Abstract
The feasibility of utilizing crude glycerol as carbon source for heterotrophic growth of the green microalgae Chlorella pyrenoidosa and Coccomyxa subellipsoidea C-169 was investigated. The highest biomass concentration of C. pyrenoidosa (6.25 g L−1) and C. subellipsoidea C-169 (7.62 g L−1) was achieved in basal medium containing 5 and 10 g L−1crude glycerol. Compared to pure glycerol and glucose, the algal cells grown in crude glycerol media obtained a higher intracellular protein content, while the microalgal lipid consists of a large amount of unsaturated fatty acids. The most abundant fatty acids in C. pyrenoidosa and C. subellipsoidea C-169 were linolenic acid (C18:3) and palmitic acid (16:0). The cetane number (49.0) of both investigated algal species corresponds to the requirements of the Standard B100. Our results indicate that crude glycerol could provide a promising alternative feedstock for heterotrophic growth of these two microalgae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the short shortage of fossil fuel and increasing global concerns about environmental issues, the biodiesel industry has become increasingly important for its sustainability. During the manufacturing process of biodiesel, crude glycerol is generated as a main by-product in considerable quantities. Generally, approximately 1 kg of crude glycerol will be generated for every 10 kg of biodiesel produced (Johnson and Taconi 2010). In the USA and EU, 6.97 million tonnes of biodiesel are being produced each year (Dasari et al. 2005), which results in a considerable amount of crude glycerol as waste and its associated disposal costs. Crude glycerol typically consists of 70–75% glycerol along with a few contaminants in small quantities such as soap, oils, methanol, salts, and solid organic materials (Kumar et al. 2015). However, the refining process of this product to commercial pharmaceutical grade is time-consuming and not economically feasible (Zhang et al. 2013). In the current stage, crude glycerol is mostly considered as an industrial waste that causes severe environmental problems. Therefore, developing a sustainable and efficient approach to utilize crude glycerol is crucial.
Microalgae, which have the ability to grow autotrophically, mixotrophically, or heterotrophically, are considered as prolific producers of value-added products including pigments, proteins, polysaccharides, and fatty acids (Lu et al. 2016). Moreover, a variety of biofuels (e.g., biodiesel, bioethanol, and bio-hydrogen) have been generated from microalgal biomass in lab scale. Several limitations, such as low biomass yields and low photosynthetic efficiency, have largely prevented the autotrophic algal cultivation from achieving commercial success (Cheirsilp and Torpee 2012). Hence, heterotrophic cultivation has received increasing attention because it is easier to maintain and does not depend on sunlight (Brennan and Owende 2010). However, the industrialization of microalgal fermentation is restricted mostly due to the high price of glucose feedstock which accounts up to 80% of the total substrate cost. Renewable, affordable, and effective alternatives to glucose for heterotrophic cultivation of microalgae are desirable.
Recently, extensive research has been conducted on the production of value-added metabolites from microalgae using crude glycerol as feedstocks. Bioactive compounds extracted from microalgae, like the polyunsaturated fatty acids, pigments, and functional polysaccharides, could be used as green and natural alternatives for chemicals in health care and cosmetics. Protein is an important component in microalgal biomass and has been recommended by the World Health Organization as a well-balanced protein source for humans and animals (Joseph et al. 1960). Heterotrophic cultivation of C. pyrenoidosa based on straw substrate was favorable in composition. The highest protein in microalgal biomass reached to 62% under nitrogen-excessive conditions. Over 40% of amino acids in biomass belonged to essential amino acids (EAA) (Zhang et al. 2017). Schizochytrium limacinum has been reported to convert crude glycerol to docosahexaenoic acid (DHA) with a yield of as high as 4.91 g L−1 (Chi et al. 2007). Crude glycerol with lower price (approx. $US 0.055 kg−1) has become more competitive than sugars (Chen and Walker 2011). Furthermore, the abundant macro-elements (e.g., calcium, potassium, sulfur, and magnesium) in crude glycerol may be important supplementary nutrients for microalgal growth. Crude glycerol also contains surfactants which have been reported to exhibit growth-promoting effects on microorganisms. Applying crude glycerol as a carbon source for heterotrophic culture of microalgae might be a win-win strategy for both waste disposal and valuable biomass production.
Chlorella pyrenoidosa, a well-studied green microalga, has been approved as a new food source by the Food Safety Law of the People’s Republic of China in 2012. Besides that, it has potential applications such as feedstock for biofuels, synthesis of bioactive compounds, and feedstocks for animal feed and live feed in aquaculture (Tu et al. 2016; Wells et al. 2017). Coccomyxa subellipsoidea C-169 is the first sequenced eukaryotic microalgae from the polar environment (Blanc et al. 2012). Due to its fragile cell wall and high lipid content, C. subellipsoidea C-169 is an attractive and promising candidate for biodiesel production (Peng et al. 2016). However, no studies have investigated the growth of these two green microalgae on crude glycerol. In the current study, the feasibility of using crude glycerol as a carbon source for heterotrophic cultivation of these two strains is examined. This study will provide a potential route to integrate the microalgal biomass production with economical crude glycerol disposal.
Materials and methods
Crude glycerol characterization
The trace elements in crude glycerol were determined by inductively coupled plasma (ICP) (Linge 2010). The parameters were as follows: radio frequency power 1050 kW, atomized gas pressure 4.25 × 106 Pa, the auxiliary airflow speed 1 L min−1, the sample flow rate 1.70 mL min−1, the sample washed for 1 min, the high wave scanning 5 s, and the low wave scanning 30 s. Glycerol and methanol content were determined by colorimetric methods (Anthon and Barrett 2004).
Microalgae strains and seed culture conditions
The green microalga Chlorella pyrenoidosa SJTU-2 was provided by Professor Feng Chen of Peking University; Coccomyxa subellipsoidea C-169 was from the Japan National Institute of Environmental Research (NIES), Japan. Crude glycerol was collected from Guangdong Wuzhou Pharmaceutical Co., Ltd.
For cultivation, 250-mL Erlenmeyer flasks with a working volume of 100 mL were used. Chlorella pyrenoidosa and C. subellipsoidea were selected from single colonies and cultured in basal medium with 10 g L−1 glucose. The initial concentrations of inoculated microalgae 0.21 g L−1. After inoculation, the culture was placed in a 150-rpm, 28 ± 1 °C dark shaking chamber for 8 days. All treatments were carried out in triplicate.
The dry biomass concentration was calculated by gravimetric analysis. Two milliliter of individual culture liquid was centrifuged for 3 min at 8000 rpm, then washed three times with distilled water and dried in an oven at 60 °C to a constant weight.
Heterotrophic culture
Heterotrophic growth was carried out by grown algae species in modified basal medium (Ogbonna et al. 1997) containing 1250 mg L−1 NaNO3, 1250 mg L−1 KH2PO4, 1000 mg L−1 MgSO4·7H2O, 500 mg L−1 EDTA (disodium salt), 114.2 mg L−1 H3BO3, 111 mg L−1 CaCl2, 4.98 mg L−1 FeSO4·7H2O, 8.82 mg L−1 ZnSO4·7H2O, 1.42 mg L−1 MnCl2·4H2O, 1.19 mg L−1 NaMoO4·2H2O, 1.57 mg L−1 CuSO4·5H2O, and 0.49 mg L−1 Co(NO3)2·6H2O. The pH was adjusted to 6.1 ± 0.1. Different carbon sources (glucose, pure glycerol, and crude glycerol) were added to the medium.
Analysis of protein, polysaccharide, and fatty acid profiles
Lyophilized biomass (20 mg) was prepared in a 2-mL centrifuge tube and then completely disrupted by a bead beater with 50-mg ceramic beads. After that, the tube was cooled in liquid nitrogen. The supernatant extraction solvent-containing protein was collected by centrifugation. Protein was determined by the Bradford method (Bradford 1976).
The content of intracellular polysaccharides was measured with phenol sulfuric acid (Xi et al. 2010); the preprocessing method was the same as for intracellular protein. The supernatant was collected for polysaccharide determination.
The determination of fatty acid composition was carried out by GC-MS. Fatty acid methyl esters (FAMEs) were prepared according to the previous procedure (Lu et al. 2012). Nonadecanoic acid (C19:0) (Sigma, USA) was added in each sample as internal standard. The chromatographic parameters were as follows: DB-5 column (30 m × 0.25 mm), inlet temperature at 260 °C, programmed temperature rise, 2 min at 60 °C, 30 min−1 rise to 120 °C, 1.5 min−1 increased to 250 °C retained for 2 min, high-purity helium gas, and column flow rate was 1.2 mL min−1. Mass spectrum conditions: the ionization mode was EI, and the ionization voltage was 70 EV.
Statistics
All values in figures and tables are shown as mean ± standard deviation (SD). One-way ANOVA was used to evaluate the mean difference between groups. A value of p < 0.05 was considered statistically significant.
Results
Characteristics of crude glycerol
Table 1 shows the elements and components of crude glycerol determined by inductively coupled plasma. As expected, sodium and potassium were the main elements in crude glycerol, accounting for 6359 and 1044 ppm, respectively. Additionally, it is worth noting the high levels of elements such as phosphorus (647 ppm) and sulfur (423 ppm) in the crude glycerol, which are essential nutrients in cell proliferation. The crude glycerol obtained during biodiesel production from soybean oil had the following chemical composition (w/w): glycerol 75%; methanol 8.9%; water 11.27%; other impurities in ash such as non-glycerol organic matter and salts, about 3–4%.
Growth of C. pyrenoidosa and C. subellipsoidea
Different concentrations of crude glycerol affected the growth of C. pyrenoidosa and C. subellipsoidea (Fig. 1). A lag phase in C. pyrenoidosa growth was observed for all crude glycerol concentrations in the first 3 days. There was a sharp increase in biomass from day 3 to day 6 in the medium with an initial glycerol concentration of 5 g L−1 and this increase was significantly higher than that at 10 g L−1 (P < 0.05). Maximum biomass concentrations of C. pyrenoidosa (6.25 g L−1) and C. subellipsoidea C-169 (4.97 g L−1) were obtained in the stationary growth phase in the media containing 5 and 10 g L−1 crude glycerol, respectively. Rapid initial growth was observed for C. subellipsoidea at all crude glycerol concentrations. During the first 5 days, the growth curves of C. subellipsoidea were very similar in all media with different concentrations of crude glycerol. The maximum biomass concentration of C. subellipsoidea C-169 (7.62 g L−1) in the medium containing 10 g L−1 crude glycerol was 34.38% higher than that in the medium with a crude glycerol concentration of 20 g L−1.
The biomass concentration obtained in the culture grown with crude glycerol was less than that in glucose, whereas there is no significant difference in biomass concentration of C. pyrenoidosa between crude glycerol and pure glycerol (Fig. 2). Likewise, the highest biomass concentration of C. subellipsoidea C-169 was in basal medium with 10 g L−1 glucose.
Proximate composition of C. pyrenoidosa and C. subellipsoidea
The highest protein content of C. pyrenoidosa was 19.37% in the group with 5 g L−1 crude glycerol, which was significantly higher than that of glucose (P < 0.05) (Fig. 3). A similar result was obtained in C. subellipsoidea where the highest protein content was 15.61% obtained in the basal medium with 10 g L−1 crude glycerol, whereas for the other two carbon sources the protein contents were 12.44 and 13.11%.
The intracellular polysaccharide contents of C. pyrenoidosa and C. subellipsoidea under heterotrophic cultures with different carbon sources are shown in Fig. 4. Chlorella pyrenoidosa grown in pure glycerol contained more intracellular polysaccharide (24.31%) than C. subellipsoidea (15.13%). Generally, there was no significant effect of glucose and crude glycerol on the accumulation of polysaccharide in the two microalgae.
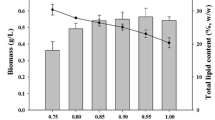
The total lipid contents of C. pyrenoidosa cultured in crude glycerol, pure glycerol, and glucose were 28.66, 26.37, and 30.24%, respectively. The total lipid content in C. pyrenoidosa was not significantly affected by the addition of 5.0 g L−1 crude glycerol or glucose (p > 0.05). The total lipid contents of C. subellipsoidea cultivated in the three different carbon sources were 20.41, 18.87, and 22.37%, respectively. The total lipid content did not differ statistically between the crude glycerol and glucose groups.
Fatty acid composition
The fatty acid composition of algae under heterotrophic condition with crude glycerol is shown in Table 2. Chlorella pyrenoidosa and C. subellipsoidea grown in 5 and 10 g L−1 crude glycerol, respectively, were used for analysis due to the maximum biomass obtained in the different conditions. The most abundant fatty acids in the two microalgae were palmitic acid (16:0), linoleic acid (18:2), and linolenic acid (18:3), accounting for over 85% of the total fatty acids. The proportions of total unsaturated fatty acids in C. pyrenoidosa and C. subellipsoidea accounted for 75.59 and 69.57%, respectively. Palmitic acid (16:0) predominates the fatty acid profiles in the two microalgae.
Discussion
There is a slight variation in the composition of crude glycerol depending on the different industrial processes. Generally, biodiesel-generated crude glycerol with various physical properties contains many impurities such as alcohol, spent catalyst, ash, and fatty acids in different proportions. The color of crude glycerol ranges from yellow to dark brown, which contains salt and free fatty acid. According to Table 1, sodium and potassium occupied a large proportion of chemical elements in the crude glycerol. Hu et al. (2012) reported that the glycerol content in the crude glycerol generated from different types of feedstock ranged from 60 to 70%. In the current study, glycerol accounted for 76% in crude glycerol, which could be considered as a high content. According to the literature, high concentrations of crude glycerol (over 15 g L−1) showed inhibitory effects on microalgal growth, which might be caused by the presence of methanol, ash, and salinity. For example, high concentration of methanol has been reported to have unfavorable effects on the growth of algae (Nakai et al. 1999). Salt stress can interfere with physiological processes of microalgae inhibiting algal growth and photosynthesis (Xia et al. 2004). On the other hand, trace elements such as iron, magnesium, and zinc may promote cell growth. Magnesium is an important component of chlorophyll and an activator of many enzymes (Kaplan et al. 1986). Iron is a component of photosystem II and a co-factor for some enzymatic reactions (Quigg 2016). Liu et al. (2008) found that supplementation of chelated Fe3+ in the late exponential growth phase increased the final cell density and induced lipid accumulation in C. vulgaris.
In the current study, the highest biomass concentration of C. pyrenoidosa was 6.25 g L−1. Similar results were obtained of C. vulgaris under mixotrophic condition by Kong et al. (2013). Compared to autotrophic condition, the addition of glycerol at lower concentration to the culture has a positive effect on biomass production. There is limited information on the feasibility of C. subellipsoidea C-169 as a potential strain for lipid production. Our results show that C. subellipsoidea C-169 has similar growth rates as Chlorella sp.
Many algal species can grow mixotrophically and heterotrophically with the addition of various carbon sources (Neilson et al. 1973). Glucose is the most widely used organic carbon source to cultivate microalgae in heterotrophic conditions (Mohan et al. 2015). As shown in Fig. 5, compared to pure glycerol and waste glycerol, glucose was advantageous for total lipid accumulation in algal cell. Nevertheless, in comparison to pure glycerol and glucose, a significantly higher protein content was achieved (p < 0.05) in cultures utilizing crude glycerol. Therefore, in order to obtain higher intracellular protein in C. pyrenoidosa and C. subellipsoidea C-169 in heterotrophic culture, basal medium supplemented with 5 and 10 g L−1 crude glycerol is recommended.
Numerous heterotrophically grown algae have been reported as potential sources of biodiesel. For example, Chlorella vulgaris (Sharma et al. 2016) and Chlorococcum sp. (Sabeela Beevi and Sukumaran 2015) can accumulate large amount of lipids when the culture medium is supplemented with glycerol as carbon source. The properties of biodiesel are highly related to the fatty acid profile. The property of biodiesel can be greatly affected by the composition of fatty acid methyl esters, such as the carbon chain length and the unsaturation degree. For instance, the presence of unsaturated bonds will reduce the melting point of biodiesel and increase its low-temperature fluidity. Excess double bonds also affect its stability and combustion property (Knothe 2013). According to the European standards (Han et al. 2016), unsaturated fatty acids with four or more double bonds are easily to be oxidized during storage, which is unfavorable for biodiesel production. The fatty acid composition of the heterotrophic growth of C. pyrenoidosa and C. subellipsoidea C-169 using crude glycerol is shown in Table 2. In addition, the quality of biodiesel is highly related to the cetane number, which influences the ignition quality in engines. In the current study, the cetane numbers of C. pyrenoidosa and C. subellipsoidea C-169 were 49.14 and 50.29, which suggested that the fatty acids of these two microalgae might be suitable for biodiesel production. It is also noteworthy that the proportion of linolenic acid (18:3) content of C. pyrenoidosa is above (49.27% of total fatty acids), which is higher than other algae cultivated with crude glycerol (Liang et al. 2009). Linolenic acid is an essential fatty acid needed for human health and has been reported to have anti-cancer, neuro-protective, cardiovascular-protective, anti-osteoporotic, and antioxidative, anti-inflammatory effects (Callaway et al. 2005; Moranis et al. 2012; Kim et al. 2014). Palmitic acid has been utilized as cosmetics, food additives, and lubricant, which have wide broad application prospects in various areas (Rose-Monde and Sébastien 2015; Hurtado-Benavides et al. 2016).
In conclusion, biodiesel-derived crude glycerol was demonstrated as an effective, low-cost, and renewable carbon substrate for heterotrophic growth of microalgae and value-added metabolite production. Compared with pure glycerol and glucose, crude glycerol is more beneficial to protein accumulation in the algae cells. The lipids from the microalgae growing on crude glycerol contained C16 and C18 as the main fatty acid components. Additionally, he cetane numbers of the two investigated algae species complied with the requirements of the Standard B100 (Lu et al. 2016) (> 49.0). This study provides new insights into the integration of economical cultivation of heterotrophic microalgae with industrial waste disposal.
References
Anthon GE, Barrett DM (2004) Comparison of three colorimetric reagents in the determination of methanol with alcohol oxidase. Application to the assay of pectin methylesterase. J Agric Food Chem 52:3749–3753
Blanc G, Agarkova I, Grimwood J, Kuo A, Brueggeman A, Dunigan DD, Gurnon J, Ladunga I, Lindquist E, Lucas S (2012) The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol 13(5):R39
Bradford M (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energy Rev 14:557–577
Callaway J, Schwab U, Harvima I, Halonen P, Mykkanen O, Hyvonen P, Jarvinen T (2005) Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat 16:87–94
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516
Chen YH, Walker TH (2011) Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol Lett 33:1973–1983
Chi Z, Pyle D, Wen Z, Frear C, Chen S (2007) A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem 42:1537–1545
Dasari MA, Kiatsimkul PP, Sutterlin WR, Suppes GJ (2005) Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl Catal A 281:225–231
Han SF, Jin W, Tu R, Abomohra AE, Wang ZH (2016) Optimization of aeration for biodiesel production by Scenedesmus obliquus grown in municipal wastewater. Bioprocess Biosyst Eng 39:1073–1079
Hu S, Luo X, Wan C, Li Y (2012) Characterization of crude glycerol from biodiesel plants. J Agric Food Chem 60:5915–5921
Hurtado-Benavides A, Daniela DA, Sánchez-Camargo ADP (2016) Study of the fatty acid profile and the aroma composition of oil obtained from roasted Colombian coffee beans by supercritical fluid extraction. J Supercrit Fluids 113:44–52
Johnson DT, Taconi KA (2010) The glycerin glut: options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ Prog Sustain Energy 26:338–348
Joseph K, Rao MN, Swaminathan M, Indiramma K, Subrahmanyan V (1960) The nutritive value of protein blends similar to FAO reference protein pattern in amino acid composition. Ann Biochem Exp Med 20:243–250
Kaplan D, Richmond AE, Dubisnky Z, Aaronson A (1986) Algal nutrition. In: Richmond A (ed) Handbook of Microalgal Mass Culture CRC Press, Boca Raton, pp 147–198
Kim KB, Nam YA, Kim HS, Hayes AW, Lee BM (2014) α-Linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem Toxicol 70:163–178
Knothe G (2013) Production and properties of biodiesel from algal oils. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 207–221
Kong WB, Hong Y, Cao YT, Hao S, Hua SF, Xia CG (2013) Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotrophic culture. Food Technol Biotechnol 51:62–69
Kumar P, Sharma R, Ray S, Mehariya S, Patel SKS, Lee JK, Kalia VC (2015) Dark fermentative bioconversion of glycerol to hydrogen by Bacillus thuringiensis. Bioresour Technol 182:383–388
Liang Y, Sarkany N, Yi C (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Linge KL (2010) Trace element determination by ICP-AES and ICP-MS: developments and applications reported during 2006 and 2007. Geostand Geoanal Res 32:453–468
Lu L, Pohnert G, Dong W (2016) Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar Drugs 14(10):191
Lu N, Wei D, Jiang X-L, Chen F, Yang S-T (2012) Fatty acids profiling and biomarker identification in snow alga Chlamydomonas nivalis by NaCl stress using GC/MS and multivariate statistical analysis. Anal Lett 45:1172–1183
Neilson AH, Blankley WF, Lewin RA (1973) Growth with organic carbon and energy sources. In: Stein JR (ed) Handbook of Phycological Methods. Cambridge University Press, Cambridge, pp 275–285
Mohan SV, Rohit MV, Chiranjeevi P, Chandra R, Navaneeth B (2015) Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: progress and perspectives. Bioresour Technol 184:169–178
Moranis A, Delpech J-C, Smedt-Peyrusse VD, Aubert A, Guesnet P, Lavialle M, Joffre C, Layé S (2012) Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain Behav Immun 26:721–731
Nakai S, Inoue Y, Hosomi M, Murakami A (1999) Growth inhibition of blue–green algae by allelopathic effects of macrophytes. Water Sci Technol 39:47–53
Ogbonna JC, Masui H, Tanaka H (1997) Sequential heterotrophic/autotrophic cultivation—an efficient method of producing Chlorella biomass for health food and animal feed. J Appl Phycol 9:359–366
Peng H, Dong W, Gu C, Feng C (2016) Transcriptome analysis reveals global regulation in response to CO 2 supplementation in oleaginous microalga Coccomyxa subellipsoidea C-169. Biotechnol Biofuels 9(1):151
Quigg A (2016) Micronutrients. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 211–231
Rose-Monde M, Sébastien N (2015) Improving the optimized shea butter quality: a great potential of utilization for common consumers and industrials. Springerplus 4(1):667
Sabeela Beevi U, Sukumaran RK (2015) Cultivation of the fresh water microalga Chlorococcum sp. RAP13 in sea water for producing oil suitable for biodiesel. J Appl Phycol 27:141–147
Sharma AK, Sahoo PK, Singhal S, Patel A (2016) Impact of various media and organic carbon sources on biofuel production potential from Chlorella spp. 3 Biotech 6(2):116
Tu R, Jin W, Wang M, Han S, Abomohra EF, Wu WM (2016) Improving of lipid productivity of the biodiesel promising green microalga Chlorella pyrenoidosa via low-energy ion implantation. J Appl Phycol 28:2159–2166
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME, Brawley SH (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29:949–982
Xi X, Wei X, Wang Y, Chu Q, Xiao J (2010) Determination of tea polysaccharides in Camellia sinensis by a modified phenol-sulfuric acid method. Arch Biol Sci 62:669–676
Xia J, Li Y, Zou D (2004) Effect of salinity stress on PSII in Ulva lactuca as probed by chlorophyll fluorescence measurements. Aquat Bot 80:129–137
Zhang TY, Wang XX, Yin-Hu WU, Wang JH, Deantes-Espinosa VM, Zhuang LL, Hong-Ying HU, Guang-Xue WU (2017) Using straw hydrolysate to cultivate Chlorella pyrenoidosa for high-value biomass production and the nitrogen regulation for biomass composition. Bioresour Technol 244:1254–1260
Zhang Z, Wong HH, Albertson PL, Doherty WOS, O’Hara IM (2013) Laboratory and pilot scale pretreatment of sugarcane bagasse by acidified aqueous glycerol solutions. Bioresour Technol 138:14–21
Liu Z-Y, Wang G-C, Zhou B-C (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Funding
This work was funded by the program of Sciences and Technology of Guangzhou (Grant No. 201704030084), the Science and Technology Program in Marine and Fishery of Guangdong (Grant No. A201401C01), and the Science and Technology Program of Guangdong (Grant Nos. 2015A020216003, 2016A010105001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Z., Liu, L., Chen, J. et al. Effect of crude glycerol on heterotrophic growth of Chlorella pyrenoidosa and Coccomyxa subellipsoidea C-169. J Appl Phycol 30, 2989–2996 (2018). https://doi.org/10.1007/s10811-018-1551-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1551-x