Abstract
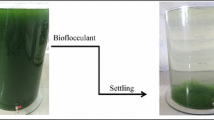
Microalgal biomass production has been investigated since the 1950s by academic and industry sectors due to its potential and for biodiesel production. One of the main bottlenecks in microalgae production is biomass recovery and its separation from aqueous medium. For this reason, the use of a flocculation process is an important step to promote microalgae recovery in large scale. Here we provide a strategy for the harvesting of microalgal biomass using chitosan as flocculating agent in pilot scale cultures performed in flat plate photobioreactor. The results show that chitosan was effective in inducing microalgae flocs and separating them by settling. In the jar test scale the best clarification efficiency was around 99% and when scaled up using a 100-L photobioreactor, the biomass recovery efficiency was close to 98%. Comparison of the compositions of biomasses obtained by flocculation and centrifugation indicates no significant differences in terms of carbohydrates, proteins, lipids, and ashes, showing that the flocculation process is not affecting the biomass characteristics and its potential biotechnological applications. Based on the presented results, flocculation using chitosan as flocculant agent can be considered as an efficient method to harvest Desmodesmus subspicatus biomass cultured in pilot scale photobioreactors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae present valuable features for bioindustry as their metabolic plasticity, tolerance to environmental changes and potential to be used in genetic engineering. These characteristics make microalgae an important source of high-value compounds such as carotenoids, proteins, polyunsaturated fatty acids, polysaccharides, and other compounds useful to agriculture and food, pharmaceutical, and chemical industries (Valverde et al. 2016). Microalgae cultures can also be used to promote wastewater treatment and CO2 fixation from diverse fossil fuel emissions (Correa et al. 2017).
Biomass separation from the liquid medium represents one of the main costs in biomass production and can account for up to 30% of production costs (Hansel et al. 2014). Microalgae cultures are relatively diluted, so large volumes need to be manipulated for biomass recovery (Grima et al. 2003). The technique used to recover microalgae biomass depends on the characteristics of microalgae cells, such as shape and size, crop density, biomass application, and costs involved (Brennan and Owende 2010). Essentially, the main techniques used are centrifugation, filtration, and sedimentation preceded by flocculation. Various flocculants have been studied in biomass recovery, including inorganic and organic flocculants. Inorganic flocculants commonly include salts of polyvalent cations, such as Al2(SO4)3 and Fe2(SO4)3 (Gerde et al. 2014; Chatsungnoen and Chisti 2016). Coagulants of vegetable and animal origin, such as tannin, cationic starch, and chitosan have been used for water treatment and microalgal biomass recovery (Vandamme et al. 2010; Vieira et al. 2012; Escapa et al. 2017).
Chitosan is a linear polysaccharide composed of randomly distributed β-(1 → 4)-linked d-glucosamine and N-acetyl-glucosamine obtained from chitin partial deacetylation. Modification of chitin to chitosan alters its properties, so that chitosan is insoluble in water but soluble in most organic acids and some inorganic acids. Due to the large amount of primary amines in its chain, chitosan has several applications in the biomedical area, such as carrier of drugs or genes. Chitosan is also widely used in food, cosmetics, and agriculture, and because it is a long-chain polymer and has a high cationic charge density, chitosan can be used as a flocculant agent (Dong et al. 2014). The recovery of the microalgae biomass using chitosan requires low concentration of this polymer and is applicable in cultures of the most variable microalgae species; however, the optimal dosage of chitosan depends on the species used (Heasman et al. 2000). In addition, cell density and pH of the culture may influence the required concentration of chitosan (Divakaran and Pillai 2002; Şirin et al. 2012; Chen et al. 2013). The addition of chitosan flocculation preceding the final centrifugation step may represent a significant reduction in energy consumption during the biomass recovery process. Xu et al. (2013) achieved a 95% reduction in energy expenditure by adding a chitosan flocculation step in Chlorella sorokiniana cultures.
Most of flocculation studies with chitosan focus on the evaluation of isolated parameters, such as efficiency biomass recovery, flocculant concentration or pH influence. For this reason the main novelty of the present study is the evaluation of the whole process, from the microalgae culture in a photobioreactor to the recovery of the biomass using chitosan, determining the most important parameters for the process, such as flocculating concentration, flocculation efficiency, zeta potential, floc morphology, and biochemical composition of the recovered biomass. Therefore, the aim of this work was to evaluate the microalgae biomass production of Desmodesmus subspicatus cultivated in a pilot scale flat plate photobioreactor and harvested by in situ flocculation using chitosan as flocculant agent. The flocculation efficiency was determined in a jar test scale and in scaled up cultures using a 100-L photobioreactor.
Material and methods
Flat plate photobioreactor
The microalgae culture was carried out in a 100-L flat plate photobioreactor developed in our lab, patent pending in Brazil (Noseda et al. 2016). The developed photobioreactor is characterized by its rectangular-shaped regular hexahedron geometric structure, constructed with translucent material, illumination provided by removable side plates with variable-intensity LED lamps, and an air injection system. The photobioreactor construction features allow the biomass recovery by in situ flocculation.
Microalgae culture
The green freshwater microalga Desmodesmus subspicatus was obtained from the Elizabeth Aidar Microalgae Culture Collection of the Fluminense Federal University (Brazil). The algae were photoautotrophically cultivated for 14 days in a 100-L photobioreactor at room-controlled temperature (23 ± 1 °C), without pH-control system, under continuous compressed air supply (0.25 vvm without supplementary CO2), and continuous illumination (24 h) at an intensity of 155 μmol photons m−2 s−1. Bold’s basal medium (Nichols and Bold 1965) modified was used, prepared in deionized water containing the following chemicals: NaNO3 (250 mg L−1), CaCl2·2H2O (25 mg L−1), MgSO4·7H2O (75 mg L−1), K2HPO4 (75 mg L−1), KH2PO4 (175 mg L−1), NaCl (25 mg L−1), EDTA (50 mg L−1), KOH (31 mg L−1), FeSO4·7H2O (5 mg L−1), H3BO3 (11.4 mg L−1), ZnSO4·7H2O (8.8 × 10−3 mg L−1), MnCl2·4H2O (1.4 × 10−3 mg L−1), Na2MoO4·2H2O (1.2 × 10−3 mg L−1), CuSO4·5H2O (1.6 × 10−3 mg L−1), Co(NO3)2·6H2O (0.49 × 10−3 mg L−1). Parameters such as dry weight (APHA 2005) and pH were daily measured to describe the microalgae growth in culture.
Jar test flocculation assays
Fifteen assays (triplicate) were performed with samples collected from the photobioreactor in the culture stationary phase. The samples’ pH was adjusted to: (a) 8.0 (with 1 M HCl), (b) 9.0, and (c) 10.0 (with 1 M NaOH), to investigate the effect of different pH values and chitosan concentrations. For each assay, 200 mL of culture samples was poured into 250-mL beakers, and the tests were carried out adding chitosan aqueous solution (pKa 6.5) to obtain final concentrations of 10, 20, 30, 40, and 50 mg of chitosan per liter of culture. Chitosan stock solution (5 g L−1) was prepared before the experiments, dissolving 0.5 g of medium molecular weight chitosan (Sigma-Aldrich catalog no. 448877) in 100 mL of 1% (v/v) aqueous acetic acid. To emulate the flocculation in the photobioreactor, the individual beaker samples were vigorously mixed with air injection for 15 s, then the chitosan solution was added and gently homogenized for 60 s to promote floc formation, and finally, the flocs were left settling for 15 min. After the precipitation time, the flocculation efficiency was determined as follows:
where Bf is the biomass at half of the height of the clarified phase after settling and Bi is the biomass in the fresh culture, before chitosan addition.
After the settling time the sludge volume was determined to evaluate the flocculation effectiveness in concentrating the microalgal biomass. The concentration factor (CF) was calculated according to Xu et al. (2013), as follows:
where V is the total volume of the sample, Vs is the volume of supernatant, Bi is biomass density of the initial sample, and Bs is biomass density of the supernatant after flocculation and sedimentation. A higher CF value indicates lower energy consumption for biomass harvesting, considering the centrifugation step after flocculation.
Zeta potential measurements
To understand the influence of chitosan addition in the cell surface charges and flocculation efficiency, zeta potential (ZP) of the fresh culture and all the flocculated biomasses were determined with Stabino (Colloid Metrix GmbH). ZP measurements were carried out with 10 mL of fresh culture cell suspension and with the same volume of all the samples obtained after jar test flocculation assays.
Scanning electron microscopy (SEM)
Microalgae flocculated samples were analyzed by SEM to evaluate the floc morphology. The flocculated microalgae were fixed in 2.5% (v/v) glutaraldehyde in 1.0-M sodium phosphate buffer (PBS) pH 7.4 for 1 h; after fixation, a small aliquot was filtered using polycarbonate membrane (1 μm) and washed for 1 h with PBS; then the flocs samples were dehydrated with increasing concentration ethanol solutions (30, 40, 50, 60, 70, 80, 90, and 95% v/v) for 10 min each solution (Shubert and Wilk-Wozniak 2003). The cells were CO2 critical point dried, metalized with gold, and analyzed by SEM (JEOL mod. JSM-6360 LV).
Photobioreactor biomass recovery
The best flocculation conditions as determined by the jar test assays were reproduced in the 100-L photobioreactor. Chitosan homogenization in the photobioreactor culture was performed by the air injection system. After chitosan addition, the culture was vigorously mixed for 30 s, followed by 5 min of low flow air injection, and finally the air was turn off and the biomass settled for 30 min. Before flocculation, a sample was collected directly from the photobioreactor culture and was centrifuged for 30 min (2600 ×g). Both biomass samples were then freeze dried and stored in freezer for later biomass composition analysis.
Biomass composition
Biomass composition of the samples obtained by two different harvesting processes (flocculation and centrifugation) was determined by thermogravimetric analysis using the thermal analyzer STA 449 S3 Jupiter (Netzsch) with temperature program from 20 to 800 °C at a heating rate of 10 °C min−1 (Larrosa et al. 2018). The chemical composition of microalgae biomasses was also determined using the following methods: total carbohydrates by the phenol-sulfuric acid colorimetric method (Dubois et al. 1956), proteins using the colorimetric method described by Lowry et al. (1951), and total lipids were determined by the gravimetric method using a solvent mixture of chloroform:methanol (2:1 v/v) for lipid extraction as described by Bligh and Dyer (1959).
Statistical methods
All the results were obtained from at least three biological replicates and expressed as mean value ± standard deviation. The biomass composition was analyzed using Student t test, and statistical significance was set at P < 0.05. All graphs were performed using GraphPad Prism 7.
Results and discussion
The microalga D. subspicatus was cultivated in a pilot scale flat plate photobioreactor under photoautotrophic conditions. The growth profile (Fig. 1) shows that biomass increased approximately 12 times from the inoculation of the system until the end of the period, where a concentration of 0.7 ± 0.01 g L−1 was recorded. The growth rate was faster during early 4 days showing exponential tendency, while during the other 10 days, the growth profile was more linear. This may indicate that microalgae multiply more rapidly at the beginning of cultivation, and after that period, they begin to gain mass more slowly by the accumulation of energetic reserve molecules. These data are comparable with those presented by Jiang et al. (2013) who obtained similar results in autotrophic culture of Scenedesmus dimorphus in a 300-mL bubble column photobioreactor, and Mattos et al. (2015) who cultivated Scenedesmus bijuga in Erlenmeyer flasks of 4 L and obtained 0.7 g L−1 in different lighting regimes. Although comparable to the literature results, it is worth mentioning the total volume of the photobioreactor used in this work, which results in a higher total biomass produced in the same time interval. The optical path that the light traverses inside the reactor, the two lateral plates with LED illumination, and the existence of an aeration system for culture homogenization are the differentials of the photobioreactor used in this work and may explain the high biomass production capacity under photoautotrophic conditions.
Another important factor shown in Fig. 1 is the pH profile over time. The cultivation of microalgae begins with pH close to neutrality (7.26 ± 0.02), but from its inoculation, the pH increases rapidly until the fourth day of cultivation (8.66 ± 0.03). From that moment, the variations become less, reaching the highest pH at the end of the culture period (9.00 ± 0.03). The pH variation is important in the evaluation of the recovery of the biomass obtained by flocculation with chitosan. Therefore, the understanding of the pH behavior throughout the culture is fundamental to determine the flocculation conditions, since the solubility and density of chitosan are strongly influenced by this factor. At neutral and alkaline pH, chitosan can precipitate with co-precipitation of algae (Renault et al. 2009). Although the pH at the end of the culture is alkaline, the addition of chitosan in acetic acid solution decreases the pH of the medium, favoring the protonation of the amino groups in its structure, making the polymer capable of interacting with the negative charges on the microalgae surface, increasing flocculation efficiency.
At the end of the culture period, aliquots of the photobioreactor culture were collected to evaluate the flocculation efficiency using chitosan at different concentrations and pH values to determine the best biomass recovery conditions and to carry out the process scaling up for the total volume of photobioreactor. For this reason, three pH values (8, 9, and 10) as well as five concentrations of chitosan (10, 20, 30, 40, and 50 mg L−1) were evaluated in experiments similar to the Jar test methodology. The objective of the choice of the pH range was to reproduce the values usually found in previously photobioreactor cultures and to make the analysis closer to the reality of biomass production. For the jar tests at pH 8, the higher flocculation efficiency (97.64 ± 0.14%) corresponds to the lower concentration of chitosan used (10 mg L−1); for the tests performed at pH 9, the highest biomass recovery efficiency was recorded using 20 mg L−1 of chitosan; whereas for the pH 10 tests, the highest efficiency was related to the use of 40 mg L−1 (Fig. 2). The need for addition of higher amounts of chitosan in the tests at higher pH is a consequence of the structural characteristics of this polymer as its deprotonation constant. Chitosan tends to present higher densities of positive charges at more acidic pH (Renault et al. 2009). Thus, the more alkaline the initial pH of the samples, the greater the amount of chitosan needed to neutralize the negative charges present on the surface of the microalgae cells. This dose-dependent effect presents a balance that involves the amount of positive charges present in the polymer, the pH of the medium, and its influence on the ionization of the chitosan amino groups and the density of negative charges resulting from the concentration of biomass present in the system. Studies using chitosan as a flocculating agent to recover biomass of Scenedesmus sp. showed results of flocculation efficiency in the order of 97.4% at pH 11.5 (Chen et al. 2013), while Ahmad et al. (2011) observed an efficiency higher than 99.0% for Chlorella sp. with 20 ppm of chitosan. These results reinforce the reproducibility and viability of the use of chitosan as a flocculating agent in the recovery of microalgae biomass.
The zeta potential, another parameter frequently used to study the flocculation process, is defined as a measure of the electric potential in the double layer at the interface of the particles and is dependent on the surface charge of the particles and the nature and composition of the surrounding medium in which they are suspended (Atkins 1990). Figure 3 shows the results of the zeta potential measurements performed on the flocculated fractions of each jar test experiment. The zeta potential of the culture without addition of the flocculating agent was − 23.11 ± 0.27 mV, like that observed by Selesu et al. (2016), which registered a value of − 26.30 ± 1.55 mV for Scenedesmus sp. cultivated under similar conditions and close to the value observed by Lama et al. (2016), with − 24 ± 1.0 mV for S. obliquus. The observation of negative zeta potential values is related to the negative surface charge of the suspended microalgae cells and for most species it is between − 10 and − 35 mV.
For the tests carried out at pH 8, the zeta potential of the samples varied from − 9.47 ± 0.13 mV in the lowest concentration of chitosan up to − 2.92 ± 0.10 mV in the highest flocculant concentration; for the tests at pH 9, the zeta potential values ranged from − 8.37 ± 0.10 mV up to − 1.47 ± 0.59 mV; while for the assays at pH 10, the results ranged from − 9.67 ± 0.33 mV up to − 1.21 ± 0.02 mV. Although the zeta potential values do not show a direct correlation with the flocculation efficiency results, all the values recorded show a tendency to load neutrality due to the addition of positively charged chitosan. Similar results were obtained by Gerchman et al. (2017) in flocculation assays using chitosan at different concentrations to flocculate Chlorella vulgaris. Different to what was initially expected, the observed zeta potential values do not reflect the complete neutrality of surface charges, even though they present high biomass-recovery efficiency results. In our results, this effect may be related to the chemical nature of the culture medium, since the experiments showed in the present work were performed with aliquots taken directly from the photobioreactor culture after 14 days of cultivation, and may contain cell debris, secondary metabolites, and other compounds produced by algae.
Together with the results of biomass recovery, efficiency, and zeta potential, the concentration factor (CF) was calculated for each sample, in order to determine the effectiveness of concentrating the suspended biomass in the smallest possible volume, thus reducing the energy demand for the harvesting at the end of the growing period. In summary, the concentration factor correlates the flocculation efficiency with the volume generated by the precipitate at the end of the sedimentation period. Table 1 summarizes all the results obtained in the assays performed in jar test scale. Similar to the results of flocculation efficiency, the highest CF was recorded for the assay performed at pH 9 with the addition of 20 mg L−1 of chitosan showing a value of 79.33 ± 0.08.
With the results obtained in the jar test scale, it was possible to evaluate the biomass recovery conditions in the photobioreactor. Since the pH found at the end of the microalgae cultivation is pH 9 (see Fig. 1) and the best flocculation result for this pH in the jar tests was observed with chitosan at 20 mg L−1 (see Table 1), we decided to choose this concentration to perform the flocculation scale up in a 100-L photobioreactor. Using the aforementioned conditions, the flocculation efficiency in the photobioreactor reached 98.7% with a concentration factor of 69.6 and biomass recovery of 7.0 g L−1. These results indicate a great efficiency in the flocculation carried out using a 100-L photobioreactor, as the pilot scale process allowed a biomass recovery with few losses when compared with the jar test scale. The geometry and construction characteristics of the photobioreactor allowed the in situ biomass recovery, which avoids the need of volumes transfer to additional systems, such as settling tanks, thus reducing the associated biomass losses. After addition of the flocculating agent, homogenization of the cells in suspension, and precipitation, the biomass is deposited in the lower portion of the reactor, available for harvesting through a register that allows the complete flow of the material.
To compare the characteristics of the biomasses recovered by centrifugation and flocculation, scanning electron microscopy (SEM) analyses were performed (Fig. 4). In fresh culture, the microalgae present isolated cells or colonial growth usually with four cells arranged parallel to the longitudinal axis and with elongated spines at their ends (Fig. 4a, b). After flocculation, the microalgae present the appearance of cellular and colonial agglomerates bound together by a network of interactions resulting from the action of chitosan, along with the aggregation of culture medium salts and dead cell fragments (Fig. 4c, d).
This aggregation pattern can be explained by the interactions between the chitosan polymer and cells surface. Different flocculation mechanisms were proposed such as simple charge neutralization, charge patching, bridging, and sweeping. It is possible that more than one flocculation mechanism occurs simultaneously, and this depends on the characteristics of particles in suspension and the chemical properties of flocculant (Yang et al. 2016). The chitosan polymer, through its positive charges, binds to the microalgal cells, thereby bridging them and resulting in a network of polymers and cells (Salim et al. 2011). Based on the SEM analysis, we suggest that bridging is the mechanism responsible for the floc formation between the microalgae cultivated in photobioreactor and the flocculant agent chitosan (Fig. 5).
In addition to the morphological analysis using SEM, biomasses recovered by centrifugation and flocculation were evaluated for their chemical composition. Thermogravimetric analysis is a methodology in which the weight of the samples is determined continuously during a continuous and controlled heating process. The correlation between the sample weight and temperature changes allows the physical and chemical properties of the samples to be described through the TG and DTG curves (Bach and Chen 2017). In summary, TG shows the mass loss of the samples, while DTG indicates the temperature ranges between which the mass loss events are characterized. The results show great similarity between the thermal decomposition pattern of flocculated and centrifuged biomasses (Fig. 6). DTG curves indicate three different stages in decomposition process (Fig. 6a, b). Stage 1, with a temperature range from 20 to 135 °C, is characterized by slight weigh loss due to the moisture present in the samples. The main mass loss occurred during stage 2, with a temperature range from 135 to 500 °C, where most of the organic compounds, such as carbohydrates, proteins, and lipids, were lost. In stage 3, with temperature between 500 and 800 °C, the composition of other carbonaceous matters contributes to slight weigh loss as show in TG curves (Wu et al. 2014).
The composition of the flocculated and centrifuged biomasses was calculated based on the mass degradation during thermogravimetric analysis (Table 2). The moisture of the samples was 4.2 and 3.5% for flocculated and centrifuged biomasses, respectively. For the main organic compound composition (carbohydrates, proteins, and lipids), the content was 59.3% for flocculated biomass and 60.2% for the centrifuged biomass. These values represent a variation coefficient of 1.1% indicating a small difference between samples’ compositions. The content of other carbonaceous compounds was 14.8% for flocculated biomass and 14.2% for centrifuged biomass, which represents 2.9% of variation coefficient. The content of ash and fixed carbon for flocculated biomass was 21.7%, while for centrifuged, it was 22.1%, with variation of 1.3%. These results indicate negligible differences between the biomasses recovered by flocculation and centrifugation and show that chitosan does not interfere on the biomass main composition.
To corroborate and complement the thermogravimetric analysis, the carbohydrate, protein, and lipid contents were analyzed in both biomass lots by colorimetric and gravimetric methods (Fig. 7). The results showed the presence of approximately 33% of carbohydrates in the flocculated biomass and about 31% for the centrifuged biomass. Proteins showed values of 11% for both centrifuged and flocculated lots. Similarly, the lipid content was 16% for both biomasses. The sum of carbohydrate, protein, and lipid contents represents the main organic compounds present in the biomass and the comparison of these results with those obtained from thermogravimetric analysis indicates that flocculation with chitosan did not interfere or modify the biomass composition. The stability in the composition of the centrifuged and flocculated biomasses corroborates the data of Gupta et al. (2018), who evaluated the lipid extraction of Scenedesmus sp. to produce biodiesel in microalgae flocculated with chitosan and aluminum salts. They reported the maintenance of lipid contents after the use of chitosan, unlike the results observed when aluminum salts were used as flocculant agent.
Evaluating the influence of chitosan on biomass composition is fundamental to define the use of microalgae biomass and its compounds. Ideally, flocculating agents should not modify the biomass composition of microalgae or alter the physicochemical properties of their constituents. Chitosan does not interfere in the metabolite extraction from microalgal biomass unlike the use of metals such as aluminum sulfate that cause a significant loss of microalgae metabolite contents (Chatsungnoen and Chisti 2016). According to Gutiérrez et al. (2015), the presence of inorganic coagulants such as aluminum or iron salts can consume alkalinity and reduce pH providing a negative impact on subsequent biomass reuse for feed-supplement production for animals and aquatic organisms or biofuel production.
Conclusions
The present study gives an overview of the cultivation of the microalga D. subspicatus in photobioreactor, evaluating one of the main bottlenecks in this process: the biomass recovery. The use of chitosan for biomass flocculation enabled the scale up of the production process to a 100-L photobioreactor. The results indicate the feasibility of the use of this polymer, since in addition to the high efficiency in biomass recovery, it did not affect the microalgae composition, allowing its use in purposes that can range from the pharmaceutical industry to food supplementation. Thus, the use of chitosan to recover the biomass of D. subspicatus via flocculation is a functional alternative that enables the biomass production of this microalga.
References
Ahmad AL, Yasin NHM, Derek CJC, Lim JK (2011) Optimization of microalgae coagulation process using chitosan. Chem Eng J 173:879–882
APHA (2005) Standard methods for the examination of water and waste water, 21st edn. American Public Health Association, Washington
Atkins PW (1990) Physical chemistry. WH Freeman and Company, New York
Bach Q-V, Chen W-H (2017) Pyrolysis characteristics and kinetics of microalgae via thermogravimetric analysis (TGA): a state-of-the-art review. Bioresour Technol 246:88–100
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brennan L, Owende P (2010) Biofuels from microalgae - a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Chatsungnoen T, Chisti Y (2016) Oil production by six microalgae: impact of flocculants and drying on oil recovery from the biomass. J Appl Phycol 28:2697–2705
Chen L, Wang C, Wang W, Wei J (2013) Optimal conditions of different flocculation methods for harvesting Scenedesmus sp. cultivated in an open-pound system. Bioresour Technol 133:9–15
Correa DO, Santos B, Dias FG, Vargas JVC, Mariano AB, Balmant W, Rosa MP, Savi DC, Kava V, Glienke C, Ordonez JC (2017) Enhanced biohydrogen production from microalgae by diesel engine hazardous emissions fixation. Int J Hydrog Energy 42:21463–21475
Divakaran R, Pillai VNS (2002) Flocculation of algae using chitosan. J Appl Phycol 14:419–422
Dong C, Chen W, Liu C (2014) Flocculation of algal cells by amphoteric chitosan based flocculant. Bioresour Technol 170:239–247
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Escapa C, Coimbra RN, Paniagua S, García AI, Otero M (2017) Comparison of the culture and harvesting of Chlorella vulgaris and Tetradesmus obliquus for the removal of pharmaceuticals from water. J Appl Phycol 29:1179–1193
Gerchman Y, Vasker B, Tavasi M, Mishael Y, Kinel-Tahan Y, Yehoshuan Y (2017) Effective harvesting of microalgae: comparison of different polymeric flocculants. Bioresour Technol 228:141–146
Gerde JA, Yao L, Lio JY, Wen Z, Wang T (2014) Microalgae flocculation: impact of flocculant type, algae species and cell concentration. Algal Res 3:30–35
Grima EM, Belarbi EH, Fernández FGA, Medina AR, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515
Gupta SK, Kuma NM, Guldhe A, Ansari FA, Rawat I, Nasr M, Bux F (2018) Wastewater to biofuels: comprehensive evaluation of various flocculants on biochemical composition and yield of microalgae. Ecol Eng 117:62–68
Gutiérrez R, Ferre I, García J, Uggetti E (2015) Influence of starch on microalgal biomass recovery, settleability and biogas production. Bioresour Technol 185:341–345
Hansel PA, Riefler RG, Stuart BJ (2014) Efficient flocculation of microalgae for biomass production using cationic starch. Algal Res 5:133–139
Heasman M, Diemar J, O’Connor W, Sushames T, Foulkles L (2000) Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve mollusks - a summary. Aquac Res 31:637–659
Jiang Y, Zhang W, Wang J, Chen Y, Shen S, Liu T (2013) Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour Technol 128:359–364
Lama S, Muylaert K, Karki TB, Foubert I, Henderson RK, Vandamme D (2016) Flocculation properties of several microalgae and a cyanobacterium species during ferric chloride, chitosan and alkaline flocculation. Bioresour Technol 220:464–470
Larrosa APQ, Camara AS, Pohndorf RS, Rocha SF, Pinto LAA (2018) Physicochemical, biochemical, and thermal properties of Arthrospira (Spirulina) biomass dried in spouted bed at different conditions. J Appl Phycol 30:1019–1029
Lowry OH, Rosebrough NJ, Farr AL, Randall KL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mattos ER, Singh M, Cabrera ML, Das KC (2015) Enhancement of biomass production in Scenedesmus bijuga high-density culture using weakly absorbed green light. Biomass Bioenergy 81:473–478
Nichols HW, Bold HC (1965) Trichosarcina polymorpha gen. et sp. nov. J Phycol 1:34–38
Noseda MD, Correa, DO, Noseda MED, Oliveira AC, Dominiz, BSN, Rodrigues, JM (2016) Fotobiorreator para cultivo de micro-organismos fotossintetizantes. INPI patent BR1020160294851
Renault F, Sancey B, Badot PM, Crini G (2009) Chitosan for coagulation/flocculation processes - an eco-friendly approach. Eur Polym J 45:1337–1348
Salim S, Bosma R, Vermuë MH, Wijffels RH (2011) Harvesting of microalgae by bio-flocculation. J Appl Phycol 23:849–855
Shubert LE, Wilk-Wozniak E (2003) SEM investigation of several non-motile coccoid green algae isolated from aquatic habitats in Poland. Biol Bratisl 58:459–466
Selesu NFH, de Oliveira TV, Correa DO, Miyawaki B, Mariano AB, Vargas JVC, Vieira RB (2016) Maximum microalgae biomass harvesting via flocculation in large scale photobioreactor cultivation. Can J Chem Eng 94:304–309
Şirin S, Trobajo R, Ibanez C, Salvadó J (2012) Harvesting the microalgae Phaeodactylum tricornutum with polyaluminum chloride, aluminium sulphate, chitosan and alkalinity-induced flocculation. J Appl Phycol 24:1067–1080
Valverde F, Romero-Campero FJ, León R, Guerrero MG, Serrano A (2016) New challenges in microalgae biotechnology. Eur J Protistol 55:95–101
Vandamme D, Foubert I, Meesschaert B, Muylaert K (2010) Flocculation of microalgae using cationic starch. J Appl Phycol 22:525–530
Vieira RB, Vieira PA, Cardoso SL, Ribeiro EJ, Cardoso VL (2012) Sedimentation of mixed cultures using natural coagulants for the treatment of effluents generated in terrestrial fuel distribution terminals. J Hazard Mater 231-232:98–104
Wu K, Liu J, Wu Y, Chen Y, Li Q, Xiao X, Yang M (2014) Pyrolysis characteristics and kinetics of aquatic biomass using thermogravimetric analyzer. Bioresour Technol 163:18–25
Xu Y, Purton S, Baganz F (2013) Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour Technol 129:296–301
Yang R, Li H, Huang M, Yang H, Li A (2016) A review on chitosan-based flocculants and their applications in water treatment. Water Res 95:59–89
Acknowledgments
DOC acknowledges CNPq for a doctoral fellowship. MRD and MDN are Research Members of CNPq. The authors wish to acknowledge the Elizabeth Aidar Microalgae Culture Collection of the Fluminense Federal University (UFF) for the kindly supply of the microalgae used in this work. In addition, we are grateful to the Electronic Microscopy Center of the Paraná Federal University (CME-UFPR) for the SEM analyses.
Funding
This work was financed by the National Council for Scientific and Technological Development, CNPq, Brazil (project nos. 403869/2013-7 and 462414/2014-0).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Corrêa, D.O., Duarte, M.E.R. & Noseda, M.D. Biomass production and harvesting of Desmodesmus subspicatus cultivated in flat plate photobioreactor using chitosan as flocculant agent. J Appl Phycol 31, 857–866 (2019). https://doi.org/10.1007/s10811-018-1586-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1586-z