Abstract
Two different strains of microalgae, one raphidophyte and one dinoflagellate, were tested under different abiotic conditions with the goal of enhancing lipid production. Whereas aeration was crucial for biomass production, nitrogen deficiency and temperature were found to be the main abiotic parameters inducing the high-level cellular accumulation of neutral lipids. Net neutral lipid production and especially triacylglycerol (TAG) per cell were higher in microalgae (>200% in Alexandrium minutum, and 30% in Heterosigma akashiwo) under treatment conditions (25°C; 330 μM NaNO3) than under control conditions (20°C; 880 μM NaNO3). For both algal species, oil production (free fatty acids plus TAG fraction) was also higher under treatment conditions (57 mg L−1 in A. minutum and 323 mg L−1 in H. akashiwo). Despite the increased production and accumulation of lipids in microalgae, the different conditions did not significantly change the fatty acids profiles of the species analyzed. These profiles consisted of saturated fatty acids (SAFA) and polyunsaturated fatty acids (PUFA) in significant proportions. However, during the stationary phase, the concentrations per cell of some PUFAs, especially arachidonic acid (C20:4n6), were higher in treated than in control algae. These results suggest that the adjustment of abiotic parameters is a suitable and one of the cheapest alternatives to obtain sufficient quantities of microalgal biomass, with high oil content and minimal changes in the fatty acid profile of the strains under consideration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are microscopic autotrophic, mixotrophic, or heterotrophic organisms that live in fresh, brackish, or sea water. At the cellular level, they are composed of varying percentages of lipids, proteins, and carbohydrates. Lipids in eukaryotic photoautotrophic cells function as a structural component of cell membranes, modulate cellular activity, and serve as energy storage compounds. In fact, one of the main biological functions of neutral lipids (triacylglycerol, TAG) in microalgal cells is to provide energy for immediate and delayed metabolic requirements. Under specific circumstances, microalgae accumulate high concentrations of carbon in the form of TAG [8, 26, 27, 31]. These high-carbon lipids have been proposed as a source of sustainable oil production and thus as a highly feasible alternative in the development of third-generation biofuels. Microalgae also produce other metabolites, such as astaxanthin, lutein, arachidonic, eicosapentaenoic, and docosahexaenoic acids, which are of high economic value, and even toxins such as yessotoxins [5, 6, 17, 36, 40]. Recent research has focused on the production and isolation of commercially interesting metabolites, including those derived from microalgae, for a broad range of applications. These efforts have led to improvements in processes targeting microalgal biomass production [22, 36]. Currently, one of the major goals in microalgae research is to identify new species or strains with high TAG storage capabilities for use in biofuel production [16, 18] or the production of high-value molecules, e.g., polyunsaturated fatty acids (ω3 and ω6), anti-oxidants, and toxins, that can be further processed as nutritional supplements or used in biomedical or cosmetics applications.
Dinoflagellates and raphidophytes are microalgae that produce high concentrations of biomass within short periods of time in their natural environments. Both groups are present worldwide and have been well studied for decades in the field [3] and, recently, in controlled systems [12, 13]. In general, when algae are exposed to stress conditions (e.g., nitrogen (N) or phosphorus (P) depletion, high light irradiance), growth ceases, synthesis of polar lipids for use in structural membranes stops, and the cells begin to accumulate oil in the form of cytoplasmic lipid bodies, specifically TAG [21, 38]. The energy stored in TAG is used to maintain cellular growth and the specific metabolic requirements of the cells during the stationary phase. For example, according to the growth curve of some species of chlorophyceae, differences in the fatty acids profile, i.e., enhanced saturated (SAFA) and monounsaturated (MUFA) contents, are seen with increasing culture age, whereas in other species there are changes in the polyunsaturated (PUFA) portion of the profile [28]. These characteristic physiological responses were mainly proved experimentally in chlorophyceae [2] but have not been documented in other groups, including dinoflagellates and raphidophytes. Although it is well established that these microalgae increase their lipid quantities with culture age [30], it is not known whether their lipid profiles also change when they are submitted to stress abiotic conditions.
Some microalgal strains (especially those of the chlorophyceae group), when placed in N-depleted media or under other stress conditions, can be exploited to produce large quantities of neutral lipids that can be used as oil for biodiesel production [23]. Whether this approach can be applied to other microalgal groups, including dinoflagellates and raphidophytes, remains to be determined.
Different techniques are used to modify the biochemical composition of microalgae, including altered environmental parameters, such as light, salinity, or nutrients [29, 37, 39], and different CO2 flow regimes during the aeration of photobioreactors, open ponds, or raceway ponds [11]. These techniques enhance the productivity of specific microalgal strains and have been successfully applied to obtain new sources of natural products, e.g., labeled lipids (13C) [1], toxins [14], secondary metabolites (antioxidants, e.g., lutein), pigments (astaxanthin), or PUFAs such as arachidonic acid, eicosapentanoic acid, and docosahexanoic acid [5, 6, 17].
Some dinoflagellate species are known to naturally increase their intracellular concentration of TAG during the stationary phase [30]; however, the conditions required for optimal lipid production and the influence of abiotic factors (e.g., air, temperature, nutrients, and light) are poorly understood. Previous studies on the raphidophyte Heterosigma akashiwo and the dinoflagellates Alexandrium minutum and Karlodinium veneficum showed that the growth of these organisms in a variety of production systems (photobioreactors, flat panels, bubble column) promotes the synthesis and accumulation of large quantities of biomass, oils, and certain metabolites [12, 32]. The rapid proliferation of these and other dinoflagellate species, their capacity to grow in seawater, and their rich lipid profile (e.g., high abundance of saturated and polyunsaturated fatty acids) support their use as a novel source of biomass for biodiesel production. The aims of the present study were (1) to evaluate biomass and lipid production in H. akashiwo and A. minutum, (2) to determine the changes in the fatty acids profile of these species with culture age or other applied treatments, and (3) to identify the optimal abiotic conditions required for the maximum synthesis of lipids. The experimental conditions consisted of constant aeration regimes but allowed changes in temperature and in growth medium composition, mainly the concentration of inorganic dissolved nitrogen (NaNO3).
Materials and methods
Culture conditions
All of the microalgal strains were cultured in batch cultures. The dinoflagellate Alexandrium minutum (AMP4) and the raphidophyte Heterosigma akashiwo (ICMB 830) were isolated from natural samples collected from the northwest Mediterranean Sea.
Experiments were designed to test the effects of abiotic parameters (aeration, temperature and nitrogen limitation) on batch cultures of the target microalgae with respect to growth rate, fatty acid composition, TAG accumulation, and final biomass concentration. Species, culture conditions, and variables measured in each assay are presented in Table 1. The following treatments were tested: aeration with a flow rate of ca. 0.1 v/v min−1 and an average of 420 ± 16 ppm of CO2 (measured by a Qubit Systems S151 CO2 Analyzer) was provided, or not. NaNO3 limitation was compared to control conditions and full L1 medium [20]. The composition of the medium was as follows: NaH2 PO4·H2O, 36.3 μM; Na2 EDTA·2H2O, 11.7 μM; FeCl3·6H2O, 11.7 μM; CuSO4·5H2O, 0.01 μM; Na2MoO4·2H2O, 0.09 μM; ZnSO4·7H2O, 0.08 μM; CoCl2·6H2O, 0.05 μM; MnCl2·4H2O, 0.9 μM; H2SeO3, 0.01 μM; NiSO4·6H2O, 0.01 μM; Na3VO4, 0.01 μM; K2CrO4, 0.001 μM. The nitrogen source was NaNO3, provided at different molar concentration (880, 660, 440, 220 μM) depending on the treatment. In addition, the strains were exposed to different temperatures (15, 20, and 25°C).
Stock, control, and experimental cultures were kept under a 12:12 h light:dark (L:D) cycle (light period starting at 08:00 a.m.), with illumination provided by a combination (1:1 proportion) of Gyrolux fluorescence tubes (58 W; Sylvania, Erlangen, Germany) and cool-white bulbs (58 W; Philips, Eindhoven, the Netherlands) emitting a photon irradiance of 110 μmol photons m−2 s−1 (measured with a Licor sensor). Stock, control, and experimental cultures were carried out in autoclaved seawater of salinity 37 and neutral pH, obtained from the Zona de Acuarios Experimentales (ZAE). Stock and control cultures were grown in full L1-enriched seawater without added silicate [20].
Experimental cultures were inoculated after several transfers of exponentially growing stock cultures to new L1 medium, using the exponential phase of the parent culture at an average concentration of 104 cells mL−1 per replicate. The microalgae were grown in 2-L Nalgene flasks, and the cultures incubated with prefiltered air (Iwaki filter, 0.2-μm pore size) provided at a flow of 0.1 v/v min−1. The air flux had been previously tested for optimal growth and determined not to create deleterious turbulence to the cells, instead allowing for a well-mixed supply of nutrients and avoiding the formation of reactive oxygen species (ROS) [13]. The final concentration of NaNO3 in the L1 medium was modified in order to induce N limitation in the treatment experiment. Preliminary experiments were conducted to evaluate the minimal concentration of dissolved NaNO3 needed to maintain cell growth. All strains were submitted to a range of NaNO3 concentrations (880, 660, 440, 220 μM). A final concentration of 330 μM NaNO3 (maintaining the original concentrations of phosphorus, vitamins, and metals in the L1 medium) was used in the combined nitrogen limitation and high temperature (25°C) experiments (Table 1).
Growth rates
The growth rates of the two strains were determined in triplicates, with 10-mL subsamples from each culture removed every 2 or 3 days and fixed in Lugol’s iodine solution. Cell abundances were estimated using a Sedgewick-Rafter slide under an inverted optical microscope (Leica-Leitz DM-II, Leica Microsystems GMbH, Wetzlar, Germany) at ×200–400 magnification. Net exponential growth rates, μ (day−1) [19], were calculated as the slope of the regression line of ln N versus time (t), where N is defined as the estimated cell concentration.
Lipid extraction and fatty acid analyses
Lipids produced by the strains under control and treatment conditions were analyzed at two different times during growth: exponential phase and stationary phase. Triplicates of 50 mL were filtered on precombusted (450°C 4 h) GF/F Whatman glass-fiber filters, immediately frozen in liquid N2, freeze-dried for 12 h, and then stored at −20°C until analysis (5–10 days approx.). The filters were placed in a tube with 3:1 dichloromethane/methanol (DCM/MeOH) spiked with an internal standard (2-octyldodecanoic acid and 5β-cholanic acid). Lipids were extracted using a microwave-assisted technique (5 min at 70°C), previously identified as the simplest and most effective for microalgal lipid extraction [10, 15, 25]. After centrifugation, the extract was concentrated to near dryness in a vacuum centrifuge maintained at constant temperature and then fractionated by solid-phase extraction (SPE), according to a previously published method [35]. The sample was subsequently redissolved in 0.5 mL of chloroform and eluted through a 500-mg aminopropyl mini-column (Waters Sep-Pak® cartridges) previously activated with 4 mL of n-hexane. The first fraction (neutral lipids) was eluted with 3 mL of chloroform/2-propanol (2:1) and the fatty acids recovered with 8.5 mL of diethyl ether/acetic acid (98:2). Despite reported concerns regarding background levels of free fatty acids (FFA) in aminopropyl columns [35], the concentrations of target FFA in the SPE cartridges were below the detection limit. The FFA fraction was methylated using a 20% solution of MeOH/BF3 followed by heating at 90°C for 1 h, yielding fatty acid methyl esters (FAMEs). The reaction was quenched with 4 mL of NaCl-saturated water. FAMEs were recovered by extracting the samples twice with 3 mL of n-hexane. The combined extracts were concentrated to near dryness, redissolved in 1.5 mL of chloroform, eluted through a glass column filled with Na2SO4 to remove residual water, and, after chloroform removal, subjected to nitrogen evaporation. The extracted sample was stored at −20°C until gas chromatography (GC) analysis (no more than 5 days later). At that time, the extracts were redissolved in 30 μL of iso-octane and then analyzed in a Thermo Finnigan Trace GC Ultra instrument, equipped with a flame ionization detector and a splitless injector, fitted with a DB-5 Agilent column (30-m length, 0.25-mm internal diameter, 0.25-μm phase thickness). Helium was used as the carrier gas, delivered at a rate of 33 cm s−1. The oven temperature was programmed to increase from 50 to 320°C at 10°C min−1. Injector and detector temperatures were 300 and 320°C, respectively. FAMEs were identified by comparison of their retention times with those of standard fatty acids (37 FAME compounds, Supelco® Mix C4-C24), and quantified by integrating the areas under the curves in the gas chromatograph traces (Chromquest 4.1 software), using calibrations derived from internal standards.
Neutral lipid fluorescence in microalgae
The intracellular neutral lipid distribution in microalgal cells was examined along the entire growth curve (lag, exponential, and stationary phases) by staining a 3-mL suspension of each of the strains (in triplicate) with 10 μL (7.8 × 10−4 M) of Nile Red fluorescent dye (Sigma-Aldrich) dissolved in acetone, yielding a final concentration of 0.26 μM [9]. The samples were examined by epifluorescent microscopy (Leica-Leitz DM-II, Leica Microsystems GMbH, Wetzlar, Germany). Photographs were taken using the Sigmapro software image analyzer. To quantify the concentration of cellular neutral lipids, all strains were stained and then left in darkness for 15 min before they were read in an LS55 PerkinElmer fluorescence spectrometer at an excitation wavelength of 486 nm and with emission measured at 570 nm.
Oil concentration
Oil concentration was measured by GC analysis as the sum of the FFA concentration plus the TAG concentration in the stationary phase, and expressed in milligrams per liter.
Dry weight biomass
Dry weight (DW) was determined by filtering duplicate subsamples (10 mL) through preweighed glass-fiber filters (Whatman GF/F 25 mm, nominal pore size 0.7 μm). The filters were dried in an oven (105°C) for 4 h and then weighed (Sartorious microbalance, precision of 0.001 g) every 2 h. We observed that 4 h at 105°C was sufficient to achieve a constant dry weight.
Data analysis
Experiments were compared using non-parametric equality tests (Kruskal–Wallis test; P < 0.05 was considered significant). Statistical analyses were conducted with Statistica© 6 software for PCs.
Results
Table 2 summarizes the net growth rate, final estimated cell numbers, TAG measurements by GC analysis and spectrofluorometric data, oil concentration, and maximum dry weight obtained with the control and the various treatments for the tested algae and shows a comparison among experiments. Aeration significantly increased H. akashiwo growth rates and maximum dry weight (P < 0.05; Table 2; comparison μ and DW exp. 1 vs. 2). Indeed, aeration resulted in 1 g more of dry weight per liter compared with the non-aerated culture (comparison DW exp. 1 vs. 2).
Higher temperature (20 and 25°C) resulted in significantly higher growth rate in H. akashiwo (comparison μ, exp. 1 vs. 4, exp. 1 vs. 3, and exp. 4 vs. 3). In terms of DW, the highest productivity was for 20°C followed by 25°C (comparison DW, exp. 1 vs. 4, exp. 1 vs. 3, and exp 4 vs. 3).
In order to choose the appropriated nitrogen concentration to not limit growth rate, TAG concentration and the growth rate (μ) were measured under a range of nitrogen concentrations (Fig. 1). The highest growth rates under different NaNO3 concentrations were for nitrogen-replete conditions (880 μM), and the lowest for nitrogen-limited conditions (220 μM), with extremely significantly differences among them (P < 0.0001). No significant differences in terms of growth rate were detected between 660 and 440 μM (Fig. 1). The highest concentrations of TAG, determined spectrofluorometrically, were measured during the stationary phase under the range of N-deficient conditions, with 440 μM NaNO3 resulting in a slightly but not significantly (P > 0.05) higher concentration than 220 μM NaNO3, whereas significantly higher than the control (P < 0.05) (Fig. 1). The concentration of 330 μM NaNO3 was chosen as it did not significantly reduce growth rates but increased the lipid concentration per cell. This was demonstrated in experiment 5 in which nitrogen deficiency of 330 μM NaNO3 in H. akashiwo did not reduce growth rate (Table 2, comparison μ, 1 vs. 5, P > 0.05), but it significantly increased the TAG concentration per cell in H. akashiwo (P < 0.05).
Growth rate and neutral lipid per cell in H. akashiwo determined spectrofluorometrically (488/570 ex/em, nm), during different growth phases. TAG r.u. triacylgycerides relative units, μ growth rate (div day−1). *Means significant differences (P < 0.05) compared to the control treatment (880 μM). **Means extremely significant differences (P < 0.0001)
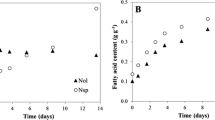
The two strains under 20°C and 880 μM NaNO3 conditions reached maximum cell concentrations on different days, according to their particular physiology and growth rate. H. akashiwo achieved the highest cell density around day 15, whereas for A. minutum the maximum concentration was reached at day 20 (Fig. 2). Under 25°C and 330 μM NaNO3 conditions, both of the tested strains reached their maximum densities between days 6 and 8 of the growth curve, after which they entered the stationary phase such that the cell abundance was maintained for a period of 10 days. It was during this stage that a significant enhancement of the cellular oil concentration occurred (Fig. 2).
Higher growth rates were achieved in H. akashiwo under treatment conditions at 25°C in medium containing 330 μM of NaNO3 (Table 2). In terms of DW, there was a significant reduction in the volumetric productivity of the biomass but, free fatty acids concentration plus TAG concentration measured by GC confirmed that these conditions resulted in larger and significant (300%) amounts of microalgal lipids by cell (ng cell−1) (Table 2), with marked accumulation after culture day 15 (Fig. 2). Neutral lipid accumulation (spectrofluorometric analysis) was 30% greater in H. akashiwo at 25°C in medium containing 330 μM of NaNO3 than under control conditions on the same day of culture (Fig. 2). The intense lipid accumulation was also observable when cells were stained with Nile Red and examined under epifluorescence microscopy (Fig. 3c, d).
The physiological response of A. minutum species to high temperature and low NaNO3 concentration was similar to that of H. akashiwo, with no significant differences found in the growth rates (Table 2, comparison μ, 6 vs. 7). Volumetric productivity was also higher in cells incubated under control conditions, differing by more than 1 g DW biomass L−1 (Table 2, comparison DW, 6 vs. 7). The accumulation of TAG, as measured by spectrofluorometry and GC analysis, in A. minutum cells submitted to treatment conditions was extremely significantly (P < 0.0001) higher (>200%) than in the control (Table 2, Fig. 2), with marked accumulation also after culture day 15. Those differences were not significant (P > 0.05) in terms of total oil produced, with a slightly (26%) higher oil concentration (comparison, 6 vs. 7). Staining of Alexandrium cells with Nile Red confirmed the presence of oil inside the cells. In the treated cells, high concentrations of lipids droplets were visible inside the cytoplasm whereas in control cells just a few stained drops could be seen (Fig. 3a, b).
Fatty acids composition
In H. akashiwo, no significant differences (P > 0.05) in terms of changes in the fatty acid profile between growth phases and control vs. treatment experiments were found. In H. akashiwo cultured under control conditions, fatty acids differed only slightly with respect to two growth phases (Table 3). The largest increase was 1.1% in saturated lauric acid C12:0, followed by 0.9% in MUFA palmitoleic acid C16:1, and 0.7% in palmitic acid C16:0. In addition, some fatty acids decreased, especially the PUFAs arachidonic acid C20:4n6 (−1.7%) and C18:5 (−0.9%). The fatty acids of H. akashiwo under treatment conditions differed from those in cells under control conditions during the stationary phase. Specifically, there was a small increase in the saturated fatty acids profiles, particularly C14:0, C15:0, C16:0 (1.0%, 0.2%, 0.1%, respectively), as well in palmitoleic acid C16:1 (0.7%), the PUFA eicosapentanoic acid C20:5n3 (0.1%), and arachidonic acid C20:4n6 (1.1%). At the same time, there was a decrease in the percentage of γ linolenic acid C18:3n6 (−3.7%), and a slightly decrease in stearic acid C18:0 (−0.1%), and lauric acid C12:0 (−0.3%).
In A. minutum, no significant differences (P > 0.05) in terms of changes in the fatty acid profiles of the different growth phases and control vs. treated experiment were found. The fatty acids profile of A. minutum cultured under control conditions differed only slightly with respect to growth phase, with decreases in the PUFAs C20:4n6, C18:5, C18:2n6 (−1.4%, −0.7%, −0.6% respectively), and in the MUFA C18:1n9 (−0.3%) (Table 3). The largest increase was in γ linolenic acid C18:3n6 (2.1%). Differences in the fatty acids of A. minutum under control vs. treatment conditions during the stationary phase consisted of a slight decrease under the latter conditions, especially in the saturated portion C16:0, and C12:0 (−1.2 and −0.1%, respectively). There also was a decrease in MUFA C14:1 (−0.2%) and PUFA C18:3n6 (−2.7%). In contrast, there was a small increase in docosahexanoic C22:6 (0.8%), arachidonic acid C20:4n6 (0.4%), linoleic acid C16:1 (0.2%), and saturated pentadecanoic acid C15:0 (0.3%) (Table 3).
Discussion and conclusion
Different approaches have been used to enhance the quantity and quality of lipid production over the last few years. All of them have led to the conclusion that improved culture strategies are needed in order to obtain an increased oil concentration in microalgae cells [22, 23]. The use of genetically modified organisms (GMO) and strain selection [31] are among the strategies that have been examined. The establishment of GMO cultures is time-consuming, requires biosafety considerations, and typically involves high costs [31], and strain selection is also problematic. Here, we combined a strategy of marine strain selection and alterations in abiotic variables to enhance the cellular oil concentration. Two strains of microalgae, one raphidophyte and one dinoflagellate, previously determined to yield optimal lipid production under control conditions [12] were subjected to physiological stress, by altering key abiotic parameters, and the effects on oil production and biomass productivity subsequently examined. In our working volume, the gentle aeration of the cultures resulted in higher growth of the raphidophyte species as well as the production of higher amounts of biomass than obtained with non-aerated cultures. This result was primarily due to the CO2 concentration in the injected air flow, which supports microalgal growth; and, secondly to the agitation or hydrodynamic turbulence provoked by the air inside the flasks that allows good mixing of the cells and the liquid medium. Dinoflagellates and raphidophytes are microalgae that require minimal amounts of turbulence to grow which is enhanced with low-level turbulence, especially hydrodynamic turbulence or air bubbling [37]. There are also other dinoflagellate species that cannot grow under turbulent conditions especially when accompanied by mechanical agitation produced by an oscillating rod, magnetic stirring, or shaker [4]. The low-volume air flow (ca. 0.1 v/v min−1) used in this study resulted in well-mixed cultures and adequate nutrient availability for the cells, with no visible cell damage. By contrast, a high-volume flow of injected air (≥0.5 v/v min−1) deforms cell membranes and induces the production of ROS, which cause visible damage to the membranes of dinoflagellates microalgal cells [4, 13]. Accordingly, the use of low-volume air flow (ca. 0.1 v/v min−1) is recommended for the culture of raphidophytes in enclosed systems, such as bubble column photobioreactors.
In the combined treatment, there were no significant differences in growth rates for the two species tested; nevertheless, the amounts of oil per cell were significantly higher in stationary-phase cells cultured under treatment rather than under control conditions. Stationary phase was previously identified as the best harvest time in terms of maximum lipid accumulation and biomass concentration [12]. At the beginning of the curve, the similar growth rates of the strains in the treated and control conditions were due to the initially abundant availability of nutrients (especially nitrogen). However, the oil concentration in cells of A. minutum and H. akashiwo under control conditions remained low during the exponential phase and did not strongly increase as the culture approached the stationary phase. The high concentration of oil in cells under treatment conditions during the stationary phase was likely triggered by the exhaustion of available nitrogen in the culture medium. These results are consistent with those of other nitrogen depletion studies carried out in freshwater microalgae from the chlorophycean group such as Neochloris oleoabundans or Chlorella vulgaris [26, 39].
The final cell concentration was strain-dependent, according to their particular physiology and growth rate. But in general, biomass concentration was highest in microalgae under control conditions, whereas TAG and total lipid accumulation per cell were greater in cultures under treatment conditions. This may have been due to the stress conditions imposed by the latter, which stimulated the cellular production of energetic reserves. Previous studies showed that lipid accumulation in the final part of the growth curve of batch cultures is greater in Dinophyceae and Raphidophyceae than in other microalgal groups, such as Prasinophyceae and Bacillariophyceae [12, 29, 30]. Whereas the cell densities of the tested strains under treatment conditions were always below those obtained under control conditions (see maximum cell concentration in Table 2), this was not the case for the oil concentration, which was higher in H. akashiwo and A. minutum under treatment rather than under control conditions. This result supports the use of a combined approach in which temperature is increased and nitrogen is limited in order to improve the oil concentration in microalgae biomass. Whereas both strains cultured under treatment conditions produced less biomass, they had higher per-cell oil contents and oil concentration than their paired controls.
The oil composition of microalgae exposed to stress (suboptimal light, temperature, air, nutrients, salinity, CO2) has been shown to vary, e.g., lipids required for growth, especially polar lipids that contribute to the cell membrane. Polar lipids are no longer produced and instead, those required for energetic reserves (neutral lipids) accumulated in order for the microalgae to avoid starvation [21, 40].
Thus, if algae are to be used as feedstock in the production of biodiesel or metabolites, it is important to determine whether and how their lipid composition varies during stress conditions, because the composition of the raw material used for the transesterification reaction determines the characteristics of the biofuel obtained [18] and the quality of the metabolites produced [36]. Our strains are highly similar in terms of lipid profile, and specifically palm oil [12, 33], which is most commonly lipid used in first-generation biodiesel production, based on its high cetane number and good oxidation stability, both of which are indicators of the high percentage of saturated fatty acids accumulated. Here, as seen in the fatty acids profile of the control and the treated microalgae, the lipid profile was not drastically altered by stress treatment, as only the PUFA portion increased in Heterosigma under treatment conditions. In Alexandrium under treatment conditions, a decrease in the principal saturated fatty acids C12:0 and C16:0 and in the PUFA portion (below 2.5%) was registered. The absence of a drastic change in the fatty acids produced by the microalgae under treatment conditions indicates that, at least qualitatively, the microalgal biomass obtained under the abiotic conditions stress used in the present study would not significantly differ in terms of lipid profiles relevant for biofuel purposes. Interestingly, treatment enhanced the production of specific PUFAs, including ω6 such as arachidonic acids, favoring greater production of these metabolites, which are of value in the nutritional and cosmetics industries [14].
According to our results, to achieve industrial-scale microalgae production with an increase temperature and nutrient limitation, we propose a hybrid strategy of growth: using a greenhouse enclosed photobioreactor (GH-EPBR) and stable environmental conditions to obtain high biomass production with controlled temperature and nutrients, followed by inoculation of the microalgae into an open pond system with warm water and deficiency of nutrient. Many thermoelectric power plants use seawater to cool their turbines; this seawater flows out of the turbines at a higher temperature (approximate increase of 5–10°C), which in most cases could be suitable for microalgal cultures. Moreover, the outflow seawater usually has a nitrogen concentration lower than that of commercial culture medium. This can be used in the same strategy as evaluated in this study, promoting microalgal growth and then N-starving the microalgae in order to enhance oil production, including high-quality raw material in amounts needed for biodiesel production or the increased production of high value molecules.
References
Acién Fernández FG, Alías CB, García-Malea López MC, Fernández Sevilla JM, Ibáñez González MJ, Gómez RN, Molina Grima E (2003) Assessment of the production of 13C labeled compounds from phototrophic microalgae at laboratory scale. Biomol Eng 20(4–6):149–162
Alonso DL, Belarbi A-H, Fernandez-Sevilla JM, Rodriguez-Ruiz J, Grima EM (2000) Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornotum. Phytochemistry 54:461–471
Anderson DM (1989) Toxic algal blooms and red tides: a global perspective. In: Okaichi T, Anderson DM, Nemoto T (eds) Red tides: biology, environmental science, and toxicology. Elsevier, New York, pp 11–16
Berdalet E, Peters F, Koumando VL, Roldán C, Guayadol O, Estrada M (2007) Species-specific physiological response of dinoflagellates to quantified small-scale turbulence. J Phycol 43:965–977
Bigogno C, Khozin-Goldberg I, Cohen Z (2002) Accumulation of arachidonic acid-rich triacylglycerols in the microalga Parietochloris incisa (trebuxiophyceae, chlorophyta). Phytochemistry 60(2):135–143
Blanco A, Moreno J, Del Campo J, Rivas J, Guerrero M (2007) Outdoor cultivation of lutein-rich cells of Muriellopsis sp. In open ponds. Appl Microbiol Biotechnol 73(6):1259–1266
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Converti A, Casazza AA, Ortiz E, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Elsey D, Jameson D, Raleigh B, Cooney MJ (2007) Fluorescent measurement of microalgal neutral lipids. J Microbiol Methods 68(3):639–642
Escala M, Rosell-Melé A, Masqué P (2007) Rapid screening of glycerol dialkyl glycerol tetraethers in continental Eurasia samples using HPLC/APCI-ion trap mass spectrometry. Org Geochem 38:161–164
Fu F-X, Zhang Y, Warner ME, Feng Y, Sun J, Hutchins DA (2008) A comparison of future increased CO2 and temperature effects on sympatric Heterosigma akashiwo and Prorocentrum minimum. Harmful Algae 7(1):76–90
Fuentes-Grünewald C, Garcés E, Rossi S, Camp J (2009) Use of the dinoflagellate Karlodinium veneficum as a sustainable source of biodiesel production. J Indus Microbiol Biotechnol 36(9):1215–1224
Gallardo-Rodríguez JJ, Mirón AS, Camacho FG, García MC, Belarbi EH, Chisti Y, Grima EM (2009) Causes of shear sensitivity of the toxic dinoflagellate Protoceratium reticulatum. Biotechnol Prog 25(3):792–800. doi:10.1002/btpr.161
Garcia Camacho F, Gallardo Rodríguez JJ, Sánchez Mirón A, Cerón García MC, Belarbi EH, Chisti Y, Molina Grima E (2007) Biological significance of toxic marine dinoflagellates. Biotechnol Adv 25:176–194
Gómez-Brandón M, Lores M, Domínguez J (2008) Comparison of extraction and derivatization methods for fatty acid analysis in solid environmental matrixes. Anal Bioanal Chem 392(3):505–514
Greenwell HC, Laurens LML, Shields RJ, Lovitt RW, Flynn KJ (2010) Placing microalgae on the biofuels priority list: a review of the technological challenges. J R Soc Interface 7(46):703–726. doi:10.1098/rsif.2009.0322
Grewe C, Griehl C (2008) Time and media-dependent secondary carotenoid accumulation in Haematococcus pluviales. Biotechnol J 3:1232–1244
Griffiths M, Harrison S (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21(5):493–507
Guillard RRL (1973) Division rates. In: Stein JR (ed) Handbook of phycological methods. I. Culture methods and growth measurements. Cambridge University Press, Cambridge, pp 289–312
Guillard RRL (1995) Culture methods In: Hallegraeff GM, Anderson DM, Cembella AD (eds) Manual on harmful marine microalgae. IOC manuals and guides. UNESCO 33:551
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45(2):160–186
Hsieh C-H, Wu W-T (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 100(17):3921–3926
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639
Kornilova O, Rosell-Melé A (2003) Application of microwave-assisted extraction to the analysis of biomarker climate proxies in marine sediments. Org Geochem 34:1517–1523
Kuwata A, Hama T, Takahashi M (1993) Ecophysiological characterization of two life forms, resting spores and resting cells, of a marine planktonic diatom, Chaetoceros pseudocurvisetus, formed under nutrient depletion. Mar Ecol Prog Ser 102:245–255
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Liang Y, Beardall J, Heraud P (2006) Effect of UV radiation on growth, chlorophyll fluorescence and fatty acids composition of Phaeodactylum tricornotum and Chaetoceros muelleri (Bacillariophyceae). Phycologia 45(6):605–615
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99(11):4717–4722
Mansour MP, Volkman JK, Jackson AE, Blackburn SI (1999) The fatty acid and sterol composition of five marine dinoflagellates. J Phycol 35(4):710–720
Mansour MP, Volkman JK, Blackburn SI (2003) The effect of growth phase on the lipid class, fatty acid and sterol composition in the marine dinoflagellate, Gymnodinium sp. in batch culture. Phytochemistry 63(2):145–153
Melis A, Mitra M (2008) Optical properties of microalgae for enhanced biofuels production. Opt Express 16(26)
Parker NS, Negri AP, Frampton DMF, Rodolfi L, Tredici MR, Blackburn SI (2002) Growth of the toxic dinoflagellate Alexandrium minutum (dinophyceae) using high biomass culture systems. J Appl Phycol 14(5):313–324
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez A (2008) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Ruiz J, Antequera T, Andres AI, Petron MJ, Muriel E (2004) Improvement of a solid phase extraction method for analysis of lipid fractions in muscle foods. Anal Chim Acta 520(1–2):201–205
Russel JM, Werne JP (2007) The use of solid phase extraction columns in fatty acids purification. Org Geochem 38:48–51
Sanchez JF, Fernández-Sevilla JM, Acién FG, Cerón MC, Pérez-Parra J, Molina-Grima E (2008) Biomass and lutein productivity of Scenedesmum almeriensis: influence of irradiance, dilution rate and temperature. Appl Microbiol Biotechnol 79:719–729
Sullivan JM, Swift E, Donaghay PL, Rines J (2003) Small-scale turbulence affects the division rate and morphology of two red-tide dinoflagellates. Harmful Algae 2:183–199
Takagi M, Karseno, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101(3):223–226
Widjaja A, Chien CC, Ju YH (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem Eng 40:13–20
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J Phycol 38:325–331
Acknowledgments
We are grateful to the members of the L′Esfera Ambiental Laboratory, Universitat Autònoma de Barcelona, for their help in gas chromatography analyses. We thank S. Fraga for providing the clonal culture AMP4. We thank L. del Río for help with the experiments, and the Zona Acuarios Experimentales (ZAE) of the ICM-CSIC for the use of their facilities. We gratefully acknowledge the Comisión Nacional de Investigación Ciencia y Tecnología (CONICYT), Chile, for its support of the scholarship “Beca de Gestión Propia,” which finances the PhD studies of C. Fuentes-Grünewald. The work of S. Rossi is supported by the Ramon y Cajal award of the Spanish Ministry of Science and Innovation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuentes-Grünewald, C., Garcés, E., Alacid, E. et al. Improvement of lipid production in the marine strains Alexandrium minutum and Heterosigma akashiwo by utilizing abiotic parameters. J Ind Microbiol Biotechnol 39, 207–216 (2012). https://doi.org/10.1007/s10295-011-1016-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-1016-6