Abstract
A new lectin from the marine red alga Meristiella echinocarpa (MEL) was isolated and biochemically characterized. MEL is a monomeric protein of 28 kDa with specificity for yeast mannan. Hemagglutination activity of MEL was stable between pH 5 and 10, temperatures up to 50 °C, and neither EDTA nor divalent ions affected it. The complete amino acid sequence of MEL was determined through a combination of tandem mass spectrometry and DNA cloning. As a new member of the OAAH-lectin family, the primary structure of MEL consists of 267 amino acid residues distributed in four tandem repeat domains, sharing at least 48% of identity. Theoretical secondary structure of MEL was composed of 3% α-helix, 40% β-sheet, 19% β-turn, and 38% coil. Melting temperatures of the lectin in the absence and presence of mannan were 54 and 61 °C, respectively. Furthermore, MEL was able to recognize and agglutinate pathogenic bacterial strains, such as multidrug-resistant Salmonella and Vibrio alginolyticus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lectins are carbohydrate-binding proteins. Although they are found in all organisms, attention has been focused almost exclusively on land plants, whereas other groups, such as marine algae and invertebrates, were often neglected by researchers (Vasta and Ahmed 2008). Interest in algae lectins began 50 years ago. So far, the number of studies with these lectins has grown at a slow pace, mainly owing to the complexity of their isolation caused by the presence of pigments, low concentration of lectins in extracts, creating difficulty in obtaining sufficient material for study, and presence of isoforms in the preparations (Rogers and Hori 1993; Calvete et al. 2000; Nascimento-Neto et al. 2012; Chernikov et al. 2013).
Recently, marine red algae have attracted some attention as potential sources of new lectins with antiviral and anti-HIV activities through binding to viral envelope glycoproteins based on their specificity for high-mannose N-glycan (Sato et al. 2011; Hirayama et al. 2016). For instance, griffithsin (GRFT) isolated from Griffithsia sp. and KAA isolated from Kappaphycus alvarezii showed potent anti-HIV activity (Mori et al. 2005; Hirayama et al. 2016). In addition, other lectins, such as ESA-2 from Eucheuma serra, EDA from Eucheuma denticulatum, and KSA from Kappaphycus striatum, also show affinity for high-mannose N-glycans (Hori et al. 2007; Hung et al. 2011, 2015a, b).
With the exception of GFRT, which is a jacalin-like lectin (Mori et al. 2005), most marine red algae lectins with specificity for N-glycans have four tandem repeat domains of about 67 amino acids each, showing similarity to a 13-kDa lectin isolated from the cyanobacterium Oscillatoria agardhii (OAA). Moreover, theses lectins showed identity with lectins isolated from such bacteria as PFL, MBHA, and BOA from Pseudomonas fluorescens, Myxococcus xanthus, and Burkholderia oklahomensis, respectively. OAA was the first lectin of this group to show anti-HIV activity through binding to gp120. Moreover, the tridimensional structure of OAA has been determined (Koharudin et al. 2011); therefore, many authors have termed this group as the OAAH (O. agardhii agglutinin homologs) family.
Meristiella echinocarpa (Areschoug) Cheney is a marine red alga found along the northeastern Brazilian coast. Ainouz et al. (1992) showed that M. echinocarpa aqueous extract caused high agglutination of rabbit, goat, and chicken erythrocytes. Now, we have isolated and characterized a lectin from M. echinocarpa (MEL) with agglutination activity against multidrug-resistant Salmonella and Vibrio alginolyticus. Furthermore, its primary structure was determined by a combination of MS/MS and DNA cloning.
Material and methods
Collection
Specimens of Meristiella echinocarpa were collected in the intertidal zone at Paracuru Beach, Ceará, Brazil. The algae were transported in plastic bags to the lab where they were cleaned from epiphytes, washed with water, and freeze-dried. A small portion of the alga was stored at − 80 °C for DNA extraction. All collections were authorized through our registration with SISBIO (Sistema de Autorização e Informação em Biodiversidade, ID: 33913-8) and SISGEN (Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado, ID: AC14AF9).
Lectin purification
Freeze-dried algae were triturated until a fine powder was obtained. The algae powder was suspended in twenty volumes of cold ethanol 70% v/v and maintained under agitation for 30 min at room temperature. The mixture was then filtered, and solid residues were re-extracted with 70% v/v ethanol, as described above, two more times. After that, solid residues recovered by filtration were suspended in ten volumes of 20% v/v ethanol and maintained under agitation for 4 h at 4 °C. The mixture was filtrated and centrifuged at 8000×g for 20 min at 4 °C, and supernatant (crude extract) was stored at − 20 °C.
Crude extract was dialyzed against sodium phosphate buffer, 20 mM, pH 7 (PB), and loaded onto a DEAE (Diethylaminoethyl)-Sephacel column (1.0 × 7.0 cm), previously equilibrated with PB. Unbound proteins were washed with equilibrium buffer (fraction D1), and retained proteins were eluted in two steps: PB containing 0.5 M NaCl (fraction D2) and PB containing 1 M NaCl (fraction D3). The chromatography was monitored at 280 nm, 3-mL fractions were collected, and flow rate was maintained at 1.5 mL min−1.
Biochemical properties of MEL
Hemagglutination activity and inhibition by sugars
Hemagglutination activity and inhibition assays were conducted as described by Sampaio et al. (1998), using human (A, B, and O) and rabbit erythrocytes in their native form and treated with the proteases trypsin and papain.
The following sugars and glycoproteins were used in the inhibition assay: d-xylose, d-ribose, l-fucose, l-arabinose, l-rhamnose, d-galactose, d-mannose, d-glucose, d-glucosamine, d-galactosamine, N-acetyl- d-glucosamine, N-acetyl- d-galactosamine, N-acetyl-d-manosamine, d-galacturonic acid, d-fructose, d-sucrose, d-melibiose, α-d-lactose, β-d-lactose, d-lactulose, d-maltose, d-raffinose, mannan from Saccharomyces cerevisiae (yeast mannan), methyl-α-d-galactopyranoside, methyl-β-d-galactopyranoside, methyl-β-d-thiogalactose, phenyl-β-d-galactopyranoside, 4-nitrophenyl-α-d-galactopyranoside, 4-nitrophenyl-β-d-galactopyranoside, 2-nitrophenyl-β-d-galactopyranoside, bovine fetuin, and type 2 porcine stomach mucin (PSM).
The effects of pH, temperature, EDTA (ethylenediaminetetraacetic acid) and divalent cations on hemagglutination activity were evaluated, as described in pre-established methods (Sampaio et al. 1998).
Carbohydrate content
Neutral carbohydrate content in MEL was estimated as described by Dubois et al. (1956), using lactose as standard.
Molecular mass determination
Homogeneity and molecular mass of MEL were estimated by SDS-PAGE (Laemmli 1970) in the presence and absence of 2-mercaptoethanol (2-ME). LMW-SDS Marker kit (GE Healthcare, UK) was used as molecular weight marker.
Native molecular mass of MEL was estimated by gel filtration chromatography in BioSuite 250, using a 5-μm HR column coupled to an Acquity UPLC system (Waters Corp, USA). Chromatography was performed in sodium phosphate buffer 20 mM, pH 7, containing 0.15 M NaCl (PBS) at a flow rate of 0.4 mL min−1. The column was previously calibrated with a gel filtration marker kit for protein weights between 29 and 700 kDa (Sigma-Aldrich, USA).
Average molecular mass of MEL was determined by MALDI-ToF on an Autoflex III mass spectrometer (Bruker Daltonics, Germany), using matrix solution (10 mg mL−1 of CHCA (α-cyano-4-hydroxycinnamic acid) acetonitrile, water, and TFA (trifluoroacetic acid), 50:47:3% v/v). The spectra were acquired in linear positive mode and processed with Flex Analysis 3.4 software (Bruker Daltonics, Germany).
Primary structure determination
Tandem mass spectrometry (MS/MS)
First, MEL was subjected to 1D-SDS PAGE as described above. Protein spots were digested with trypsin and chymotrypsin, and peptides were extracted from gel according to Shevchenko et al. (2006). Peptides were separated on a reverse phase C-18 nanocolumn (0.075 × 100 mm) coupled to a nanoAcquity system. The eluates were directly infused in a hybrid mass spectrometer (ESI-Q-ToF) (Synapt HDMS, Waters Corp, USA). The instruments’ parameters were adjusted as described by Carneiro et al. (2013). MS/MS spectra were manually interpreted, and sequenced peptides were searched online against NCBI and UniProt databanks.
DNA extraction and purification
Small pieces of algae were ground in dry ice until a fine powder was obtained. Powdered alga was incubated with CTAB extraction buffer (2% cetyl trimethylammonium bromide, 1.4 M NaCl, 0.1 M Tris-HCl, 20 mM EDTA, and 2% 2-ME; Sigma-Aldrich) containing 1% PVPP (polyvinylpolypyrrolidone) in water bath at 60 °C for 1 h. The extract was centrifuged at 9000×g for 20 min, and the supernatant was transferred to a clean tube. One volume of chloroform/isoamyl alcohol solution (24:1) was added, and the mixture was centrifuged at 9000×g for 20 min. The upper phase was collected, washed with chloroform/isoamyl alcohol solution, centrifuged, and transferred to a clean tube. A 0.3 volume of isopropyl alcohol was added; then, the mixture was incubated for 20 min at − 20 °C and centrifuged at 9000×g for 10 min. The supernatant was removed, and 1 mL of 70% cold ethanol was added to the precipitate. Again, the mixture was centrifuged at 9000×g for 10 min, and the supernatant was removed. The precipitate was dried at room temperature and then rehydrated with a small volume of nuclease-free water.
Primers, PCR, and product sequencing
Degenerate primers were designed based on the amino acid sequences obtained by MS/MS: VQNQWGG (primer-Upst-VQ-FW: 5′- GTI CAG AAT CAR TGG GGI GG -3′) and EGPIGF (primer-Down-Rv: 5′- RAA GCC GAT BGG WCC TTC -3′). PCR was performed with a 25-μL reaction mixture containing 100 ng of the genomic DNA, 0.4 μM of each primer, 0.4 mM of dNTP Mix (Promega, USA), and 1 U of Platinum Taq DNA polymerase (Invitrogen, USA) in 1X PCR buffer with 3 mM of MgCl2. The amplification protocol included an initial denaturation step at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 60 s, extension at 72 °C for 60 s, and the final extension step at 72 °C for 5 min.
The PCR product was purified by the PureLink Quick Gel Extraction Kit (Invitrogen). Then, the PCR product was cloned into pGEM-T Easy Vector, transformed into Escherichia coli strain DH5α (Novagen, Brasil), and screened with blue-white selection in LB agar containing 100 μg mL−1 ampicillin (Thermo Scientific, USA), 0.5 mM IPTG (isopropyl β-d-1-thiogalactopyranoside; Thermo Scientific), and 80 μg mL−1 of X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Thermo Scientific). The cloning was performed in a biosafety laboratory certified in accordance with governmental requirements (CQB: R007-2016).
Recombinant plasmids were extracted by the illustra plasmid Prep Mini Spin Kit (GE Healthcare, UK) and confirmed by restriction digestion with EcoRI (Promega). Finally, constructions were sequenced in the MegaBACE automatic sequencer (GE Healthcare), using primers containing the T7 (5′- TAA TAC GAC TCA CTA TAG GG-3′) and SP6 promoter (5′- ATT TAG GTG ACA CTA TAG-3′) sequences.
Sequencing was performed with at least six clones from PCR cloning amplification to avoid any PCR errors. The reads were analyzed by the Phred-Phrap-Consed program. The contigs formed were translated into amino acids, using the ExPASy translation tool (https://web.expasy.org/translate/).
Circular dichroism
MEL (0.2 mg mL−1 in 20 mM phosphate buffer, pH 7.0, containing 100 mM NaCl) was placed in a rectangular quartz cuvette with 0.5 mm path length. Spectra were acquired at a scan speed of 50 nm min−1 with a bandwidth of 1 nm in a Jasco J-815 spectropolarimeter (Jasco International Co., Japan) connected to a peltier module with controlled temperature. The acquisitions were performed at 190–250 nm (far UV). The DICHROWEB web server (Whitmore and Wallace 2008) was used to perform analyses of secondary structure prediction.
The thermodynamic parameters of lectin folding and unfolding in the presence and absence of ligands were calculated by monitoring the changes in ellipticity at 214 nm as a function of temperature (Greenfield 2007). The lectin was evaluated in the presence and absence of yeast mannan (0.1 mg mL−1).
Antibacterial activity and agglutination assays
The following standard strains were used in tests: Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923, multidrug-resistant Salmonella ser. Brandeirup Lamap 18 (Lamap collection), Vibrio cholerae IOC 19582, V. parahaemolyticus IOC 18950, V. harveyi ATCC 14126, and V. alginolyticus ATCC 17749.
Antibacterial activity of MEL was performed in microtiter plates according to the broth dilution method as described by Balouiri et al. (2016). Bacterial inocula were prepared in tubes containing 0.85 and 1% NaCl solutions. Turbidity was adjusted to 0.5 according to the McFarland scale. Each well containing Mueller-Hinton broth received 50 μL of bacterial inoculum and 50 μL of MEL at 500, 250, 100, and 50 μg mL−1. Kanamycin A (Affymetrix USB products, USA) at 30 μg mL−1 was used as positive control, and bacterial inoculum without lectin was used as negative control. The procedure was performed in triplicate. The plates were incubated at 28 °C for 48 h, and then triphenyl tetrazolium chloride (TTC) (Sigma-Aldrich) was added at the final concentration of 0.5%. The reaction was incubated at 28 °C for 3 h. The change of color to red was considered negative activity.
The agglutination assay was conducted according to Melo et al. (2014). The lectin (100 μg mL−1) was incubated with bacteria in the presence and absence of yeast mannan at 100 μg mL−1. Results were observed after incubation for 1 h under a light microscope.
Results
Lectin purification
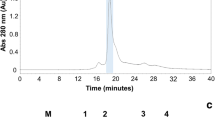
The crude extract of M. echinocarpa showed hemagglutination activity against trypsin-treated rabbit erythrocytes. After ion exchange chromatography, hemagglutination activity was concentrated in fraction D2. Fractions D1 and D3 showed residual activity (Fig. 1). The purification procedure increased by 6.4-fold the lectin activity compared to the crude extract, and MEL represented 75% of the total hemagglutination activity present in the crude extract (Table 1).
Ion exchange chromatography. Approximately 40 mL of dialyzed extract were applied onto a DEAE-Sephacel column equilibrated with phosphate buffer, pH 7. The column was washed with the equilibrium buffer, and two adsorbed fractions (D2 and D3) were eluted with 0.5 and 1 M of NaCl in the phosphate buffer
Biochemical properties of MEL
MEL could agglutinate only rabbit erythrocytes treated with papain (data not shown).
Simple sugars could not inhibit hemagglutination activity caused by MEL. However, the lectin was inhibited by yeast mannan with minimal inhibitory concentration (MIC) of 62.5 μg mL−1 (Table 2). Hemagglutination activity of MEL was stable between pH 5 and 10. Lectin activity was unaltered up to 50 °C, but after that, activity was gradually lost and then completely abolished at 70 °C (data not shown). The presence of EDTA, CaCl2, MnCl2, and MgCl2 did not affect hemagglutination activity caused by MEL.
The phenol-sulfuric acid method suggested that MEL is a glycoprotein with less than 2% of neutral sugar in its composition. In SDS-PAGE, MEL showed a broad band of 30 kDa in the presence and absence of 2-ME (Fig. 2). Under native conditions (gel filtration chromatography), MEL was determined to be a monomeric protein with relative molecular mass (Mr) of 21 kDa (data not shown).
MALDI-ToF analysis revealed a broad signal between 28,000 and 30,000 Da with maximal intensity at 28,907 Da (Fig. 2).
Amino acid sequence determination
The primary structure of MEL was determined by a combination of mass spectrometry and DNA cloning. The amplification product obtained by DNA cloning was approximately 400 bp. This DNA sequence encodes a polypeptide chain of 124 amino acids, which corresponds to an amino acid sequence between 4 and 127 residues. The amino acid sequences of peptides obtained by digestion with trypsin and chymotrypsin were sufficient to cover of the primary structure of MEL, in addition to confirming the deduced sequence obtained by DNA (Fig. 3).
The primary structure of MEL consisted of 267 residues, including one cysteine, which did not appear to be involved in disulfide bond formation. Three N-glycosylation sequences were found along the sequence, but no carbohydrates were found attached to the polypeptide backbone during MS or MS/MS analysis. Theoretical pI and Mr of MEL were 4.9 and 27,942 Da, respectively. The amino acid sequence of MEL was similar to several members of the OAAH-family (Fig. 4), including MPAs from Meristotheca papulosa [BAX08602.1; BAX08602.1], ASLs from Agardhiella subulata [BAX08599.1; BAX08598.1], KAA [BAU19431.1; BAU19430.1], KSA-2 [BAR91206.1], ESA-2 [P84331.1], EDA-2 [BAR91516.1], and SfL-1 and -2 from Solieria filiformis (Chaves et al. 2018).
Alignment of MEL and OAAH-lectin family. Alignment obtained by comparison of MEL, Meristotheca papulosa agglutinins (MPA-1 and -2), Agardhiella subulata lectins (ASL-1 and -2), Kappaphycus alvarezii agglutinins (KAA-1 and -2), K. striatum agglutinins (KSA-2), Eucheuma serra agglutinin (ESA), E. denticulatum agglutinin (EDA), and Solieria filiformis lectins (SfL-1 and -2). ESPript 3.0 was used as alignment tool
Circular dichroism
Native MEL exhibited minimum absorption at 207 and 217 nm in CD measurements (Fig. 5). As determined by the CONTIN prediction method, a general-purpose constrained regularization method for continuous distributions (Van Stokkum et al. 1990), the theoretical secondary structure of MEL was composed of 3% α-helix, 40% β-sheet, 19% β-turn, and 38% coil.
The protein-ligand complex (MEL-mannan) showed greater resistance to thermal denaturation than the lectin in the absence of ligand (Fig. 6). Melting temperatures (Tm) of the lectin in the absence and presence of mannan were 54 and 61 °C, respectively.
Antibacterial and agglutination assays
MEL showed no antibacterial activity against strains tested. However, MEL was able to agglutinate cells of V. alginolyticus and multidrug-resistant Salmonella. Agglutinations were not observed when the lectin was previously incubated with yeast mannan (Fig. 7). MEL showed no agglutination activity against E. coli, S. aureus, V. cholerea, V. parahaemolyticus, or V. harveyi.
Discussion
A new lectin isolated from the marine red alga M. echinocarpa (MEL) showed several characteristics common to other marine red algae lectins, such as Ca2+-independent activity, no inhibition for simple sugars, and relative stability to pH and temperature variation (Hori et al. 1990; Rogers and Hori 1993).
In marine red algae, at least three families of lectins can be considered. The first family is composed of lectins isolated from Hypnea japonica (HJA) and lectins from the genus Bryothamnion, BTL and BSL from B. triquetrum and B. seaforthii, respectively. This lectins show low molecular weight (~ 9 KDa), four conserved cysteines, specificity for complex carbohydrates, and similarity of sequence (Ainouz et al. 1995; Calvete et al. 2000; Hori et al. 2000; Nascimento-Neto et al. 2012). The second family includes lectins isolated from Hypnea musciformis (HML) and H. cervicornis (HCA), which, together, present 14 conserved cysteines, a complex post-translational processing with proteolytic cleavage and rebinding of chains by disulfide bond, and specificity for fucose cores (Nagano et al. 2002, 2005). Finally, the third family, OAAH, is represented by lectins with primary structure similar to OAA. Our finding strongly indicated that MEL could be grouped into this family. MEL showed identity with MPA-2 (97%), MPA-1 (93%), ASL-1 (84%), ASL-2 (83%), KAA-2 (84%), KSA-2 (84%), EDA-2 (84%), SfL-1 (78%), and SfL-2 (78%). Moreover, like other OAAH-family members, MEL has four tandem repeat domains, which shared at least 48% of identity. Interestingly, two short sequences are present in all domains: “NQWGGSSAPW” and “EGPIGF.” These sequences are also present in BOA and correspond to carbohydrate-binding regions (Whitley et al. 2013).
Sequence “EGPIGF” was chosen to design primers in the cloning step. However, owing to the repetition of this sequence in the four domains, several amplification products of varied lengths were obtained after PCR. Curiously, a fragment containing four domains was not observed among the amplification products. Therefore, MS/MS was applied to complete the primary structure of MEL, and results revealed the remainder of the sequence and confirmed the deduced DNA sequence.
MEL showed specificity similar to other OAAH-family members. Like MEL, Eucheuma and Kappaphycus lectins were inhibited by small amounts of yeast mannan (Kawakubo et al. 1999; Hung et al. 2011; Hirayama et al. 2016). Lectins from the OAAH-family exclusively recognize high-mannose N-glycans. Yeast mannan is a highly branched oligomannoside with α(1 ➔ 2)- and α(1 ➔ 3)-linked side chains attached to an α(1 ➔ 6)-linked backbone (Jones and Ballou 1969). Lectins from the OAAH-family have demonstrated interesting biological properties. For instance, ESA induced cell death against several cancer cell lines, such as colon cancer Colo201 cells and cervix cancer HeLa cells (Sugahara et al. 2001). SfLs showed in vitro effect against human breast cancer (Chaves et al. 2018), and KAA-2 inhibited infection of various influenza strains with EC50 of nanomolar levels (Sato et al. 2011).
ESA-2 and EDA-2 were able to inhibit growth of Vibrio vulnificus and V. alginolyticus, respectively (Liao et al. 2003; Hung et al. 2015a). EDA activity against V. alginolyticus was inhibited by yeast mannan. Similarly, MEL was able to recognize V. alginolyticus, but no agglutination effect was observed in the presence of yeast mannan, suggesting that mannoside, or mannoside-like structure(s), must be found on the bacterial surface, acting as receptors for lectins (Hung et al. 2015a). Additionally, the inhibition of MEL by mannan in the bacterial agglutination of multidrug-resistant Salmonella also suggested the presence of mannoside, or mannoside-like, structure(s) on its cell surface.
The bacterial agglutination displayed by some lectins resides in the recognition and interaction with polysaccharide or lipopolysaccharide on the bacterial cell surface, and this ability can be used to identify and distinguish microorganisms according to the different compounds exposed on their cell surfaces. Thus, lectins can be an alternative method for typing microorganisms with minimum specialized facilities. In particular, the agglutination assay is a rapid, inexpensive, reproducible, and simple assay to perform and a useful method for epidemiological studies and detection of bacteria (Ottensooser et al. 1974; Schaefer et al. 1979; Davidson et al. 1982; Khin et al. 2000; Templier et al. 2016).
In conclusion, we have isolated a new lectin from the OAA-family 25 years after the initial report of Ainouz et al. (1992), who demonstrated hemagglutination activity in aqueous extracts of the M. echinocarpa. MEL showed the ability to select and type pathogenic microorganisms.
References
Ainouz IL, Sampaio AH, Benevides NMB, Feritas ALP, Costa FHF, Carvalho MR, Pinheiro-Joventino F (1992) Agglutination of enzyme treated erythrocytes by Brazilian marine algal extract. Bot Mar 35:475–479
Ainouz IL, Sampaio AH, Freitas ALP, Benevides NMBB, Mapurunga S (1995) Comparative study on hemagglutinins from the red algae Bryothamnion seaforthii and Bryothamnion triquetrum. R Bras Fisiol Veg 7:15–19
Balouiri MM, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79
Calvete JJ, Costa FHF, Saker-Sampio S, Murciano MPM, Nagano CS, Cavada BS, Grangeiro TB, Ramos MV, Jr CB, Silveira SB, Freitas BT, Sampio AH (2000) The amino acid sequence of the agglutinin isolated from the red marine algae Bryothamnion triquetrum defines a novel lectin structure. Cell Mol Life Sci 57:343–350
Carneiro RF, Melo AA, Almeida AS, Moura RM, Chaves RP, Sousa BL, Nascimento KS, Sampaio SS, Lima JP, Cavada BS, Nagano CS, Sampaio AH (2013) H-3, a new lectin from the marine sponge Haliclona caerulea: purification and mass spectrometric characterization. Int J Biochem Cell Biol 45:2864–2873
Chaves RP, da Silva SR, Nascimento Neto LG, Carneiro RF, Coelho da Silva AL, Sampaio AH, Lopes de Sousa B, Cabral MG, Videira PA, Teixeira EH, Nagano CS (2018) Structural characterization of two isolectins from the marine red alga Solieria filiformis (Kützing) P.W. Gabrielson and their anticancer effect on MCF-7 breast cancer cells. Int J Biol Macromol 107(Pt A):1320–1329
Chernikov OV, Molchanova VI, Chikalovets IV, Kondrashina AS, Li W, Lukyanov PA (2013) Lectins of marine hydrobionts. Biochem Mosc 78:760–770
Davidson SK, Keller KF, Doyle RJ (1982) Differentiation of coagulase-positive and coagulase-negative staphylococci by lectins and plant agglutinins. J Clin Microbiol 15:547–553
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Greenfield NJ (2007) Determination of the folding of proteins as a function of denaturants, osmolytes or ligands using circular dichroism. Nat Protoc 1:2733–2741
Hirayama M, Shibata H, Imamura K, Takemasa Sakaguchi T, Hori K (2016) High-mannose specific lectin and its recombinants from a carrageenophyta Kappaphycus alvarezii represent a potent anti-HIV activity through high-affinity binding to the viral envelope glycoprotein gp120. Mar Biotechnol 18:144–160
Hori K, Miyazawa K, Ito K (1990) Some common properties of lectins from marine algae. Hydrobiologia 204/205:561–566
Hori K, Matsubara K, Miyazawa K (2000) Primary structures of two hemagglutinins from the marine red alga, Hypnea japonica. Biochim Biophys Acta 1474:226–236
Hori K, Sato Y, Ito K, Fujiwara Y, Iwamoto Y, Makino H, Kawakubo A (2007) Strict specificity for high-mannose type N-glycans and primary structure of a red alga Eucheuma serra lectin. Glycobiology 17:479–491
Hung LD, Sato Y, Hori K (2011) High-mannose N-glycan-specific lectin from the red alga Kappaphycus striatum (carrageenophyte). Phytochemistry 72:855–861
Hung LD, Hirayama M, Ly BM, Hori K (2015a) Purification, primary structure, and biological activity of the high-mannose N-glycanspecific lectin from cultivated Eucheuma denticulatum. J Appl Phycol 27:1657–1669
Hung LD, Hirayama M, Ly BM, Hori K (2015b) Biological activity, cDNA cloning and primary structure of lectin KSA-2 from the cultivated red alga Kappaphycus striatum (Schmitz) Doty ex Silva. Phytochem Lett 14:99–105
Jones GH, Ballou CE (1969) Studies on the structure of yeast mannan. J Biol Chem 244:1043–1051
Kawakubo A, Makino H, Ohnishi J, Hirohara H, Kanji H (1999) Occurrence of highly yielded lectins homologous within genus Eucheuma. J Appl Phycol 11:149–156
Khin MM, Hua JS, Ng HC, Wadström T, Ho B (2000) Agglutination of Helicobacter pylori coccoids by lectins. World J Gastroenterol 6:202–209
Koharudin LMI, Furey W, Gronenborn AM (2011) Novel fold and carbohydrate specificity of the potent anti-HIV cyanobacterial lectin. J Biol Chem 286:1588–1597
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680–683
Liao WR, Lin JY, Shieh WY, Jeng WL, Huang R (2003) Antibiotic activity of lectins from marine algae against marine vibrios. J Ind Microbiol Biotechnol 30:433–439
Melo AA, Carneiro RF, Silva WM, Moura RM, Silva GC, Sousa OV, Saboya JS, Nascimento KS, Saker-Sampaio S, Nagano CS, Cavada BS, Sampaio AH (2014) HGA-2, a novel galactoside-binding lectin from the sea cucumber Holothuria grisea binds to bacterial cells. Int J Biol Macromol 64:435–442
Mori T, O’Keefe BR, Sowder RC, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, McMahon JB, Boyd MR (2005) Isolation and characterization of griffithsin, a novel HIV inactivating protein, from the red alga Griffithsia sp. J Biol Chem 280:9345–9353
Nagano CS, Moreno FBMB, Bloch C, Prates MV, Calvete JJ, Sampaio SS, Farias WRL, Tavares TD, Nascimento KS, Grangeiro TB, Cavada BS, Sampaio AH (2002) Purification and characterization of a new lectin from red marine alga Hypnea musciformis. Protein Pept Lett 9:159–165
Nagano CS, del Sol FG, Cavada BS, Nascimento KSD, Nunes EV, Sampaio AH, Calvetea JJ (2005) Crystallization and preliminary X-ray diffraction analysis of HML, a lectin from the red marine alga Hypnea musciformis. Acta Cryst F 61:997–999
Nascimento-Neto LG, Carneiro RF, da Silva SR, da Silva BR, Arruda FVS, Carneiro VA, do Nascimento KS, Saker-Sampaio S, da Silva VA Jr, Porto ALF, Cavada BS, Sampaio AH, Teixeira EH, Nagano CS (2012) Characterization of isoforms of the lectin isolated from the red algae Bryothamnion seaforthii and its pro-healing effect. Mar Drugs 10:1936–1954
Ottensooser F, Nakamizo Y, Sato M, Miyamoto Y, Takizawa K (1974) Lectins detecting group C streptococci. Infect Immun 9:971–973
Rogers DJ, Hori K (1993) Marine algal lectins: new developments. Hydrobiologia 260/261:589–593
Sampaio AH, Rogers DJ, Barwell CJ (1998) A galactose-specific lectin from the red alga Ptilota filicina. Phytochemistry 48:765–769
Sato Y, Morimoto K, Hirayama M, Hori K (2011) High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. Biochem Biophys Res Commun 405:291–296
Schaefer RL, Keller KF, Doyle RJ (1979) Lectins in diagnostic microbiology: use of wheat germ agglutinin for laboratory identification of Neisseria gonorrhoeae. J Clin Microbiol 10:669–672
Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860
Sugahara T, Ohama Y, Fukuda A, Hayashi M, Kawakubo A, Kato K (2001) The cytotoxic effect of Eucheuma serra agglutinin (ESA) on cancer cells and its applications to molecular probe for drug delivery system using lipid vesicles. Cytotechnology 36:93–99
Templier V, Roux A, Roupioz Y, Livache T (2016) Ligands for label-free detection of whole bacteria on biosensors: a review. TrAC Trend Anal Chem 79:71–79
Van Stokkum IHM, Spoelder HJW, Bloemendal M, Van Grondelle R, Groen FCA (1990) Estimation of protein secondary structure and error analysis from CD spectra. Anal Biochem 191:110–118
Vasta GR, Ahmed H (eds) (2008) Animal lectins: a functional review. CRC Press, Boca Raton
Whitley MJ, Furey W, Kollipara S, Gronenborn AM (2013) Burkholderia oklahomensis agglutinin is a canonical two-domain OAA-family lectin: structures, carbohydrate binding, and anti-HIV activity. FEBS J 280:2056–2067
Whitmore L, Wallace BA (2008) Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers 89:392–400
Acknowledgments
This work was supported by the Brazilian agencies CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico), and FINEP (Financiadora de Estudos e Projetos). The authors thank CETENE by the availability of the mass spectrometer, and, especially, the authors are grateful to Dr. Julia Campos for MALDI-ToF experiments. AHS, CSN, and OVS are senior investigators of CNPq.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated in memory of Wladimir Ronald Lobo Farias.
Rights and permissions
About this article
Cite this article
Chaves, R.P., da Silva, S.R., da Silva, J.P.F.A. et al. Meristiella echinocarpa lectin (MEL): a new member of the OAAH-lectin family. J Appl Phycol 30, 2629–2638 (2018). https://doi.org/10.1007/s10811-018-1473-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1473-7