Abstract
Algae have long been acclaimed as the attractive renewable source for generating third-generation biofuels, particularly biodiesel. Under the present investigation, the trends of production of biomass and lipid during the autotrophic and heterotrophic growth of newly isolated blue-green algae, Leptolyngbya subtilis JUCHE1, were compared and correlated with the variation in C-sources. In the autotrophic and heterotrophic growth studies, CO2 and glycerol were respectively used as the inorganic and organic C-sources maintaining equivalence in the initial amount of carbon. Light was used as the source of energy in both cases. The concentration of CO2 in the feed gas stream was varied from 5 to 20% (% v/v). Equivalent quantity of carbon was supplied through glycerol during heterotrophic growth. Small-scale closed algal bioreactors were used for growing the algae at 37 °C and 2.5 kLux light illumination in batch mode for 0–4 days. Primarily, higher biomass production from glycerol compared with CO2 was observed. In case of photoautotrophic growth, the maximum values of biomass and lipid productivity, obtained at 15% CO2, were 0.1857 g/L/d and of 0.020 g/L/d respectively. The maximum biomass productivity of 0.2733 g/L/d was obtained for photoheterotrophic growth at a glycerol concentration equivalent to 15% CO2 (v/v). Under photoheterotrophic growth of Leptolyngbya subtilis JUCHE1, lipid productivity of 0.0702 g/L/d was obtained at glycerol concentration equivalent to 5% (v/v) CO2, which is 4.66-fold higher than that obtained under corresponding photoautotrophic condition. The “switch-over” from the autotrophy to the photoheterotrophy instigated the oleaginous anabolism and consequent lipid enrichment in L. subtilis JUCHE1, which can be extracted and converted to biodiesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, there is a threat of global warming due to high extent of CO2 emission in the atmosphere worldwide by industry and transport sectors. For the reduction of atmospheric CO2 level, algae have been assessed as potential biosequestrators. Due to the capability of high rates of growth compared with terrestrial plants and storage potential of lipids, they can also serve as a sustainable feedstock for generating alternative biofuel, namely biodiesel. Most of the algae are also rich in valuable biochemicals such as omega 3-fatty acids namely, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and alpha-linolenic acid (ALA). Hence, the concept of third-generation biorefinery generating biofuel and biochemicals based on algal biomass has been established by the International Energy Agencies (IEA). Algal biodiesel, i.e., fatty acid methyl esters (FAME), is obtained through the transesterification of fatty acids (C12-C24) present in algal oil (Mandotra et al. 2014). Algae follow several mechanisms for production of biomass as well as lipid and can be grown under different metabolic modes: (i) photoautotrophic (with light energy and inorganic carbon source, e.g., CO2, Na2CO3, and NaHCO3); (ii) photoheterotrophic growth (with light energy and organic carbon source, e.g., glucose and glycerol); and (iii) mixotrophic mode (with light energy and carbon sources of both organic and inorganic ones) (Chen et al. 2011; Zheng et al. 2012; Kim et al. 2013). Heterotrophic growth of different algae has also been reported in the absence of light under a dark condition (Zheng et al. 2012; Sachdeva et al. 2016).
Phototrophic growth of algae, whether autotrophs, heterotrophs, or mixotrophs, involves light-dependent photosynthetic metabolic pathway occurring in the thylakoid membrane. In this pathway, light energy is utilized for producing ATP and NADPH. When CO2 is assimilated to form sugar, ATP and NADPH act respectively as energy source and reducing agents in the light-independent Calvin cycle occurring in the stroma part of chlorophyll. The light-dependent and -independent reactions occur simultaneously; however, the light-independent reactions can be freely regulated in presence of exogenous organic carbon. In photoheterotrophic mode, ATP and NADPH produced in the thylakoid enable algae to utilize the energy-efficient active transport of the available organic carbon source. The light-dependent products can also be utilized in cellular metabolic pathways and can be used for accumulation of energy-rich storage products, namely fatty acids and TAGs (Abomohra et al. 2018). As reported in the literature, glycerol, a byproduct of biodiesel production from fatty acids obtained from vegetable and algal sources, has been used as an inexpensive organic carbon source (Abomohra et al. 2018). In most of the studies, enhanced growth of biomass and lipid has been observed for glycerol, compared with CO2 as the carbon source (Yang et al. 2011; Leite et al. 2015). A few pioneering studies are available where the auto- and heterotrophic growth of algae using photons as energy source are compared from the perspective of the products, namely, biomass and lipid. However, in most of the cases, the amount of carbon supplied from glycerol in photoheterotrophic experiments is much higher than that from CO2 used in autotrophic growth. Reports of the comparative experimental results of biomass growth and lipid production obtained from equivalent amount of carbon supplied by the inorganic (CO2) and the organic (glycerol) source are, however, important from the perspective of strategic decision on the selection of carbon substrate. Under the present investigation, the photoautotrophic and the photoheterotrophic growth of Leptolyngbya subtilis JUCHE1, a newly isolated power plant algal strain, have been studied using CO2 and glycerol respectively (xChowdhury et al. 2018). The content of lipid and biomass productivity of Leptolyngbya subtilis JUCHE1 during auto- and heterotrophic modes of growth has been compared, providing equivalent number of moles of carbon from CO2 and glycerol. The lipid content of the algal strain under nitrogen-stressed autotrophic growth condition has also been compared with the results obtained under the present condition. An analysis of the biomass productivity and the lipid content of this power plant algal strain has also been made by comparing the values with those of other algae, as reported in the literature.
Materials and methods
Algal strain and culture medium
A blue-green alga, Leptolyngbya subtilis JUCHE1, isolated from water bodies of a coal-fired power plant situated in Sagardighi, Berhampur, West Bengal, was used. Modified 18 medium was used as the minimal salt medium for all experiments (Pradhan et al. 2015). The composition of M18 is as follows (Basis 1 L): 1.5 g NaNO3, 0.38 g MgSO4.7H2O, 0.12 g K2HPO4, 0.11 g CaCl2.2H2O and minor salts such as 0.07 g NaCl, 0.01 g Fe2(SO4)3.4H2O, 0.003 g H3BO3, 0.002 g MnSO4.4H2O, 0.0003 g ZnSO4.7H2O, 0.00008 g CuSO4.5H2O, and 0.00004 g CoCl2.6H2O. The pH was 7. The cultures were grown in an incubator at 37 °C with a light intensity of 2.5 kLux.
Analytical methods
CHN analysis
The CHNS elemental analyzer (Perkin Elmer 2400 series II) was used for elemental analysis of dry algal biomass. The analysis was performed by the experts of Indian Association for the Cultivation of Science (IACS), Kolkata.
GC-MS analysis
The fatty acid analysis was carried out using GC-MS analyzer (Thermo-Scientific) in TR-WAX column (30 m × 0.25 mm × 0.25 μm). The oven temperature was set at 175 °C for 5 min and then raised to 230 °C. The injector and MS detector temperatures were set at 230 °C and 280 °C respectively. Helium was used as a carrier gas. The mass line range was programmed from 25 to 800 amu (Mandotra et al. 2014).
Spectrophotometric analysis
The glycerol concentrations in the culture medium (M-18)/sample was quantified using spectrophotometric analysis, and the method is suggested by Bondioli and Della Bella (2005). The spectrophotometric analysis was carried out in a UV-Vis spectrophotometer (PerkinElmer). The detailed method is provided in the supplementary section.
Culture medium using CO2 as carbon source
Maintenance medium
The culture medium used for maintenance of algae was prepared by sparing pure CO2 into M18 medium for 10 h. The algal strain was maintained in this medium under constant illumination of 2.5 kLux at 37 °C. Culture medium for experiments with varying CO2 concentration was prepared by sparging M18 medium with CO2-air mixture having different partial pressure of CO2 for 10 h. The values of equilibrium concentration of CO2 in the aqueous phase were determined using Henry’s law (Sander 2015).
Effect of CO2 bubbling on medium pH
During the bubbling of gas containing CO2 at different concentration levels (5–50%), reduction of pH of the medium was observed. The values of pH after 20 h of bubbling have been provided in Table 1.
The drop in pH clearly indicates acidification. When CO2 reacts with H2O to form carbonic acid H2CO3, two protons are lost to form bicarbonate, HCO3− , and carbonate, CO32− as shown in the reaction given below.
Similar observations was reported by Ota et.al (2009) which showed that with the increase in inlet CO2 concentrations, there was a decrease in the values of pH (Ota et al. 2009). Another research article reported that when CO2 concentration was as low as 0.04%, pH increased with the increase in algal growth. However, when the same algal biomass was transferred to higher concentration of CO2, i.e., 40%(v/v), the value of pH suddenly decreased to 6 within 4 h and constantly varied between the range of 5.8 and 6.4 in another culture period of 9 days (Li et al. 2020).
Batch experiments under photoautotrophic growth mode
Conical flasks of 250-mL capacity were used as algal bioreactors. The working volume was maintained at 150 mL for all the experiments. Each bioreactor was equipped with two glass tubes: one for transfer of inoculum and another for gas transfer. An outlet was also provided at the bottom of the conical flask as shown in Fig. 1. At first, the bioreactors were filled to the brim with minimal salt medium (M18) saturated with CO2, as described in the earlier section. Air-CO2 mixture having different concentrations of (5–20% v/v) CO2 was subsequently introduced in the conical flask by displacement of M18 medium through the gas transfer tube. Algal mass weighing 1 g (equivalent to 0.1 g dry mass) was introduced into the inoculation tube, and an injection vial containing M18 medium was held above it. Algal inoculum was ultimately transferred to the flask by pushing the M18 medium using the movement of the piston of the injection vial. Algal growth in culture medium having saturated liquid phase concentration of CO2 against each gas phase concentration 5–20% (v/v) was conducted for different time periods up to 4 days. For each gas phase concentration (5–20%) of CO2, four sets of experiments were conducted for 1, 2, 3, and 4 days. Constant illumination of 2.5 kLux and temperature of 37 °C were maintained during all growth experiments. The growth medium for each time duration was analyzed for algal mass and its lipid content.
Determination of liquid phase CO2 Concentration under equilibrium
Assuming the validity of Henry’s law and the attainment of equilibrium, aqueous phase concentration of CO2 corresponding to different inlet concentrations or partial pressure of CO2 in the inlet air-CO2 mixture has been determined as follows (Sander 2015):
where \( {C}_{{\mathrm{L}}_{{\mathrm{CO}}_2}}^{\ast } \) = liquid phase concentration of CO2 under equilibrium, M; \( {p}_{{\mathrm{CO}}_2} \) = partial pressure of CO2 in gas phase, kPa; and \( {H}_{{\mathrm{CO}}_2}^{\mathrm{CP}} \) = Henry’s constant for CO2, M/kPa.
Temperature dependence of
where TRef = 298K and \( {\mathrm{H}}_{{\mathrm{CO}}_2}^{\operatorname{Re}f} \) = Henry’s constant at 298 K.
From a standard table (Sander 2015), \( {H}_{{\mathrm{CO}}_2}^{\operatorname{Re}f} \) = 3.3 × 10−4 M/kPa, \( \frac{-{\Delta \mathrm{H}}_{{\mathrm{Sol}}_{{\mathrm{CO}}_2}^n}}{R}=\frac{d\ln {\mathrm{H}}_{{\mathrm{CO}}_2}}{d\left(\frac{1}{T}\right)}=2400K \), and \( {H}_{{\mathrm{CO}}_2}^{310K} \) = 2.448 × 10−4 M/kPa.
The values of equilibrium concentration of CO2 in aqueous phase corresponding to each gas phase concentration in CO2-air mixture has been provided in Table 2.
Batch experiments under photoheterotrophic condition
The culture tubes of 60-mL capacity were filled with 30 mL glycerol supplemented M-18 medium. The values of Vgly used for substitution of C supplied by gas phase having different CO2 concentrations have been provided in Table 2. A total of 0.2 g of wet algal biomass was inoculated in each of the tubes. The remaining headspace was sparged with argon to remove any trace of carbon dioxide and other gases present in the headspace. The tubes were closed using caps and sealed with parafilm. The tubes were then carefully placed as slants inside an incubator and incubated at 37 °C for duration up to 4 days under illumination of 2.5 kLux. For each concentration of glycerol, 4 sets of experiments varying time duration of 1, 2, 3, and 4 days were conducted. Each growth medium was analyzed for biomass concentration and the corresponding lipid content. The glycerol in the culture medium (M18) after respective intervals of algal growth was quantified by analyzing its liquid part using a spectrophotometric method suggested by Bondioli and Della Bella (2005). The detailed method is provided in the supplementary section. All experiments were conducted in triplicate. The moisture content and the elemental composition of algal cell mass were determined through drying and C–H–N analysis respectively.
Determination of equivalent glycerol concentration against each gas phase concentration of CO2
Growth medium for photoheterotrophic growth was prepared by supplementing M18 medium with glycerol instead of CO2 as a carbon source. The values of concentrations of glycerol were maintained at levels to supply equal number of gram atoms of carbon which was available in the aqueous phase corresponding to different gas phase concentrations (5–20%) of CO2, as used in photoheterotrophic growth. Equivalent glycerol concentration against each gas phase concentration of CO2 has been calculated using the following equation.
-
Cgly = glycerol concentration, M
-
\( {n}_{{\mathrm{C}}_{\mathrm{gly}}} \) = number of g. atom of carbon in mol gly
-
\( {n}_{{\mathrm{C}}_{{\mathrm{C}\mathrm{O}}_2}} \) = number of g. atom of carbon in mol CO2
-
\( {p}_{{\mathrm{CO}}_2} \) = partial pressure of CO2 in gas phase, kPa
-
\( {H}_{{\mathrm{CO}}_2}^{\mathrm{CP}} \) = Henry’s constant for CO2, M/kPa
The values of Cgly was maintained for substitution of C, available at different gas phase concentrations (5–20%) of CO2 used under the present study. Volume of glycerol (Vgly) required for 1 L photoheterotrophic solution (glycerol supplemented M-18) was as follows,
MWgly = molecular weight of glycerol
ρgly = density of glycerol (g/mL)
Calculations
Biomass production rate
The biomass production rate (Px) has been defined as follows:
where mx(t) = mass of biomass (g) at time, t; mx0 = mass of biomass (g) at time, t0; VR = liquid volume in the reactor, L; and t = culture time, day.
Specific growth rate
The specific growth rate (μ) can be calculated using Eq. (9),
where Cx = biomass concentration; Cx, average = average biomass concentration
Conversion of glycerol
Conversion of glycerol at each culture time has been determined using the following equation:
where XGly = conversion of glycerol, CGly(t) = glycerol concentration at any culture time, t; and \( {C}_{{\mathrm{Gly}}_0} \) = initial glycerol concentration.
Carbon capture
The carbon capture (%) (CC) through biomass generation has been determined using the following equation:
Or
where, MC = weight fraction of moisture in algal mass; xC = weight fraction of carbon in algal mass; and AWC = atomic weight of carbon = 12
Lipid extraction
The de-pigmented algal biomass was used for lipid extraction. For depigmentation process, the dry biomass was treated with a 20 mL mixture of 1% NaOH and acetone in a 3:4 ratio (%v/v) and placed inside the hot air oven at 60 °C for around 1 h as followed from the protocol reported by Li et al. (2016). The dry and de-pigmented algal biomass was added to 15 mL of chloroform:methanol (2:1 v/v) solvent mixture and homogenized for 10 min for rupturing the algal cell mass (Mandotra et al. 2014). After homogenization, the heterogeneous mixture was centrifuged at 10,000 rpm for 15 min to separate supernatant containing the lipids and the pellet containing the algal debris. The supernatant was collected in a beaker, and the solvent part was removed inside a hot air oven at 60 °C. The lipid part was left in the beaker. The lipid content of algal mass was determined using the following equation:
where Lf is the mass of empty beaker + extracted lipid (g), Li is the mass of empty beaker (g), and X is the weight of algal mass from which the lipid is extracted.
Lipid production rate (PL ) has been determined as follows:
Results and discussions
Moisture content and elemental composition of algal mass
The moisture content of algal mass is 90% (w/w). The weight fractions of C, H, and N in dry algal mass are 0.35, 0.0575, and 0.0669 respectively.
Effect of inorganic and organic carbon sources on growth of algal biomass
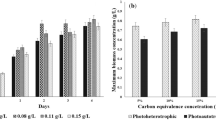
In Fig. 2, experimental time histories of algal biomass concentration have been provided using gas phase concentration of CO2 (5–20% v/v) as a parameter. Similar plots were constructed for photoheterotrophic growth using equivalent glycerol concentrations, as described in the “Materials and methods” section, as a parameter. From the analysis of the figure, it is clear that the span of exponential phase is 2 days for photoautotrophic growth using inorganic source of C-atom from CO2. On the other hand, the exponential phase for photoheterotrophic growth extends up to 4 days. The short length of lag phase ascertains that the blue-green alga Leptolyngbya subtilis JUCHE1 is well-adapted to the medium of both photoautotrophic and photoheterotrophic growth. The batch experiments were conducted in 150 mL and 30 mL respectively during photoauto- and photoheterotrophy, in once-charged mode. Therefore, the nutrients got depleted due to their high rate of uptake, as evident from the sharply increasing trend of growth curve over 2 days and the appearance of plateau on either the 3rd or the 4th day (96 h) for both modes of growth. Although in many cases, a long period of growth is reported for algae, early saturation has been observed in case of growth of microalga Chlorella sorokiniana, cyanobacteria Aphonothece microscopica Naegeli, Chlorococcum littorale, and so on ( Leite et al. 2015; Li et al. 2014; Jacob-Lopes et al. 2008; Ota et al. 2009). It is also noticed that the maximum value of biomass concentration increases with the increase of gas phase CO2 concentration up to 15%(v/v) used for saturation of aqueous medium and their glycerol equivalents. Beyond 15%, the maximum value decreases. Similar trends have been observed for time history plots. The time history plot obtained at higher gas phase concentration of CO2 and its glycerol equivalent lies above its counterpart obtained at lower concentration up to 15% with the exception of the plots obtained at 20% CO2 and the glycerol equivalent.
In Table 3, the maximum values of biomass concentration and the productivity has been provided corresponding to the gas phase CO2 concentration and their glycerol equivalents. The culture time corresponding to the maximum values has also been mentioned for each case. The maximum values of biomass concentration and productivity of 0.7286 gL−1 on the 3rd and 0.1857 gL−1d−1 on the 1st day respectively have been obtained at 15% (v/v) gas phase CO2 concentration for photoautotrophic growth. A report on experimental studies on Spirulina sp. LEB18 algal strain using different types of carbon dioxide sources, namely, CO2 from flue gas and commercial CO2 further indicated maximum biomass productivity of 0.04 ± 0.01 gL−1d−1 and 0.15 ± 0.04 gL−1d−1 respectively (Duarte et al. 2017). Another article reported the maximum biomass productivity of 0.167.23 ± 0.00689 gL−1d−1 for a batch type experimental study on Scenedesmus abundans under photoautotrophic condition using CO2 from air as carbon source and KNO3 as inorganic nitrogen source (0.0 gL−1, 0.08 gL−1, 0.16 gL−1, 0.24 gL−1, 0.32 gL−1, and 0.4 gL−1) under photo-illumination and temperature of 8 kLux and 28 °C ± 2 °C respectively (Mandotra et al. 2014). It appears that as the CO2 concentration in air is much lower than the concentration level used under the present study and the aqueous medium is not saturated with CO2, the maximum biomass productivity is also less than the present value.
For photoheterotrophic growth with glycerol concentration equivalent to 15% CO2 in the feed gas, the maximum biomass concentration and productivity are respectively 0.817 gL−1 on the 4th day and 0.2733 gL−1d−1 on the 1st day. From the literature review the maximum biomass productivity using treated waste glycerol at 5 gL−1, 10 gL−1, and 20 gL−1 are 0.192 ± 0.002 gL−1d−1, 0.196 ± 0.009, and 0.198 ± 0.009 gL−1d−1 respectively (Abomohra et al. 2018). This clearly indicates that even at much higher concentrations of glycerol compared with the present case, the biomass productivity of algal strain Scenedesmus obliquus is lower than that (0.2733 gL−1d−1) of Leptolyngbya subtilis JUCHE1 under study (Abomohra et al. 2018). One research study on different newly isolated strains of Chlorella using glycerol at 20 mM under constant illumination of 14 kLux indicates a variation of biomass concentration in the range of 0.4–0.8 g/L (Leite et al. 2015). Therefore, the transformation of carbon to algal biomass is achieved more efficiently by Leptolyngbya subtilis JUCHE1 in comparison with other algal strains. In another study on Chlorella minutissima using 9.02–25.2 gL−1 C, supplied from glycerin, i.e., 0.752–2.1 g-atom C/L and hence 250–700 mM glycerol, the biomass concentration has been reported to vary from 5.31 to 7.28 gL−1 (Yang et al. 2011). Narayan et al. (2005) reported some observations on a cyanobacterium Spirulina platensis CFTRI grown on both bicarbonate and glycerol carbon sources. In case of photoheterotrophic growth mode, 2.5 mM of glycerol concentration was used for the production of biomass, lipid, and pigments (chlorophyll, phycocyanin, etc.). It was reported that 0.1157 ± 0.0049 g/L of maximum biomass concentration was achieved (Narayan et al. 2005).
It is also noticed that the maximum value of biomass concentration increases with the increase of gas phase CO2 concentration up to 15% (v/v) used for saturation of aqueous medium and their glycerol equivalents. Beyond 15%, the maximum value decreases. Similar trends have been observed for time history plots. The time history plot obtained at higher gas phase concentration of CO2 and its glycerol equivalent lies above its counterpart obtained at lower concentration up to 15% with the exception of the plots obtained at 20% CO2 and the glycerol equivalent. The values of initial specific growth rate (μ, d−1), using the data of t = 0 and t = 1d, and % carbon capture (CC) for each concentration of CO2 and equivalent glycerol, respectively calculated using Eqs. 9 and 13 have been provided in Table 4.
The comparison of values of μ and CC obtained for CO2 and equivalent glycerol reveals that the growth rate and carbon capture are always higher for glycerol compared with its CO2 equivalent regardless of the concentration. The carbon capture data shows that this strain of blue-green alga Leptolyngbya subtilis JUCHE1 has a high potential of carbon sequestration. This may be due to the fact that this strain has been isolated from a water source of a power plant which environment is inherently rich in CO2. The results also suggest that for Leptolyngbya subtilis JUCHE1 glycerol is a favorable carbon substrate besides CO2 which is reflected in ultimate higher concentration of biomass obtained in the heterotrophic system, as described above. This can be because of the difference in the mechanisms of the uptake of carbon under photoautotrophic and mixotrophic growth as depicted in Fig. 3.
Cyanobacteria usually consists three bicarbonate transporters which are responsible for the trans-membrane passage of HCO3− into the cytoplasm. Cytoplasmic bicarbonate diffuses to carboxysome, containing carbonic anhydrase and enzyme 1,5 bisphosphate carboxylase (RubisCO). CO2 is generated through the reaction catalyzed by carbonic anhydrase. OH− ion is transported outside the cell and H+ is sequestered inside the thylakoid. CO2 is assimilated in the photosynthetic system using a RubisCO enzyme and biomass is formed (Zeebe and Wolf-Gladrow 2001; Badger et al. 2006). The higher biomass productivity under mixotrophic condition can be attributed to simultaneous assimilation of glycerol as carbon source and re-utilization of CO2 liberated through photosynthesis (Aubert et al. 1994). The literature review suggests that under photoheterotrophic/mixotrophic growth, there is a possibility of algal cell concentration and volumetric productivity (Li et al. 2014; Wang et al. 2016; Heredia-Arroyo et al. 2011). It was proposed that ATP formed in the photochemical reactions accelerated the anabolism using organic carbon source as glucose in the mixotrophic culture of Euglena gracilis (Yamane et al. 2001).
Glycerol consumption
The time histories of conversion of glycerol have been plotted in Fig. 4 using initial glycerol concentration as a parameter.
As per expectation, the conversion of glycerol increases with time at all values of initial concentration. The trends are in agreement with those of time histories of biomass concentration under heterotrophic growth. Similar trend was observed by Abomohra et al. (2018), during their studies on Scenedesmus obliquus using glycerol as the carbon source (Abomohra et al.2018).
Lipid production in both photo–auto and heterotrophic mode of cultivation
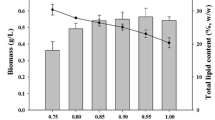
From the graphical representation (Fig. 5a) of time history of lipid content (% w/w) in case of photoautotrophic growth with gas phase CO2 concentration of 5–20% (v/v) as parameter, it is clear that the lipid content increases with increase in CO2 concentration up to 15% beyond which it decreases up to 20% CO2. As revealed from Fig. 5b, the cultivation of L. Subtilis JUCHE1 under photoheterotrophic condition using equivalent glycerol concentrations shows a reverse trend with the maximum lipid content at the minimum glycerol concentration equivalent to 5% (v/v).
Qualitative analysis of the extracted fat was performed using GC-MS analyzer (Thermo-Scientific). By following the proper methodology, the lipid was extracted, filtered, and injected for analysis. The process has been carried out using TR-Wax column. Finally, the compounds were identified from the MS library. The following compounds are as shown below (Table 5):
C16 fatty acids were also detected to be predominant in the lipid produced by the cyanobacteria Spirulina platensis. The production of C15-C19 fatty acids by cyanobacteria Nostoc muscorum, Trichodesmium erythaeum, and C15-C-C18 alcohols in case of mutant strains of cyanobacteria, Synechocystis PCC6803, Synechococcus elongatus PCC7942, and Anabaena sp. PCC7120, having an overexpression of Acetyl CoA Carboxylase (Accase) for inhibiting fatty acid biosynthesis also prove the same chain length pattern of cyanobacteria (Tan et al. 2011). As suggested by Choi et al. (2014), DHA was extracted using acid-catalyzed hot-water extraction treatment process. The algal biomass was initially treated with different concentrations of H2SO4 in the range of 0.05–3.00% w/w. The reaction temperatures were 80, 100, and 120 °C. After that, the lipid was extracted from the acid-treated biomass, using solvent extraction method using a mixture of methanol and hexane in 7:3 ratios (v/v) and chloroform, methanol solution in 2:1 ratio (v/v) respectively (Choi et al. 2014). In this lipid sample, DHA and other polyunsaturated fatty acids were qualitatively detected using GC-MS. More detailed quantitative analysis of lipid is, however, required and will be conducted in the future. In the overall range of carbon concentration, the lipid content obtained with organic carbon source (glycerol) is always higher than that obtained with inorganic one (CO2). The maximum values of lipid concentration obtained under photoautotrophic and photoheterotrophic conditions are 12.5% and 56.3% respectively. It is also quite interesting to note that the lipid accumulation does not decline after 4 days of incubation in photoheterotrophic mode in contrast to the photoautotrophic mode. In the photoautotrophic mode, the algae utilize CO2 in the presence of light to produce energy for metabolic needs using Photo System I. The excess of carbon is stored as lipid in the cells. Since glycerol is easily available to the cells, the majority of carbon assimilated is stored in form of lipids (Jajesniak et al. 2014; Khanra et al. 2017). Therefore, lipid content of algal strains in photoheterotrophic mode is much higher as compared with the photoautotrophic mode, as observed in the Fig. 2. Similar observations have been reported by previous researchers (Yang et al. 2011). Moreover, the CO2 produced during glycerol assimilation is assimilated through photosynthesis, as shown in Fig. 3 (Li et al. 2014). Some typical reported values of lipid content of different algal strains under photoautotrophic and photoheterotrophic conditions have been provided in Table 6.
From the analysis of Table 6, it is revealed that similar to the observation with the present cyanobacteria, the lipid accumulation is also favored for photoheterotrophic/mixotrophic growth of different algae using glycerol as the carbon source. Wang et al. (2016) reported a study where the lipid content obtained under mixotrophic condition is many folds higher than that obtained under autotrophic condition. Similar observation has been reported by Li et al. (2014) during their studies on Chlorella sokinianana using glucose under mixotrophic condition. Besides refixation of CO2 through photosynthesis, the light-dependent non-cyclic electron flow through PSI and PSII responsible for the formation of ATP and NADPH has been identified as some of the key factors responsible for enhanced lipid production during mixotrophic growth (Li et al. 2014). However, the exact mechanism behind the enhanced lipid content needs more focused studies in this area.
Comparison of lipid content under nitrogen-stressed autotrophic growth
As reported by the present group, nitrogen stress is also beneficial from the perspective of lipid accumulation by the same blue-green alga Leptolyngbya subtilis JUCHE1 (Das et al. 2020). During a study by the present group varying the concentration of NaNO3 in the range of 1–2.5 g/L, keeping the CO2 concentration fixed at 15% (v/v), it was observed that with the increase of NaNO3 up to 2 g/L, there was an increasing trend of biomass concentration. In contrary, an ever-increasing trend of lipid content with the declining ratio of N:C was reported. The lipid content of biomass was reported to be the highest 53.87% (w/w) when the stress of nitrogen was the maximum, i.e., at 1 g/L NaNO3. Since the productivity of biomass suffers at low values of N:C, a 2-stage strategy at 15% (v/v) CO2 with the first one producing sufficient quantity of biomass under nitrogen sufficient condition (2 g/L), followed by another stage of growth under nitrogen stress (1 g/L) was recommended to achieve high lipid productivity using Leptolyngbya subtilis JUCHE1 (Drapcho et al. 2008; Das et al. 2020). The value of lipid content at minimum N:C ratio is comparable with the lipid content (56.3%) obtained under photoheterotrophic condition using glycerol at concentration level equivalent to 5% (v/v) CO2.
Conclusions
The present study focuses on the trends of biomass productivity and lipid productivities of the cyanobacterial strain L. subtilis JUCHE1, under photoautotrophic as well as photoheterotrophic culture conditions. In course of the experiment, it was observed that under similar incubation conditions, the algal strain grown in glycerol-amended media exhibits higher lipid content in compared with the CO2-assisted growth supplying equal amount of carbon. The lipid content obtained under photoheterotrophic mode is approximately 4.66-fold higher than that of the photoautotrophic mode. The results obtained in the present study have also been compared with the literatures data and similar trends have been observed. The comparison revealed that the strain L. subtilis JUCHE1 is able to produce higher biomass as well as lipid in comparison with some other algal strains studied earlier. Significantly higher lipid production by L. subtilis JUCHE1 from glycerol compared with other algal strains also confirms the oleaginous nature of this algal strain. Since the power plant blue-green alga, Leptolyngbya subtilis JUCHE1, can produce lipid at a high rate both under photoautotrophic condition with nitrogen stress and under photoheterotrophic condition using glycerol, a zero-effluent biodiesel plant can be integrated with its cultivation process. The integrated process can be housed in a power plant, and the algal reactor can be primarily operated under autotrophic condition following a 2-stage strategy. The algal lipid can be converted to biodiesel through transesterification. Since glycerol is produced as a byproduct of transesterification process, it can further be utilized by the same alga under photoheterotrophic condition. Through this holistic approach, a very efficient sequestering of CO2, emitted in the power plant, can be possible for the production of an energy vector, namely biodiesel. Pigments and other biochemicals can also be recovered from the same algal strain. Hence, a complete biorefinery can actually be integrated with a CO2-emitting power plant which will ensure sequestration of greenhouse gas and simultaneous production of biofuels and biochemicals.
References
Abomohra AEF, Eladel H, El-Esawi M, Wang S, Wang Q, He Z, Hanelt D (2018) Effect of lipid-free microalgal biomass and waste glycerol on growth and lipid production of Scenedesmus obliquus: innovative waste recycling for extraordinary lipid production. Bioresour Technol 249:992–999. https://doi.org/10.1016/j.biortech.2017.10.102
Aubert S, Gout E, Bligny R, Douce R (1994) Multiple effects of glycerol on plant cell metabolism. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem 269(34):21420–21427 https://www.jbc.org/content/269/34/21420.full.pdf
Badger MR, Price GD, Long BM, Woodger FJ (2006) The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J Exp Bot 57(2):249–265. https://doi.org/10.1093/jxb/eri286
Bondioli P, Della Bella L (2005) An alternative spectrophotometric method for the determination of free glycerol in biodiesel. Eur J Lipid Sci Technol 107(3):153–157. https://doi.org/10.1002/ejlt.200401054
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102(1):71–81. https://doi.org/10.1016/j.biortech.2010.06.159
Choi SA, Jung JY, Kim K, Lee JS, Kwon JH, Kim SW, Park JY (2014) Acid-catalyzed hot-water extraction of docosahexaenoic acid (DHA)-rich lipids from Aurantiochytrium sp. KRS101 Bioresour Technol 161(469):472. https://doi.org/10.1016/j.biortech.2014.03.153
Chowdhury R, Das S, Ghosh S (2018) CO2 Capture and utilization (CCU) in coal-fired power plants: prospect of in-situ algal cultivation. In Sustainable energy technology and policies, Springer, Singapore, pp 231-254. https://doi.org/10.1007/978-981-10-7188-1_10.
Das S, Mahato S, Chowdhury R (2020) Studies on growth kinetics and lipid accumulation of a power plant algae: L. Subtilis JUCHE1 by variation of nitrogen concentrations (NaNO3). 9th ICONSWM-CE2019, 25:20.
Drapcho CM, Nhuan NP, Walker T H (2008) Biofuels engineering process technology. McGraw-Hill, New York.
Duarte JH, de Morais EG, Radmann M, Costa JAV (2017) Biological CO2 mitigation from coal power plant by Chlorella fusca and Spirulina sp. Bioresour Technol 234(472):475. https://doi.org/10.1016/j.biortech.2017.03.066
Eloka-Eboka AC, Inambao FL (2017) Effects of CO2 sequestration on lipid and biomass productivity in microalgal biomass production. Appl Energy 195(1100):1111. https://doi.org/10.1016/j.apenergy.2017.03.071
Heredia-Arroyo T, Wei W, Ruan R, Hu B (2011) Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 35(5):2245–2253. https://doi.org/10.1016/j.biombioe.2011.02.036
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252. https://doi.org/10.1016/j.biortech.2011.11.133
Jajesniak P, HEMO A, Wong TS (2014) Carbon dioxide capture and utilization using biological systems: opportunities and challenges. J Bioprocess Biotech 4(155):2. https://doi.org/10.4172/2155-9821.1000155
Jacob-Lopes E, Lacerda LMCF, Franco TT (2008) Biomass production and carbon dioxide fixation by Aphanothece microscopica Nägeli in a bubble column photobioreactor. Biochem Eng J 40(1):27–34. https://doi.org/10.1016/j.bej.2007.11.013
Khanra A, Vasistha S, Rai MP (2017) Glycerol on lipid enhancement and fame characterization in algae for raw material of biodiesel. Int J Renew Energy Res 7(4):1970–1978 https://www.ijrer.com/index.php/ijrer/article/view/6367
Kim S, Park JE, Cho YB, Hwang SJ (2013) Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour Technol 144:8–13. https://doi.org/10.1016/j.biortech.2013.06.068
Li T, Xu J, Wu H, Wang G, Dai S, Fan J, He H, Xiang W (2016) A saponification method for chlorophyll removal from microalgae biomass as oil feedstock. Mar Drugs 14(9):162. https://doi.org/10.3390/md14090162
Leite GB, Paranjape K, Abdelaziz AE, Hallenbeck PC (2015) Utilization of biodiesel-derived glycerol or xylose for increased growth and lipid production by indigenous microalgae. Bioresour Technol 184:123–130. https://doi.org/10.1016/j.biortech.2014.10.117
Li T, Zheng Y, Yu L, Chen S (2014) Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 66:204–213. https://doi.org/10.1016/j.biombioe.2014.04.010
Li J, Tang X, Pan K, Zhu B, Li Y, Ma X, Zhao Y (2020) The regulating mechanisms of CO2 fixation and carbon allocations of two Chlorella sp. strains in response to high CO2 levels. Chemosphere 125814. https://doi.org/10.1016/j.chemosphere.2020.125814
Mandotra SK, Kumar P, Suseela MR, Ramteke PW (2014) Fresh water green microalga Scenedesmus abundans: a potential feedstock for high quality biodiesel production. Bioresour Technol 156:42–47. https://doi.org/10.1016/j.biortech.2013.12.127
Narayan MS, Manoj GP, Vatchravelu K, Bhagyalakshmi N, Mahadevaswamy M (2005) Utilization of glycerol as carbon source on the growth, pigment and lipid production in Spirulina platensis. Int J Food Sci Nutr 56(7):521–528. https://doi.org/10.1080/09637480500410085
Pradhan L, Bhattacharjee V, Mitra R, Bhattacharya I, Chowdhury R (2015) Biosequestration of CO2 using power plant algae (Rhizoclonium hieroglyphicum JUCHE2) in a Flat Plate Photobio-Bubble-Reactor–Experimental and modeling. Chem Eng J 275:381–390. https://doi.org/10.1016/j.cej.2015.04.037
Ota M, Kato Y, Watanabe H, Watanabe M, Sato Y, Smith RL Jr, Inomata H (2009) Effect of inorganic carbon on photoautotrophic growth of microalga Chlorococcum littorale. Biotechnol Prog 25(2):492–498. https://doi.org/10.1002/btpr.123
Sachdeva N, Kumar GD, Gupta RP, Mathur AS, Manikandan B, Basu B, Tuli DK (2016) Kinetic modeling of growth and lipid body induction in Chlorella pyrenoidosa under heterotrophic conditions. Bioresour Technol 218:934–943. https://doi.org/10.1016/j.biortech.2016.07.063
Sander R (2015) Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos Chem Phys 15(8). https://pdfs.semanticscholar.org/d4fc/8ef222674503cde2058657992646a7809197.pdf
Taher H, Al-Zuhair S, Al-Marzouqi A, Haik Y, Farid M (2014) Growth of microalgae using CO2 enriched air for biodiesel production in supercritical CO2. Renew Energy 82:61–70. https://doi.org/10.1016/j.renene.2014.08.013
Tan X, Yao L, Gao Q, Wang W, Qi F, Lu X (2011) Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab Eng 13(2):169–176. https://doi.org/10.1016/j.ymben.2011.01.001
Thimijan RW, Heins RD (1983) Photometric, radiometric, and quantum light units of measure: a review of procedures for interconversion. HortScience 18(6):818–822 https://www.plantgrower.org/uploads/6/5/5/4/65545169/light_conversion_paper_thimijan_1983_ocr.pdf
Wang YZ, Hallenbeck PC, Leite GB, Paranjape K, Huo DQ (2016) Growth and lipid accumulation of indigenous algal strains under photoautotrophic and mixotrophic modes at low temperature. Algal Res 16:195–200. https://doi.org/10.1016/j.algal.2016.03.017
Yang J, Rasa E, Tantayotai P, Scow KM, Yuan H, Hristova KR (2011) Mathematical model of Chlorella minutissima UTEX2341 growth and lipid production under photoheterotrophic fermentation conditions. Bioresour Technol 102(3):3077–3082. https://doi.org/10.1016/j.biortech.2010.10.049
Yamane YI, Utsunomiya T, Watanabe M, Sasaki K (2001) Biomass production in mixotrophic culture of Euglena gracilis under acidic condition and its growth energetics. Biotechnol Lett 23(15):1223–1228. https://doi.org/10.1023/A:1010573218863
Zheng Y, Chi Z, Lucker B, Chen S (2012) Two-stage heterotrophic and phototrophic culture strategy for algal biomass and lipid production. Bioresour Technol 103(1):484–488. https://doi.org/10.1016/j.biortech.2011.09.122
Zeebe RE, Wolf-Gladrow D (2001). CO2 in seawater: equilibrium, kinetics, isotopes (No. 65). Gulf Professional Publishing. http://geosci.uchicago.edu/~kite/doc/Zeebe_CO2_In_Seawater_Ch_1.pdf
Funding
The authors received financial support from the RUSA 2.0 scheme of Jadavpur University in order to conduct the experimental studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Das, S., Nath, K. & Chowdhury, R. Comparative studies on biomass productivity and lipid content of a novel blue-green algae during autotrophic and heterotrophic growth. Environ Sci Pollut Res 28, 12107–12118 (2021). https://doi.org/10.1007/s11356-020-09577-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09577-4