Abstract
The red alga Gracilaria dura is economically important due to its high-quality agarose. Previous studies with wild populations reported the existence of specific differences in functional traits as well as agar characteristics among life cycle stages. In farmed populations, such differences can be exploited for commercial gains. For that, the variation among stages still needs to be well established under farming scenarios. Here, we compared the life cycle stages of G. dura regarding morphological and anatomical structures, growth performance under preliminary field trials, characteristics of agarose of cultivated biomass with biochemical (NMR) and molecular profiling (SCoT). The male gametophyte was found to have a significantly higher growth rate of 6.23 ± 0.59% day−1 than the tetrasporophyte (5.10 ± 0.14% day−1) and cystocarpic female gametophyte (2.67 ± 0.32% day−1). A maximum agarose yield of 28.6 ± 1.53% was obtained from the tetrasporophyte, significantly higher than 27.4 ± 0.60% in cystocarpic female gametophyte and 25.2 ± 0.36% in male gametophyte. The gel strength of agarose from male gametophytes was 2384 ± 124.13 g cm−2, which was significantly higher than the 1900 ± 50 g cm−2 and 2122 ± 124.03 g cm−2 recorded from tetrasporophytes and cystocarpic female gametophytes, respectively. A metabolomic study by NMR spectroscopy showed critical differences in alanine, lactate and isethionic acid among stages. The genetic correlation studied with the SCoT marker showed an average polymorphism of 47.02%. The average heterozygosity and Shannon-Wiener index were 0.63 and 1.06 respectively. This study of characterising and differentiating isomorphic life phases of G. dura by a decisive biomarker could be a valuable reference point to select an appropriate cultivar for commercial farming and breeding programmes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seaweeds are some of the most efficient biomass producers on the planet. For some time, they have been commercially exploited, mainly for food consumption (Knoop et al. 2020) and for their gelling-type molecules (Santos and Melo 2018). The recent fiscal inertia adversely affected the polysaccharide market, with the dismal performance of carrageenan and alginate sales, but global agar trade reported an impressive 7% annual growth. About 125,200 dry tonnes of biomass have been processed to produce ca. 14,500 t of industrial agar worth 246 million US$ (Porse and Rudolph 2017). Agarose is more refined form of agar consisting of repeating disaccharide units of (1,3)-linked β-D-galactose (G) and (1,4)-linked α-l-3, 6-anhydrogalactose. Agarose has also been prepared from Gracilaria dura (Meena et al. 2007), Gelidium amansii (Wang et al. 2012; Chew et al. 2018), Ahnfeltia plicata (Yu et al. 2019) and Gracilaria gigas (Efendi et al. 2015). New products derived through functional modification of agarose are being synthesised having niche applications in biomedical, bioengineering and molecular biology domains (Kondaveeti et al. 2013; Chaudhary et al. 2014; Chudasama et al. 2016; Sharma et al. 2017; Chadar et al. 2019). An alternative to the traditional energy-intensive freeze-thaw method for agarose production has been developed by employing surfactant-induced coagulation (Meena et al. 2014). The studies pertaining to resource augmentation and improvement are thus desired for further capitalising on financial gains.
Gracilaria dura (C. Agardh) J. Agardh has been reported from Europe, Atlantic Islands, South America, Africa, the Middle East and Asia (Guiry and Guiry 2020). The collections from Indian waters have been reported to produce superior quality agarose of gel strength of 1900-2200 g cm−2 (1% gel) benchmarking it with the commercial high-quality agarose from Sigma agarose (A0576) (Meena et al. 2007, 2014). It is worth mentioning that this process is direct and solvent free which circumvents traditional protocol of fractionation of agar to obtain low-gelling, high gel-strength agarose polymer. The method has been patented (Siddhanta et al. 2005). The agarose obtained by employing this method has comparable specifications to that which are commercially available. Thus, the Council of Scientific and Industrial Research (CSIR), New Delhi has acquired “Trademark - Sagarose”; number 2123313; March 30, 2011; Office of the Registrar of Trade Marks, Govt. of India. Further, a systematic, integrated and green process was developed for complete utilisation of feedstock to obtain spectrum of commercially valuable and industrially important products, namely pigments, lipids, agar, agriculturally important nutrient-rich liquid and energy-dense cellulose (Reddy et al. 2016). The gel strength of the native agar of this alga was found to be 546 ± 25 g cm−2 (1.5% gel) from Indian waters (Siddhanta et al. 1997). Similarly, a gel strength of 318 g cm−2 has been reported for this seaweed (Marinho-Soriano and Bourret 2005).
The commercial exploitation of seaweed biomass essentially depends on a consistent supply of raw material. Nonetheless, scanty distribution (only at selected locations along the Indian coast) and short-lived population hamper the prospects of utilising this industrially lucrative resource. Pre-feasibility studies of experimental farming of this alga using various culture methods such as raft, net, net pouch and tube net were successful along with both Gujarat and Tamil Nadu coast (Mantri et al. 2009, 2020; Veeragurunathan et al. 2015a, b). Further, a viable protocol for rapid production of elite seedlings through clonal propagation has been demonstrated (Saminathan et al. 2015).
The sexual life cycle in Gracilaria is characterised by alternation between haploid as well as diploid life phases (Kain and Destombe 1995). Typical triphasic life cycle consists of morphologically isomorphic gametophytic (separate male and female individuals), tetrasporophytic and carposporophytic generation. These life cycle stages have been found to have niche partitioning and clear ecological differentiation (Guillemin et al. 2013; Vieira et al. 2018a). The variation in functional traits related to survival and growth provides ample opportunity to select appropriate cultivar in clonally propagated seaweeds (Santelices 1992). The tetrasporophytes of Gracilaria verrucosa (now Gracilariopsis longissima) which are diploid in nature have been found to perform well with attributes such as tolerance to climatic variation, survival and growth (Destombe et al. 1993). Zhang and Meer (1988) reported that female plants grow faster in Gracilariopsis lemaneiformis. The gel strength of different life cycle stages of Gracilaria has shown a differential pattern (Whyte et al. 1981). The fecundity in Agarophyton chilense (formerly Gracilaria chilensis) was found to be highest for the male gametophytes and lowest for female gametophytes, with the diploid stages displaying intermediate values. Further, spore release and survival were also likened to life cycle (Vieira et al. 2018b). The agar yield of haploid female gametophyte is lower when compared to diploid tetrasporophytes in Gracilaria bursapastoris (Marinho-Soriano et al. 1999). Better adaptive abilities of tetrasporophytes of Gracilaria caudata (now Crassiphycus caudatus) have been reported based on the physiological difference (Faria et al. 2017). Industrial feasibility of life cycle-based resource management via germplasm selection, breeding and crop improvement is possible but such studies are seldom attempted under farming conditions.
The pilot investigation in G. dura carried out in our laboratory confirmed the potential of selecting superior germplasm using life cycle stages. The high quality of agarose was reported from tetrasporophytes, while endogenous abscisic acid (ABA) content was significantly higher for haploid gametophytes (female more than male) than diploid tetrasporophytes (Gupta et al. 2011). It may be also noted that functional trait related to growth was distinct in tetrasporophyte, while those of survival (antioxidant, proximate composition and pigments) were prominent in cystocarpic female gametophyte (Sambhwani et al. 2020). In view of the initiation of commercial farming of this species in India (Supplementary resource 1 and 2), further study towards cultivar development assumes immediate importance for domestic trade. Specific trait assessment in G. dura under farming conditions remains unattempted due to a lack of expertise for precise identification of the life cycle stage in field samples before maturity. The aim of the present investigation was thus (a) provide identification features of different life cycle stages; (b) ascertain through small-scale field trails, if these stages record different growth; (c) characterise yield and quality of agarose of cultivated biomass; (d) confirm molecular (start codon targeted) and bio-chemical (nuclear magnetic resonance) differences in life cycle stages. We only attempted to establish a “proof of concept” to validate the above objectives. It may further be noted that genetic diversity of the population, genotype variation within stages and etc. were not included and thus are out-of-scope of the present study.
Materials and methods
Sample collection
Gracilaria dura was collected from its natural habitat in April 2017 during the lowest tide of chart datum at Veraval coast (N 20° 54′; E 70° 20′), Gujarat, India. Different life history stages were collected and identified in the field. It is not possible for everyone to identify them just based on external morphological features, but the first author has developed this expertise and skill during his over 10 years of field experience of working with this seaweed. The phases were identified using a magnifying glass based on morphology and branching pattern (in case of male gametophyte), external reproductive structure (in case of cystocarpic female gametophyte), rough texture and frond length (in case of tetrasporophyte). The fronds of a particular life history stage were collected from a single population. The collection was divided into two parts. The larger proportion was placed into a cool pack under dark conditions and then transported for conducting farming experiments to CSMCRI field cultivation farm located at Simar (N 20.75; E 71.13), Gujarat, India. The second part was brought to the laboratory for documenting photographic evidence to record identification characters. Further, the samples were processed for developing biochemical and molecular markers.
Field cultivation
The attempt was made to ascertain if life cycle stages record different growth. The monoline method of farming was adopted for this study from April to May 2017. Polypropylene ropes (3 mm thick) were used for tying seedlings. A total of nine lines (5 m each) were seeded and planted. Each line contained fronds of only one life cycle stage and three lines were maintained for each stage, namely, male, cystocarpic female and tetrasporophyte (n = 3). Twenty healthy fragments of ca. 5 cm length were tied using a nylon thread at equidistance onto each rope. The total initial seedlings of ca. 250 g fresh weight (FW) were maintained on every rope. The cultivation lines were anchored to the seafloor at about 5 m depths, placed slightly below the water surface (approximately 20 cm), parallel to the coast, and facing wave direction in the open sea. The ropes were cleaned at regular intervals (once in 3 days) to remove adhering silt and other epiphytic seaweeds. The cultivation was carried out for only one cycle of 24 days. The final weight of each rope was taken at the end of the experiment. The daily growth rate (DGR) % day−1 was calculated using the formula given below.
where W2 is the final fresh weight in gram, W1 is the initial fresh weight in gram and t is the number of culture days.
It should be noted that, since all life cycle stages were cultivated in the same location at the same time and for the same duration, they were thus subjected to similar ambient field conditions.
Extraction of agarose
The aim was to examine if cultivated samples of life cycle stages record variation in agarose characteristics. It may be noted that such examination for individual cultivated fronds was out of the scope. The field-grown samples for different life cycle stages were harvested. Three ropes that were used for identical life cycle stages were harvested individually and shade dried in the field and brought to the laboratory. The biomass was then cleaned manually to remove dirt and sand particles and processed. It was dried to constant mass in a hot air oven at 60 °C. The agarose extraction was carried out separately for each life cycle stage following the method described by Meena et al. (2007). Each 20 g sample was washed with tap water to remove excess salt, soaked for 1 h at ambient temperature and subjected to 10% aqueous NaOH treatment for 2 h at 85 °C. This mass was further repeatedly washed (7-10 times) with running water to remove excess alkali. The residue left was then autoclaved in water. The hot extract obtained was homogenised using a pulveriser and vacuum filtered over a celite bed. The gel was freeze-thawed and agarose thus obtained was then dried and ground using mortar and pestle. The yield was calculated on the dry weight basis of seaweed containing nil moisture. The gel strength measurements were done using 1.5% w/w agarose gels on a gel tester (Kiya Seisakusho, Ltd., Japan). The gelling and melting temperatures of agarose gels were measured as described in our previous work (Meena et al. 2007). Apparent viscosity of agarose solutions was measured on a Brookfield viscometer (Synchrolectric Viscometer, USA), using Spindle No. 1 at a speed of 60 rpm (Meena et al. 2007). Sulphate content was determined as described before (Meena et al. 2007). The ash content was estimated in the residue that was obtained after igniting the agarose sample at 550 °C for 4 h. Infrared spectra of agarose samples were recorded (Perkin-Elmer Spectrum GX, FT-IR System, USA) by taking 2 mg of agarose in 600 mg of KBr to prepare the pellet. Intrinsic viscosities (η) were determined at 32 °C using an Ostwald viscometer (Prasad et al. 2005). The 13C CP-mass spectra were recorded for the extracted agarose on Bruker-Avance II 500 at 125 MHz. 13C chemical shifts were referenced to internal standard DMSO (39.4 ppm). The peaks were assigned to the polymeric structural units as per convention of Freile-Pelegrín and Murano (2005). The extraction was performed for three different samples of each life cycle stage (n = 3).
Extraction of metabolites and developing nuclear magnetic resonance-based marker
The metabolites were extracted from male, cystocarpic female and tetrasporophytic thalli following aqueous extraction procedure as described by Gupta et al. (2013). All identified samples were first cultured in PES medium under standard laboratory conditions (25 ± 1 °C under daylight white fluorescent lamps at 50 μmol photons m−2 s−1 irradiance with a 12-h light and 12-h dark photoperiod) for 3 days for acclimatisation prior to the metabolic analysis. The acclimatisation was performed to eliminate environmental effect on metabolite variation and focus on phase-specific metabolites. The aqueous extracts were prepared using 50 mM phosphate buffer at pH 6.0. Prior to start aqueous extraction, excess medium was removed from seaweed thalli by blotting them on a tissue paper. The thalli of 200 mg fresh mass corresponding to all life cycle stages were powdered using liquid nitrogen. Powdered biomass was mix with 200 mL phosphate buffer (50 mM) of pH 6.0 followed by vortexing for 1 min and sonication for 30 min at 55 °C. Aqueous extract was centrifuged at 10,000 rpm for 2 min followed by re-centrifugation for 2 min to obtain clear solution. This solution was transferred to 5-mm nuclear magnetic resonance (NMR) tubes with a few drops of D2O containing a reference standard (TSP). Bruker Avance II 500-MHz spectrometer, equipped with a 5-mm BBI probe, was used to get 1H NMR spectra. Samples were spun at 20 Hz at room temperature (25 °C) and 72 repeat scans were performed during each spectrum with 1 s acquisition time and 7000 Hz spectral width. A 0.3-Hz exponential line broadening value was used for spectral Fourier transformation. The spectra were then manually phased, baseline corrected and calibrated to the internal standard (trimethylsilyl propanoic acid set at 0.0 ppm). Recycle delay of 1 s with a low-strength RF pulse was adjusted with signal suppression at 4.8. 1H resonances were compared with Gupta et al. (2013) data for the identification of metabolites. The identified metabolites from the spectral data were for relative intensity variations by normalizing the peak intensity at ~ 3.67 ppm. The assignment of peaks was followed from the previous reference (Gupta et al. 2013) and only peaks showing significant differences were marked in the diagram.
Genomic DNA extraction and developing start codon targeted-based marker
Total genomic DNA was isolated from three different life cycle stages of G. dura using the CTAB method. A total of 20 start codon targeted (SCoT) primers developed by Collard and Mackill (2009) were screened during the present investigation. PCR reaction consisted of 2 μL DNA (15-20 ng μL−1), 0.2 μM primer, 1 U of Taq DNA polymerase, 10 mM Tris-HCL, 0.5 mM KCl, 0.2 mM dNTPs and 1.5 mM MgCl2. A biorad thermal cycler was used with programme adjusted as initial denaturation step of 3 min at 95 °C, denaturation at 95 °C for 1 min, primer annealing at respective temperature for 1 min and extension at 72 °C for 2 min, with a final extension at 72 °C for 10 min. A 3% agarose gel stained with ethidium bromide was used to analyse PCR products and bands were visualized under UV light (Baghel et al. 2011).
The three indices were employed to estimate and compare the genetic diversity, namely, (1) heterozygosity (He) =\( 1-{\sum}_{i=1}^k{p}_i^2 \) where pi represents the frequency of the ith allele of k alleles; (2) Shannon’s index (H) =\( -{\sum}_{i=1}^k{p}_{\mathrm{i}}\ \ln\ {p}_{\mathrm{i}} \); and (3) marker index = MI = PIC × EMR, where PIC is the polymorphic information content and effective multiplex ratio (EMR) is calculated as total number of polymorphic loci (per primer) multiplied by the proportion of polymorphic loci per their total number.
Statistical analysis of data
The growth rate and agar characterisation were expressed as mean ± SD (n = 3). The data normality was verified with the Shapiro-Wilk test, with p > 0.05 confirming the null hypothesis of normal distribution. A one-way ANOVA was applied for the factor stage with three treatments (males, cystocarpic females and tetrasporophytes), and the Tukey post hoc test was applied for comparison among treatments. This analysis was performed using the Infostat software (Di Rienzo et al. 2018).
Results
The detailed study of thalli collected from natural population provided key features to differentiate life cycle stages of G. dura. Being isomorphic, fronds of male, cystocarpic female and tetrasporophyte showed similar morphology and anatomy. Tetrasporophytes ca. 20 cm tall and robust were less branched than male and cystocarpic female gametophytes. Tetrasporangia measured 3–5.5 × 11–12 μm, and divided anticlinally, either decussate or cruciate. Bispore formation was also observed (Supplementary Fig. 3). The male thalli were slender and much branched, up to 10 cm in height. The conceptacles were widely distributed on the surface. The spermatangia were produced in deep conceptacles (62.5-90 μm deep, 25–45 μm wide). They further open through external opening—“verrucosa” type (Yamamoto 1984). However, at a few occasions, fusion among neighbouring conceptacles was also recorded resembling “henriquesiana” type (Supplementary Fig. 3). The thallus of cystocarpic female plants was bigger and not much branched, ca. 30-35 cm in height. The carpogonial branches were typical gracilariacean two-celled with of short trichogynes. Mature cystocarps had a single ostiole, 1-1.5 mm in height and slightly constricted at their base (Supplementary Fig. 3).
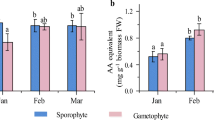
Fronds started growing within 7 days, developing branched thallus under the farming condition that differed among life cycle stages. The branching level was highest among male, followed by tetrasporophyte and cystocarpic female fronds (Fig. 1). The average daily growth rate (DGR) for male frond was found to be 6.23 ± 0.59% day−1, significantly higher from tetrasporophyte (5.10 ± 0.14% day−1) and cystocarpic female frond (2.67 ± 0.32% day−1) (F2,6 = 62.86, p = 0.0001) (Table 1, Supplementary Table 1).
The agarose yield from cultivated material, calculated from received dry seaweed containing no moisture, ranged from 25.2 ± 0.36 to 28.6 ± 1.53% (Table 1, Supplementary Table 1). The maximum yield of 28.6 ± 1.53%was obtained from the tetrasporophytic fronds, and was significantly higher than the yield obtained from male fronds of 25.2 ± 0.36% (F2,6 = 9.36, p = 0.01). The cystocarpic female fronds reported a 27.4 ± 0.60% agarose yield. The gel strength of agarose obtained from male fronds was 2384 ± 124.13 g cm−2 and was significantly higher than tetrasporophytic fronds with 1900 ± 50 g cm−2 (F(2,6) = 15.89, p = 0.004) and those of cystocarpic female frond was 2122 ± 124.03 g cm−2 (Table 1, Supplementary Table 1). The gelling and melting temperatures of agarose gels of all three phases were averaged around 38 °C and 91 °C, respectively. The sulphate content of agarose samples ranged from 0.28 ± 0.03 to 0.36 ± 0.05% obtained from male, cystocarpic female and tetrasporophytic fronds, while ash ranged between 0.94 ± 0.11 and 1.01 ± 0.09% (w/w) (Table 1).
FTIR spectra of all agarose samples reported characteristic IR bands (Fig. 2a-c). The main characteristic absorption bands of agarose were detected at 3430 cm−1 (stretching band of hydroxyl group), 1075 cm−1 (vibration of C-O-C bridge of glycosidic linkage) and 932 cm−1 (vibration of C-O-C bridge of 3,6-anhydroglactose unit). These bands were in good agreement with previously reported spectra (Garcia et al. 2000; Meena et al. 2007). The spectra thus confirmed that agarose obtained from male, cystocarpic female and tetrasporophytic fronds is chemically similar. Solid-NMR spectra revealed that a total of six chemical shifts appeared in all the three agarose samples (Fig. 3a-c). The solid-state spectra (CP-MAS) exhibited six peaks at 62.63, 69.98, 75.85, 79.89, 99.19 and 102.54 ppm for agarose from male, at 62.73, 69.88, 75.72, 79.60, 98.58 and 102.32 ppm for tetrasporophytic and at 62.82, 70.15, 75.80, 80.06, 98.39 and 100.62 ppm for cystocarpic female frond. The viscosity (η) decreased with an increase in shear rate (γ) in agarose gels obtained from all the three life cycle stages. The maximum viscosity was obtained for agarose of male fronds, while tetrasporophytic fronds reported minimum viscosity (Supplementary Fig. 4).
Figure 4 shows stack of areas of interest of 1H NMR spectra of tetrasporophyte, male and cystocarpic female samples plotted from top to bottom in different plots to highlight the differences. Carbohydrate spectra ranged from and above δ 4.0 while the amino acid region ranged from δ 0.8 to δ 4.0. Mainly studied carbohydrate compounds in seaweeds were galactose (δ 4.0 t), sucrose (δ 5.4 d), glucose (δ 4.2 d) and sorbitol (δ 3.8), whereas amino acid compounds were alanine (δ 1.5 d) and proline (δ 2.6 bm). Other compounds were lactate (δ 1.33 d), ethanolamine (δ 3.37 d) and isethionic acid (δ 3.15 t). Alanine and lactate were comparably lower in concentration in cystocarpic female fronds than male and tetrasporophytes (Fig. 4). Further, ethanolamine was completely absent in tetrasporophytic and male fronds while cystocarpic female fronds showed a significant amount. Isethionic acid was significantly low compared with male and tetrasporophyte frond while there was a negligible difference in proline (Fig. 4).
A total of 12 SCoT primers were analysed of which two primers (numbers 8 and 12) generated highly reproducible and clear polymorphic bands under optimised conditions. They were further considered for genetic analysis (Table 2). PIC values for primers studied were 0.11 and 0.25 respectively with 0.2 as an average value. A total of 48 scorable and repeatable DNA fragments were generated from male, cystocarpic female and tetrasporophytic thalli of G. dura. The size of these fragments ranged from 300 to 2000 bp (Table 2; Fig. 5). An average SCoT locus frequency was 8 loci per primers. The estimation of phenotypic diversity as allele frequency, expected average heterozygosity (He) and Shannon’s index (H) were 0.66, 0.63 and 1.06 respectively for three life cycle stages under consideration. Percentage polymorphism was 25 and 58.33%, while average polymorphism was 47.02% and marker index was 4.56. The pairwise average polymorphic loci per primer among the three types of thallus were found to be 3 between male and cystocarpic female, 3.5 between male and tetrasporophyte and 1.5 between cystocarpic female and tetrasporophyte. The pairwise estimation of phenotypic diversity showed similar diversity indices (I) as 0.43 between male and cystocarpic females, 0.71 between male and tetrasporophyte and 0.68 between cystocarpic female and tetrasporophyte. The average He for male–tetrasporophyte is 0.51 compared to 0.47 for both cystocarpic female–tetrasporophyte and 0.41 for male–cystocarpic female. The generated similarity matrix revealed a maximum genetic similarity of 0.96% between cystocarpic female and tetrasporophyte, 0.77% between male and tetrasporophyte and the lowest of 0.74% for male–tetrasporophyte.
Discussion
Although the occurrence of Gracilaria dura in Indian waters has been reported (De Toni 1900), until date, detailed morphological and anatomical information is unknown for Indian specimens. This is the first report containing detailed morpho-anatomical features of this species, including differences among life cycle phases. Its comparison with other tropical species showed the close morphological similarity to that of Gracilariopsis irregularis (as Gracilaria irregularis) from Thailand (Abbot 1988). The comparison of Indian specimen revealed that Mediterranean specimens had smaller diameter ca. 1.5 mm (Gargiulo et al. 1992). The detailed comparison of key morphological and anatomical characters of G. dura with other tropical Gracilaria species is given in Supplementary Table 2.
The growth rates recorded during the present investigation corroborated well with previous cultivation studies of this species from Indian waters (Veeragurunathan et al. 2015a, b; Mantri et al. 2020). Although these studies did not mention the life cycle stage and further the growth rate levels were different due to variable environmental conditions, growth reported in the present communication was within the range reported earlier. It may be noted that, DGR of 4.67% day−1 was reported for the tetrasporophytes of G. dura generated through carpospores under field conditions (Mantri et al. 2009). However, in laboratory culture, cystocarpic fronds reported the highest relative growth rate of 9.31 ± 3.49% day−1 in this alga (Gupta et al. 2011). Similarly, fertile female gametophytes recorded higher growth than fertile tetrasporophytes in Agrophyton chilense (as Gracilaria chilensis) (Santelices and Varela 1995). The tetrasporophytes of Gracilariopsis heteroclada (as Gracilariopsis bailinae) cultured in the sea under different depth profiles reported a 2.6–9.7% day−1 growth rate (Rabanal and Azanza 1999). Nevertheless, higher growth 13.57 and 19.7% day−1 was recorded in tetrasporophytes of Hydropuntia edulis (as Gracilaria edulis) and Agarophyton tenuistipitatum (as Gracilaria tenuistipitata) var liui cultured under laboratory conditions (Yu and Phang 2013). In A. tenuistipitatum (as G. tenuistipitata), growth rates for tetrasporophytes were higher than those of female gametophytes in the first few days; nevertheless, in subsequent week growth, attributes were similar for both the phases under controlled laboratory conditions (Barufi et al. 2010). The higher growth could be attributed to the controlled optimum culture conditions to which this seaweed was subjected. The growth rate for Gracilaria sp. has been reported in the range of 1.8-8.79% day−1 under the influence of varied environmental conditions, namely NH4: 10.37 ± 7.99 mg L−1, NO3: 4.63 ± 1.52 mg L−1 and PO4: 0.32 ± 0.22 mg L−1 (Marinho-Soriano et al. 2002). Barufi et al. (2010) reported the highest growth of 21.1% day−1 for gametophytes and 24.01% day−1 for tetrasporophytes of A. tenuistipitatum (as G. tenuistipitata), but these experiments were conducted in laboratory. The higher growth in female gametophytes was recorded when compared to male gametophytes and tetrasporophytes of Crassiphycus birdiae (as Gracilaria birdiae) (Ursi and Plastino 2001). The higher growth rate reported in the male gametophyte in the present investigation could be attributed to the fact that this gametophytic phase did not undergo reproductive maturity and as a result, the vegetative growth was enhanced. The differential growth reported for various life cycle stages in different species of Gracilaria might be a matter of genetic diversity. Further, the growth rate is related to the different nutritional needs of the respective reproductive stage (Barufi et al. 2010). To the best of our knowledge, this is the first attempt to cultivate different life cycle states of G. dura under field conditions in the open sea. The advent of protocol for selective propagation of apical fragments with the highest regeneration made it possible to produce a large number of seedlings for the selective life cycle state in the laboratory for out-planting in the sea (Saminathan et al. 2015).
There are limited studies among agrophytes where hydrocolloid characterisation was made with respect to life cycle states. Hoyle (1978) reported no significant difference in yield and gel strength of agar extracted from Gracilaria bursapastoris and Gracilaria coronopifolia. But higher yields with lower gel strength of agar were recorded in cystocarpic fronds compared to the tetrasporic fronds of Gp. longissima (as Gracilaria verrucosa) (Kim and Henriquez 1979). Yao et al. (1984) reported a 3.1% higher agar yield in cystocarpic fronds than tetrasporic fronds in Gp. longissima (as Gracilaria verrucosa). Nevertheless, the present study revealed tetrasporophytic fronds are preferable to obtain significantly high agarose yield (28.6 ± 1.53) compared to male fronds (25.2 ± 0.36). A previous study, where naturally collected fronds of G. dura were used, showed the highest yield (19%) from tetrasporophyte fronds followed by cystocarpic fronds (18.5%) and male fronds (12.5%). Similarly, agar yield of diploid tetrasporophytes has been shown to be higher (38.3%) than haploid female fronds bearing cystocarps (37.5%) in G. bursapastoris (Marinho-Soriano et al. 1999). The results of this study are in good agreement with those reported in our previous studies (Meena et al. 2007, 2011). Considerable variation in agar characterisation from three different life cycle stages of Agarophyton chilense (as Gracilaria sordida) has been reported. The agar yield ranged from 16 to 23% DW basis, while gel strength showed clear differences between life cycle stages. The highest gel strength of 423 g cm−2 was recorded for cystocarpic fronds followed by 411 g cm−2 for tetrasporic fronds and 354 g cm−2 for spermatangial fronds (Pickering et al. 1990). The viscosity, gelation and melting temperatures varied among different life cycle states. A similar trend was also reported in Gp. longissima (as Gracilaria verrucosa) (Yao et al. 1984). In our previous studies, we noted that the agarose yield and gel strength were inversely proportionate, and the same trend was also reported in the present investigation (Meena et al. 2007, 2011, 2014; Gupta et al. 2011). The agarose yield reported in our study was higher than A. plicata where 10.44 ± 0.35-14.48 ± 0.37% yield of agarose was reported (Zhang et al. 2019). Similarly, gel strength was also higher in the present study, than 853 ± 11% in Ge. amansii (Wang et al. 2012) and 1062 ± 23–1569 ± 2% in A. plicata (Zhang et al. 2019). The ash content in the present study was higher than 0.11 ± 0.07–0.80 ± 0.09% in A. plicata (Zhang et al. 2019). The sulphate content was also found to be higher in our study than 0.28 ± 0.02-0.07 ± 0.02 in A. plicata (Zhang et al. 2019) and 0.14 ± 0.01% in Ge. amansii (Wang et al. 2012). The gelling temperature was also higher than 32–36 °C in A. plicata (Zhang et al. 2019) and 34 °C in Ge. amansii (Wang et al. 2012). The melting temperature was found to be more than 75.8 ± 0.3 °C in Ge. amansii (Wang et al. 2012) and 86–93 °C in A. plicata (Zhang et al. 2019). The variation among these parameters was due to source of biomaterial besides the methods that were used for extraction of agarose.
Sex determination in seaweeds can only be achieved when thallus enters into maturity. This poses a limitation for selecting appropriate life cycle-based stage for fundamental studies including breeding and cultivar improvement. Although cytological markers are useful to certain extents, sample fixation and staining are elaborate and time-consuming. Contrary, biochemical and molecular markers are preferred due to simplified protocol and high reproducibility. Allozymes, isozymes and hormones are routinely used as biochemical markers in seaweeds, but NMR-based metabolites are seldom attempted (Sosa and Lindstrom 1999; Gupta et al. 2011). 1H NMR spectra deliver a quantitative profile of metabolites related to sugars, proteins and fatty acids, aromatics in the same spectra (Kim et al. 2011). The specific composition of metabolites unique to the life cycle phase in this study suggested the existence of dissimilar pathways. The analysis of regulatory compounds of cellular function reported a low amount of the lactate suggested circumvention of fermentative reaction of lactate and thus alanine production was significantly higher in cystocarpic female thalli. Low alanine and isethionic acid in cystocarpic female frond and the absence of ethanolamine in tetrasporophytic and male fronds suggest that they should be considered potential biomarkers for distinguishing life cycle phases. We for the first time showed the existence of metabolic differences in life cycle stages of Gracilaria through NMR-based functionality and biochemical analysis. The NMR-based metabolomic investigation has an advantage over other techniques due to high throughput and simple sample preparation. Further studies coupled with transcriptome analysis would be more appropriate to develop specialised NMR-based markers for rapid fingerprinting assay.
Similarly, restriction fragment length polymorphism (RFLP), amplified fragment length polymorphism (AFLP), random amplified polymorphic DNA (RAPD), inter simple sequence repeat (ISSR), start codon targeted (SCoT) polymorphism and sequence characterised amplified region (SCAR) molecular markers were used in seaweed for investigating population structure as well as breeding. A high productivity of G. coronopifolia strain was identified using RAPD markers (Windarsih et al. 2019). The same technique was also successfully used for detecting the increase in genetic heterogeneity of A. chilense (as G. chilensis) (Meneses and Santelices 1999). Out of 10 RAPD markers used for screening in G. lemaneiformis, 4 were found related to the phase and sex (Li et al. 1998). Gupta et al. (2011) reported ISSR marker profiling in the male cystocarpic and tetrasporophytic phase of G. dura. ISSR primers I, D and F were successfully used to differentiate male, female or tetrasporophyte. In the present study, however, two primers (codes 8 and 12) showed potential for using them as molecular markers for differentiating life cycle phases. Compared to arbitrary markers, SCoT markers were useful in genetic diversity analysis, germplasm management and genetic improvement (Gao et al. 2014). These markers with the ATG context are technically more correlated to functional genes and their corresponding traits, thus more reliable. The present investigation for the first time showed the usefulness of SCoT markers in differentiating life cycle phases in seaweeds.
Seed assortment and subsequent improvement are the basic areas of research in any agriculture crop, including seaweeds. Crop improvement in Gracilaria has been attempted by two approaches: breeding and mutagenesis. A cross between two seemingly different populations of A. tenuistipitatum (as G. tenuistipitata) was successful, but morphologically identical fronds having different geographical origins failed to breed (Zhang and Fei 1990). Nevertheless, mutants both naturally spontaneous and chemically induced by using ethyl methane sulfonate (EMS) were obtained, but their use in commercial farming has never been successful. This could be due to non-stability of mutated characters. Thus, an alternative strategy is necessary. A few other investigations were also aimed at germplasm improvement in Indian agarophytes (Subbaramaiah et al. 1990; Gupta et al. 2011; Saminathan et al. 2015; Sambhwani et al. 2020). Nevertheless, the practicability and viability of implementing such methodologies for farming are yet to be validated. The studies divulged the inherent differences existed in growth pattern and agarose characteristics among the different life cycle stages of G. dura. These trait-specific dissimilarities could be used for establishing elite germplasm that can give impetus to initiate commercial farming. The present study clearly established the “proof of concept” on the existence of dissimilarities in growth, agarose, biochemical and molecular characteristics in the life cycle stages of this industrially important seaweed. Further, the tetrasporophyte stage was found to be more competitive in terms of agarose yield and the male gametophyte in terms of agarose gel strength as well as growth, important criteria for raw material selection. These attributes are highly relevant to expand the prospects of regional agar trade, which still heavily relies on natural harvest (Mantri et al. 2019). In a quest for cultivar selection, ca. 120 specimens of different life cycle stages were collected during 2019–2020, under the Science and Engineering Research Board (Department of Science and Technology), Government of India (Jaiswar and Mantri 2019). Pilot-scale year-round farming with the more integrative approach including growth, yield and agarose gel strength of different life cycle stages shall be performed by a multivariate analysis or by a multiple ranking analysis to examine seasonality and to select elite cultivar. We believe this would certainly help to diversify the livelihood of commercial growers on one hand and the regional agar industry on the other.
References
Abbot IA (1988) Some species of Gracilaria and Polycavernosa from Thailand. In: Abbott IA (ed) Taxonomy of economic seaweeds with reference to some Pacific and Caribbean species Volume II. The California Sea Grant College Program Publication, University of California, pp 137–150
Baghel RS, Kumari P, Bijo AJ, Gupta V, Reddy CRK, Jha B (2011) Genetic analysis and marker assisted identification of life phases of red alga Gracilaria corticata (J. Agardh). Mol Biol Rep 38:4211–4218
Barufi JB, De Oliveira EC, Plastino EM, De Oliveira MC (2010) Life history, morphological variability and growth rates of the life phases of Gracilaria tenuistipitata (Rhodophyta: Gracilariales) in vitro. Sci Mar 74:297–303
Chadar DA, Chudasama NA, Vadodariya N, Meena R, Prasad K, Siddhanta AK (2019) Protein mimicking functions of nano-size monoamido amino acids derived from polysaccharides of marine origin. Macromol Chem Phys 220:201900201
Chaudhary JP, Kondaveeti S, Gupta V, Prasad K, Meena R (2014) Preparation and functional evaluation of agarose derivatives. J Appl Polym Sci 131:40630
Chew KW, Show PL, Yap YJ, Juan JC, Phang SM, Ling TC, Chang JS (2018) Sonication and grinding pre-treatments on Gelidium amansii seaweed for the extraction and characterization of agarose. Front Environ Sci Eng 12:2
Chudasama NA, Prasad K, Siddhanta AK (2016) Agarose functionalization: synthesis of PEG-agarose amino acid nano-conjugate–its structural ramifications and interactions with BSA in a varying pH regime. Carbohydr Polym 151:735–742
Collard BC, Mackill DJ (2009) Start codon targeted (SCoT) polymorphism: a simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol Biol Report 27:86
De Toni GB (1900) Sylloge algarum omnium hucusque cognitarum. Vol. IV. Florideae. Sectio II. pp. [i-iv], 387-774 + 775-776 [Index]. Patavii [Padova]: Sumptibus auctoris
Destombe C, Godin J, Nocher M, Richerd S, Valero M (1993) Differences in response between haploid and diploid isomorphic phases of Gracilaria verrucosa (Rhodophyta: Gigartinales) exposed to artificial environmental conditions. Hydrobiologia 204/205:219–223
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2018) InfoStat version. Centro de Transferencia InfoStat, Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Argentina. URL http://www.infostat.com.ar
Efendi F, Handajani R, Nursalam N (2015) Searching for the best agarose candidate from genus Gracilaria, Eucheuma, Gelidium and local brands. Asian Pac J Trop Biomed 5:865–869
Faria AV, Bonomi-Barufi J, Plastino EM (2017) Ecotypes of Gracilaria caudata (Gracilariales, Rhodophyta): physiological and morphological approaches considering life history phases. J Appl Phycol 29:707–719
Freile-Pelegrín Y, Murano E (2005) Agars from three species of Gracilaria (Rhodophyta) from Yucatán Peninsula. Bioresour Technol 96:295–302
Gao YH, Zhu Y, Tong ZK, Xu ZY, Jiang XF, Huang CH (2014) Analysis of genetic diversity and relationships among genus Lycoris based on start codon targeted (SCoT) marker. Biochem Syst Ecol 57:221–226
Garcia BG, Vidal RRL, Rinaudo M (2000) Preparation and structural characterization of O-acetyl agarose with low degree of substitution. Polímeros: Ciência e Tecnología 10:155–161
Gargiulo GM, De Masi F, Tripodi G (1992) Morphology, reproduction and taxonomy of the Mediterranean species of Gracilaria (Gracilariales, Rhodophyta). Phycologia 31:53–80
Guillemin ML, Sepúlveda RD, Correa JA, Destombe C (2013) Differential ecological responses to environmental stress in the life history phases of the isomorphic red alga Gracilaria chilensis (Rhodophyta). J Appl Phycol 25:215–224
Guiry MD, Guiry GM (2020) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available from: https://www.algaebase.org
Gupta V, Baghel RS, Kumar M, Kumari P, Mantri VA, Reddy CRK, Jha B (2011) Growth and agarose characteristics of isomorphic gametophyte (male and female) and sporophyte of Gracilaria dura and their marker assisted selection. Aquaculture 318:389–396
Gupta V, Thakur RS, Reddy CRK, Jha B (2013) Central metabolic processes of marine macrophytic algae revealed from NMR based metabolome analysis. RSC Adv 3:7037–7047
Hoyle MD (1978) Agar studies in two Gracilaria species (G. bursapastoris (Gmelin) Silva and G. coronopifolia J. Ag.) from Hawaii. I. Yield and gel strength in the gametophyte and tetrasporophyte generations. Bot Mar 21:343–346
Jaiswar S, Mantri VA (2019) Life cycle-based selection of elite germplasm in industrially important red alga Gracilaria dura: implications for commercial farming. Curr Sci 117:1177–1178
Kain J, Destombe C (1995) A review of the life history, reproduction and phenology of Gracilaria. J Appl Phycol 7:269–281
Kim DH, Henriquez NP (1979) Yields and gel strengths of agar from cystocarpic and tetrasporic plants of Gracilaria verrucosa (Florideophyceae). In: Jensen A, Stein JR (eds) Proceedings of the Ninth Internationa Seaweed Symposium. Princeton, Science Press, pp 257–262
Kim HK, Choi YH, Verpoorte R (2011) NMR-based plant metabolomics: where do we stand, where do we go? Trends Biotechnol 29:267–275
Knoop J, Griffin JN, Barrento S (2020) Cultivation of early life history stages of Porphyra dioica from the British Isles. J Appl Phycol 32:459–471
Kondaveeti S, Prasad K, Siddhanta AK (2013) Functional modification of agarose: a facile synthesis of a fluorescent agarose-tryptophan based hydrogel. Carbohydr Polym 97:165–171
Li XF, Sui ZH, Zhang XC (1998) Application of RAPD in genetic diversity study on Gracilaria lemaneiformis III. Phase and sex related markers. Chin J Oceanol Limnol 16:147–151
Mantri VA, Thakur MC, Kumar M, Reddy CRK, Jha B (2009) The carpospore culture of industrially important red alga Gracilaria dura (Gracilariales, Rhodophyta). Aquaculture 297:85–90
Mantri VA, Ganesan M, Gupta V, Krishnan P, Siddhanta AK (2019) An overview on agarophyte trade in India and need for policy interventions. J Appl Phycol 31:3011–3023
Mantri VA, Shah Y, Thiruppathi S (2020) Feasibility of farming the agarose-yielding red alga Gracilaria dura using tube-net cultivation in the open sea along the Gujarat coast of NW India. Appl Phycol 1:12–19
Marinho-Soriano E, Bourret E (2005) Polysaccharides from the red seaweed Gracilaria dura (Gracilariales, Rhodophyta). Bioresour Technol 96:379–382
Marinho-Soriano E, Bourret E, De Casabianca ML, Maury L (1999) Agar from the reproductive and vegetative stages of Gracilaria bursa-pastoris. Bioresour Technol 67:1–5
Marinho-Soriano E, Morales C, Moreira WSC (2002) Cultivation of Gracilaria (Rhodophyta) in shrimp pond effluents in Brazil. Aquac Res 33:1081–1086
Meena R, Siddhanta AK, Prasad K, Ramavat BK, Eswaran K, Thiruppathi S, Rao PS (2007) Preparation, characterization and benchmarking of agarose from Gracilaria dura of Indian waters. Carbohydr Polym 69:179–188
Meena R, Prasad K, Siddhanta AK (2011) Preparation of superior quality products from two Indian agarophytes. J Appl Phycol 23:183–189
Meena R, Chaudhary JP, Agarwal PK, Maiti P, Chatterjee S, Raval HD, Ghosh PK (2014) Surfactant-induced coagulation of agarose from aqueous extract of Gracilaria dura seaweed as an energy-efficient alternative to the conventional freeze–thaw process. RSC Adv 4:28093–28098
Meneses I, Santelices B (1999) Strain selection and genetic variation in Gracilaria chilensis (Gracilariales, Rhodophyta). J Appl Phycol 11:241–246
Pickering TD, Gordon ME, Tong LJ (1990) Seasonal growth, density, reproductive phenology and agar quality of Gracilaria sordida (Gracilariales, Rhodophyta) at Mokomoko Inlet, New Zealand. Hydrobiologia 204:253–262
Porse H, Rudolph B (2017) The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. J Appl Phycol 29:2187–2200
Prasad K, Trivedi KP, Siddhanta AK, Bhattacharya A (2005) Surface tension and fluorescence studies of polysaccharide-surfactant solutions: agar-CTAB. Indian J Chem A 44:2445–2449
Rabanal SF, Azanza RV (1999) Outplanting of laboratory-generated carposporelings of Gracilariopsis bailinae off northern Philippines. Hydrobiologia. 398/399:463–468
Reddy CRK, Baghel RS, Kumari N, Kumari P, Gupta V, Prasad K, Meena R (2016) An integrated process to recover a spectrum of bioproducts from fresh seaweeds. U.S. Patent Application No 15/109:232
Sambhwani K, Modi J, Singhala A, Bramhabatt H, Mishra A, Mantri VA (2020) Analysis of functional traits in female gametophytic and tetrasporophytic life-phases of industrially important red alga Gracilaria dura (Rhodophyta: Gracilariacae). J Appl Phycol 32:1961–1969
Saminathan KR, Ashok KS, Veeragurunathan V, Mantri VA (2015) Seedling production in the industrially important agarophyte Gracilaria dura (Gracilariales, Rhodophyta). J Appl Phycol 27:1541–1548
Santelices B (1992) Strain selection of clonal seaweeds. Prog Phycol Res 8:85–116
Santelices B, Varela D (1995) Regenerative capacity of Gracilaria fragments: effects of size, reproductive state and position along the axis. J Appl Phycol 7:501–506
Santos R, Melo RA (2018) Global shortage of technical agars: back to basics (resource management). J Appl Phycol 30:2463–2473
Sharma AK, Chudasama NA, Prasad K, Siddhanta AK (2017) Agarose based large molecular systems: synthesis of fluorescent aromatic agarose amino acid nano-conjugates–their pH-stimulated structural variations and interactions with BSA. Carbohydr Res 449:37–46
Siddhanta AK, Shanmugam M, Ramavat BK, Mody KH (1997) Agar from Gracilaria dura of the West coast of India. Seaweed Res Util 19:95–99
Siddhanta AK, Meena R, Prasad K., Ramavat BK, Ghosh PK, Eswaran K, Thiruppathi S, Mantri VA (2005) Cost-effective process for preparing agarose from Gracilaria spp. US Patent 2005/0267296; PCT Patent W0 2005/118830
Sosa PA, Lindstrom SC (1999) Isozymes in macroalgae (seaweeds): genetic differentiation, genetic variability and applications in systematics. Eur J Phycol 34:427–442
Subbaramaiah K, Thomas PC, Rao PSN (1990) Effect of ethyl methanesulfonate on growth and agar content in marine alga Gelidiella acerosa (Rhodophyta). Indian J Mar Sci 19:288
Ursi S, Plastino E (2001) Crescimento in vitro de linhagens de coloração vermelha e verde clara de Gracilaria birdiae (Gracilariales, Rhodophyta) em dois meios de cultura: análise de diferentes estádios reprodutivos. Braz J Bot 24:587–594
Veeragurunathan V, Eswaran K, Malarvizhi J, Gobalakrishnan M (2015a) Cultivation of Gracilaria dura in the open sea along the southeast coast of India. J Appl Phycol 27:2353–2365
Veeragurunathan V, Eswaran K, Saminathan KR, Mantri VA, Ajay G, Jha B (2015b) Feasibility of Gracilaria dura cultivation in the open sea on the Southeastern coast of India. Aquaculture 438:68–74
Vieira VMNCS, Engelen AH, Huanel OR, Guillemin ML (2018a) Haploid females in the isomorphic biphasic life-cycle of Gracilaria chilensis excel in survival. BMC Evol Biol 18:174
Vieira VMNCS, Engelen AH, Huanel OR, Guillemin ML (2018b) Differentiation of haploid and diploid fertilities in Gracilaria chilensis affect ploidy ratio. BMC Evol Biol 18:183
Wang TP, Chang LL, Chang SN, Wang EC, Hwang LC, Chen YH, Wang YM (2012) Successful preparation and characterization of biotechnological grade agarose from indigenous Gelidium amansii of Taiwan. Process Biochem 47:550–554
Whyte JNC, Englar JR, Saunders RG, Lindsay JC (1981) Seasonal variations in the biomass, quantity and quality of agar, from the reproductive and vegetative stages of Gracilaria (verrucosa type). Bot Mar 24:493–502
Windarsih G, Utami DW, Yuriyah S (2019) Genetic diversity and productivity of Gracilaria coronopifolia as alternative for food resource based on RAPD marker. Biodiversitas 20:3758–3765
Yamamoto H (1984) An evaluation of some vegetative features and some interesting problems in Japanese populations of Gracilaria. Hydrobiologia. 116/117:51–54
Yao SS, Xia ZY, En LZ, Qing LW (1984) The yield and properties of agar extracted from different life stages of Gracilaria verrucosa. Hydrobiologia 116:551–553
Yu CH, Phang SM (2013) Effects of irradiance and salinity on the growth of carpospore-derived tetrasporophytes of Gracilaria edulis and Gracilaria tenuistipitata var liui (Rhodophyta). J Appl Phycol 25:787–794
Yu Z, Xiaoting F, Delin D, Jiachao A, Xin G (2019) Preparation and characterization of agar, agarose, and agaropectin from the red alga Ahnfeltia plicata. Chin J Oceanol Limnol 37:815–824
Zhang XC, Fei XG (1990) Cultivation and hybridization experiments on Gracilaria tenuistipitata (Rhodophyta). In: Miyati S, Kenkyukai MB (eds) Current topics in marine biotechnology, Proceeding of the 1st International Marine Biotechnology Conference. pp 203–214
Zhang X, Meer JPVD (1988) A genetic study on Gracilaria sjoestedtii. Can J Bot 66:2022–2026
Zhang Y, Xiaoting F, Delin D, Jiachao X, Xin G (2019) Preparation and characterization of agar, agarose, and agaropectin from the red alga Ahnfeltia plicata. J Oceanol Limnol 37:815–824
Acknowledgements
The authors thank Dr. Vasco M. N. C. S. Vieira, for the critical comments on the original manuscript. We are also thankful to two anonymous reviewers for their constructive suggestions, which has improved the manuscript considerably. We are grateful to the Director, CSIR-CSMCRI for the facilities. This manuscript has CSIR-PRIS registration number 147/2019.
Funding
This work was carried out by a grant received from Council for Scientific and Industrial Research (CSIR), New Delhi, Government of India. KS received financial assistance in the form of a senior research fellow from the University Grant Commission.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1544 kb)
Rights and permissions
About this article
Cite this article
Mantri, V.A., Shah, Y., Balar, N. et al. Limited-scale field trial confirmed differences in growth and agarose characteristics in life-cycle stages of industrially important marine red alga Gracilaria dura (Gracilariales, Rhodophyta). J Appl Phycol 33, 1059–1070 (2021). https://doi.org/10.1007/s10811-020-02356-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02356-1