf tissue of different treatments. In order to know the relative expression of the genes mentioned above, a real-time PCR (CFX96, Bio-Rad) amplification was performed using SYBR Green (TaKaRa) with gene-specific primers and rice specific ubiquitin gene as a reference gene. The relative expression was calculated using the comparative cycle threshold method of Livak and Schmittgen (2001).
Efficacy of seaweed biostimulants in management of rice blast in glasshouse condition
Three-week-old rice seedlings were primed and challenge inoculated with MG-01 to determine the effectiveness of seaweed biostimulants in reducing blast disease.
Following treatments were tested based on different methods of application and concentrations of seaweed biostimulants: T1, LBD1 foliar spray (1 mL L−1); T2, LBD1 foliar spray (2 mL L−1); T3, LBD1 root dipping (1 mL L−1); T4, LBD1 root dipping (2 mL L−1); T5, LBD1 foliar spray and root dipping (1 and 2 mL L−1); and T6, pathogen control. Disease assessment was done by recording the number of lesions and lesion length at 5, 10 and 15 days post inoculation (dpi) and 5, 10 and 15 days after symptom appearance (DASA) respectively.
Statistical analysis
The statistical analysis was performed using GraphPad Prism 5 software. Each experiment was repeated thrice and the mean, standard deviations, standard error and significance level were analysed. Two-way analysis of variance (ANOVA) was calculated using Fisher’s least significant difference (LSD) at p ≤ 0.05 to determine the significance of the difference between the means of control and different treatments. Interaction between two independent (treatments and time) and one dependent variable (enzyme activity/relative expression) was analysed. The data shown in figures are means of three replicates, and error bars are based on standard deviation (SD). Mean values of treatments that were significantly different from each other are indicated by different letters in figures.
Results
Biochemical changes induced by the seaweed biostimulants in response to rice blast
The activation of defence related enzymes and total phenols in primed and non-primed plants after challenge inoculation with MG-01 was investigated to see whether seaweed biostimulants could induce plant defence.
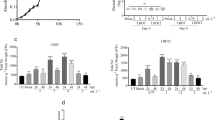
The time course study of the PAL enzyme showed that primed plants had higher activity than non-primed plants. A transient and significant (p < 0.0001) rise in PAL activity was observed in LBD-1 primed and challenge inoculated plants at 24 hpi, with a 9.99-fold shift compared to 0 h. Non-primed plants with pathogens, on the other hand, increased enzymatic activity by 4.02-fold at 24 hpi (Fig. 1a). In non-primed plants, enzyme activity decreased after 48 hpi, while enzyme activity remained constant in primed plants. At 72 hpi, LBD-1 priming and pathogen inoculation resulted in chitinase activity with a 4.06-fold change. At 24, 48 and 72 hpi, T1 (MG-01 without priming) had a fold difference of 2.05, 2.29 and 2.42, significantly lower than primed plants (Fig. 1b). PPO behaviour was typically higher in primed plants than in non-primed plants. At 24, 48 and 72 hpi, there was a shift change of 7.11-, 8.04- and 9.34-fold in LBD-1 primed and MG-01 inoculated plants, respectively (Fig. 1c). PPO activity was increased 4.27-fold in pathogen inoculated plants at 72 hpi, which was significantly lower than in primed plants (p < 0.0001). At 24 to 72 hpi, primed plants inoculated with MG-01 displayed a transient and significant increase in POD activity (Fig. 1d). In T2, enzyme activity peaked at 48 hpi (10.32-fold). Enzymatic activity increased in non-primed plants at 72 hpi with a fold shift of 5.56, which was slightly lower than primed and challenge inoculated plants. The total phenolic content of primed and unprimed samples was determined at various intervals. At 72 hpi, the phenolic content of seaweed-treated plants was higher (2.44-fold). However, an increasing pattern was observed in non-primed plants, although it was significantly lower than in primed plants (Fig. 1e).
Resistance gene (R) expression induced by the seaweed biostimulants in response to rice blast
In primed and non-primed rice plants, the response of individual OsPR1, PR1-5, PR-15 and PAL-6 genes to blast-fungus infection was studied using qPCR. At 48 hpi, primed plants inoculated with blast fungus induced OsPR1#012 transcript accumulation, with a relative expression of 7.04. At 24 hpi, LBD-1 priming and challenge inoculation of MG-01 increased the expression of OsPR1#012, OsPR1#021, OsPR1#022, OsPR1#074, OsPR1#121, PAL-6, PR1-5 and PR-15 (Figs. 2 and 3). However, both genes were upregulated in the pathogen-inoculated control but significantly lower than in the primed plants. Transcript levels of OsPR1#021, OsPR1#022, OsPR1#074, OsPR1#121 and PR1-5 were decreased in non- primed plants at 48 hpi, indicating that the up regulated genes in non-primed plants were not stable relative to primed plants. The OsPR1#021 gene expression differed over the time course studied, indicating an irregular pattern. At 24 hpi, relative expression was 7.85 compared to the water control, but at 48 hpi, it was significantly decreased and upregulated, reaching 8.09 at 72 hpi. This was evidence of pathogen entry and establishment. The pathogen attempted to bypass the defence mechanism of the primed plants; however, seaweed priming elicited the defence mechanism, which nullified the pathogen’s impact.
Efficacy of seaweed biostimulants in management of rice blast under glasshouse
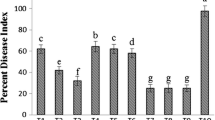
To determine the efficacy of LBD-1 in managing blast disease, rice plants were treated with seaweed biostimulants through foliar spray, root dipping, or both methods. Foliar spray + root dipping (T5) was found to be more efficient than the other two methods studied. Plants sprayed (1 mL L−1) and root dipped (2 mL L−1) with LBD-1 had the lowest number of lesions and lesions length. T1, T2, T3, T4 and T5 lesion lengths were 1.35, 1.30, 1.45, 1.22 and 0.81 cm after 15 DASA, respectively. The percentile disease reduction rate observed for various treatments were as follows: T1 (50%), T2 (64.29%), T3 (57.14%), T4 (71.43%) and T5 (78.57%). At 15 dpi, the number of lesions in pathogen control plants was 7 with a lesion length of 5.70 cm at 15 days after symptom appearance. Both methods of application provided significant defence against rice blast (Fig. 4). However, since the percentage of protection was higher in root dipping with foliar spray, it was more effective than foliar spray alone (78.57%). The reduction in disease can be due to the activation of various defence-related enzymes and genes, as estimated in previous experiments.
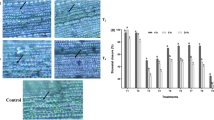
Development of blast lesions on LBD-1 primed rice plants under glasshouse conditions. (T1, LBD1 foliar spray (1 mL L−1); T2, LBD1 foliar spray (2 mL L−1); T3, LBD1 root dipping (1 mL L−1); T4, LBD1 root dipping (2 mL L−1); T5, LBD1 foliar spray and root dipping (1 and 2 mL L−1); and T6, pathogen control)
Discussion
Rice blast disease represents a significant threat to rice production and global food security worldwide (Fernandez and Orth 2018). The use of environmentally sustainable methods is more appealing in the light of the public concern on the use of chemical fungicides in agricultural production (Esserti et al. 2017). Fucans, carrageenans, ulvans and laminarins, among other bioactive compounds found in seaweeds, have been shown to trigger plant defence against several pathogens (Shukla et al. 2019). These elicitor-like molecules act as priming molecules or pathogen-associated molecular patterns (PAMPs), activating ISR and SAR responses in the process (Islam et al. 2020). In this research, we demonstrated that LBD-1 increased the activity of PAL, chitinase, PPO, POD and total phenolic content, all of which catalyse the development of lignin and other phenolic compounds that are involved in reinforcing the cell structure against pathogens (Esserti et al. 2017). Surprisingly, when rice plants were inoculated with MG-01, seaweed biostimulants prime them for increased enzymatic activity. Priming is a process that causes plants to go into a state of high alert, causing them to improve their defence responses (Conrath et al. 2015). Defence-related enzymes such as peroxidase, polyphenoloxidase, phenylalanine ammonia lyase, chitinase and β 1,3-glucanase were activated in seaweed extracts primed carrot plants (Shukla et al. 2019). We examined at the expression of four SA (OsPR1#012, OsPR1#022, OsPR1#074 and PR1-5) and three JA (OsPR1# 021, OsPR1#073 and OsPR1#121) responsive marker genes, as well as PAL-6 and PR-15. LBD-1 priming was shown to increase the expression of the aforementioned defence genes after challenge inoculation with MG-01 at different time points in the current study.
Furthermore, when the plants were only provided with seaweed extracts, the expression levels were lower. Significant upregulation of defence genes has been observed in other experiments at a single time point. According to previous studies, the expression of the PR1 gene in Arabidopsis thaliana was upregulated 24 h after treatment with seaweed extracts (Cook et al. 2018; Shukla et al. 2019). The inoculation of Macrophomina phaseolina and the priming of tomato seedlings with Kappaphycus alvarezii (K-sap) extract substantially increased transcripts of pathogenesis-related genes (PR-1b1, PR-3 and PR-5) (Agarwal et al. 2016). When opposed to non-primed plants, primed plants induced a greater preventative response against pathogen infection progression. Root dipping or foliar spray and challenge inoculation were used to evaluate LBD-1’s in vivo activity against MG-01 on rice seedlings. Rice plants were significantly protected against M. oryzae using both application methods. Crown gall disease caused by the bacterial pathogen Agrobacterium tumefaciens was reduced considerably in tomato seedlings after spraying with seaweed extracts. In comparison to the control, seaweed-treated plants had substantially higher activity of the defence enzymes polyphenol oxidase and peroxidase (Esserti et al. 2017). According to the findings of this study, the seaweed biostimulant elicited defence responses in rice against blast fungus by increasing the activity of defence-related enzymes and enhancing PR genes.
Conclusion
In context to the devastating nature of blast disease, it is necessary to adopt sustainable management practices to ensure the global food security. Considering the minimization of fungicides in agriculture, an eco-friendly approach is appreciable. The effect of seaweed biostimulants in controlling M. oryzae was investigated in this study. The enzymatic tests showed that red seaweed biostimulants triggered the plant defence mechanism and increased resistance to blast disease. Furthermore, the current work shows that the expression levels of SA and JA inducing genes increased at several critical time points after pathogen inoculation. Notably, seaweed biostimulants induced the activation of several defence-related pathways prior to pathogen infection. These findings were consistent with a primed response and were closely related to enzymatic activity and disease prevention. This ability dramatically expands the application of seaweed biostimulant to other crops. As a result, we propose that the approach used in this study be extended to commercially significant crops to determine the effect of seaweed extracts on their response to a pathogen.
Data availability
All data generated or analysed during this study are included in the manuscript.
References
Agnieszka J (2020) Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 10:173
Agarwal P, Patel K, Das AK, Ghosh A, Agarwal PK (2016) Insights into the role of seaweed Kappaphycus alvarezii sap towards phytohormone signalling and regulating defence responsive genes in Lycopersicon esculentum. J Appl Phycol 28:2529–2537
Ali O, Ramsubhag A, Jayaraman J (2021) Biostimulant properties of seaweed extracts in plants: implications towards sustainable crop production. Plants 10:531
Campos R, Nonogaki H, Suslow T, Saltveit MS (2004) Isolation and characterization of a wound inducible phenylalanine ammonia lyase gene (LsPALI) from remain lettuce leaves. Plant Physiol 121:429–438
Chatterjee S, Gangopadhyay C, Bandyopadhyay P, Bhowmick MK, Roy SK, Majumder A, Gathala MK, Tanwar RK, Singh SP, Birah A, Chattopadhyay C (2021) Input-based assessment on integrated pest management for transplanted rice (Oryza sativa) in India. Crop Protect 141:105444
Conrath U, Beckers GJM, Langenbach CJG, Jaskiewicz MR (2015) Priming for enhanced defence. Annu Rev Phytopathol 53:97–119
Cook J, Zhang J, Norrie J, Blal B, Cheng Z (2018) Seaweed extract (StellaMaris®) activates innate immune responses in Arabidopsis thaliana and protects host against bacterial pathogens. Mar Drugs 16:221
Esserti S, Amal S, Lalla AR, Tayeb K, Kacem M, Malika B, El Mostafa K, Lydia F, Lorenzo B, Nuria A, Mohamed F (2017) Protective effect of three brown seaweed extracts against fungal and bacterial diseases of tomato. J Appl Phycol 29:1081–1093
Fernandez J, Orth K (2018) Rise of a cereal killer: the biology of Magnaporthe oryzae biotrophic growth. Trends Microbiol 26:582–597
Girish T, Vantharam V, Malhotra P, Bhose SP, Sekar N, Kuruvilla S, Girija L, Khandelwal S, Sanghe N, Kumar S, Nori SS, Suryanarayan S (2020) Composition comprising sulphated galactose, and implementations thereof. Patent PCT/IN2019/050831
Islam MT, Gan HM, Ziemann M, Hussain HI, Arioli T, Cahill D (2020) Phaeophyceaean (brown algal) extracts activate plant defence systems in Arabidopsis thaliana challenged with Phytophthora cinnamomi. Front Plant Sci 11:852
Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv. PR-202) leaves during senescence. Indian J Exp Biol 20:412–416
Kumar V, Jain P, Venkadesan S, Karkute SG, Bhati J, Abdin MZ, Sevanthi AM, Mishra DC, Chaturvedi KK, Rai A (2021) Understanding rice-Magnaporthe oryzae interaction in resistant and susceptible cultivars of rice under panicle blast infection using a time-course transcriptome analysis. Genes 12:301
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Mathivana N, Kabilan V, Murugesan K (1965) Production of chitinase by Fusarium clamydosporum, a mycoparasite to groundnut rust, Puccinia arachidis. Indian Exp Biol J 35:890–893
Nori SS, Kumar S, Khandelwal S, Suryanarayan S (2017) Biostimulant formulation for improving plant growth and uses thereof. US Patent US10358391B2
Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K (2001) Essential role of the small GTPase Rac in disease resistance of rice. PNAS 98:759–764
Pal A, Dwivedi SK, Maurya PK, Kanwar P (2015) Effect of seaweed saps on growth, yield, nutrient uptake and economic improvement of maize (sweet corn). J Appl Nat Sci 7:970–975
Sadasivam S, Manickam A (1996) Phenols. In: Biochemical methods, 2nd edn. New Age International, New Delhi, p 256
Shukla PS, Tudor B, Alan TC, Balakrishnan P (2016) Carrageenans from red seaweeds as promoters of growth and elicitors of defence response in plants. Front Mar Sci 3:81
Shukla PS, Mantin EG, Adil M, Bajpai S, Critchley AT, Prithiviraj B (2019) Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front Plant Sci 10:655
Shukla PS, Borza T, Critchley AT, Prithiviraj B (2021) Seaweed-based compounds and products for sustainable protection against plant pathogens. Mar Drugs 19:59
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: MKP. Performed the experiments: SNB, PBP, PME and CG. Analysed the data: SNB. Contributed reagents/materials/analysis tools: HBM, SN, GTR and SSN. Wrote the manuscript: SNB, MKP and PBP. Edited the manuscript: SN, PBP and SSN. All authors read and approved the manuscript for publication. The authors declare that they have no conflict of interest in the publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sahana, B.N., PrasannaKumar, M.K., Mahesh, H.B. et al. Biostimulants derived from red seaweed stimulate the plant defence mechanism in rice against Magnaporthe oryzae. J Appl Phycol 34, 659–665 (2022). https://doi.org/10.1007/s10811-021-02627-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02627-5