Abstract
Metal pollution can produce many biological effects on aquatic environments. The marine diatom Amphora subtropica and the green alga Dunaliella sp. possess a high metal absorption capacity. Nickel (Ni) removal by living cells of A. subtropica and Dunaliella sp. was tested in cultures exposed to different Ni concentrations (100, 200, 300, and 500 mg L−1). The amount of Ni removed by the microalgae increased with the time of exposure and the initial Ni concentration in the medium. The metal, which was mainly removed by bioadsorption to Dunaliella sp. cell surfaces (93.63% of total Ni (for 500 mg Ni L−1) and by bioaccumulation (80.82% of total Ni (for 300 mg Ni L−1) into Amphora subtropica cells, also inhibited growth. Exposure to Ni drastically reduced the carbohydrate and protein concentrations and increased total lipids from 6.3 to 43.1 pg cell−1, phenolics 0.092 to 0.257 mg GAE g−1 (Fw), and carotenoid content, from 0.08 to 0.59 mg g−1 (Fw), in A. subtropica. In Dunaliella sp., total lipids increased from 26.1 to 65.3 pg cell−1, phenolics from 0.084 to 0.289 mg GAE g−1 (Fw), and carotenoid content from 0.41 to 0.97 mg g−1 (Fw). These compounds had an important role in protecting the algae against ROS generated by Ni. In order to cope with Ni stress shown by the increase of TBARS level, enzymatic (SOD, CAT, and GPx) ROS scavenging mechanisms were induced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are considered to be toxic and carcinogenic to living organisms and create a serious environmental problem. The contamination of soils and aquatic systems by toxic metals and organic pollutants has increased due to anthropogenic activity (Aebi 1984). Ni is a silvery white metal that takes on a high polish. It is a transition metal, hard, and ductile. As for most metals, the toxicity of Ni is dependent on the route of exposure and the solubility of the Ni compound (Anantharaj et al. 2011).
Heavy metal removal mechanisms include sedimentation, precipitation, absorption, flocculation, cation and anion exchange, oxidation/reduction, microbiological activity, and uptake. Phytoremediation is defined as a process of decontaminating soil and aquatic systems by using plants, fungi, or algae to absorb heavy metals. Algae have many features that make them ideal candidates for the selective removal and concentration of heavy metals, which include high tolerance to heavy metals, ability to grow both autotrophically and heterotrophically, large surface area/volume ratios, phototaxy, phytochelatin expression, and potential for genetic manipulation (Perales Vela et al. 2006). Microalgae remove heavy metals directly from polluted water by two major mechanisms: the first is a metabolism-dependent uptake into their cells at low concentrations and the second is biosorption which is a non-active adsorption process (Matagi et al. 1998).
Some algal species successfully can hyperaccumulate heavy metals like Cd, Zn, Pb, Hg, Ni, and Cu (Chekroun and Baghour 2013). Dunaliella salina, a green microalga, has high tendency for zinc accumulation followed by copper and cobalt, while the lowest tendency was observed for cadmium. This may be due to the low toxicity of zinc and its role as cofactor for enzymes participating in CO2 fixation (Moroney et al. 2001). Microalgae appear today as one of the best candidates for the treatment of water loaded by heavy metals. Microalgae are known for their ability to fix heavy metals by absorption or adsorption. Although the biosorption process has many advantages over conventional techniques, it remains under exploited and so far there are only a few processes that are established.
Ni and other heavy metals can also generate free radicals from molecular oxygen to produce superoxide anion. Indeed, in the presence of the heavy metal, the superoxide anions formed can then combine with protons, generating hydrogen peroxide in the process (Das et al. 2008). The cumulative production of reactive oxygen species (ROS) through either endogenous or exogenous insults is termed oxidative stress. Appropriate mechanisms are present in microalgae so that steady state concentration of potentially toxic oxygen-derived free radicals is kept in check under normal physiological condition by intrinsic antioxidant defense system (Ercal et al. 2001).
The aim of this study was to test the ability of two marine microalgae strains, the diatom Amphora subtropica and the green alga Dunaliella sp., to remove Ni and evaluate their biochemical and physiological response to Ni contamination.
Materials and methods
Amphora subtropica and Dunaliella sp. culture conditions
The marine microalgae Amphora subtropica and Dunaliella sp. isolated from the sea ports of Sfax and Chott El-Jerid, Tozeur (Tunisia), were maintained in sterilized and natural seawater F/2 Provasoli medium (Provasoli et al. 1957), with initial NaCl concentrations of 40 and 80 g L−1, respectively. The microalgae were deposited in the Centre of Biotechnology Culture Collection “CTM” and assigned the codes CTM 20013 (Amphora subtropica) and CTM 20028 (Dunaliella sp.).
Precultures were grown and maintained at 29 ± 1 °C under 24-h light/0-h dark cycle provided by cool white fluorescence lights at 80 μmol photons m−2 s−1 irradiance. Experiments were conducted in 500 mL flasks.
A stock solution of Ni was prepared by dilution of NiSO4 in Milli-Q water to a final concentration of 10 g L−1 of Ni. For the experiments, appropriate volumes of the stock solution were added to the seawater medium to obtain Ni concentrations of 100, 200, 300, and 500 mg L−1 (0.64, 1.29, 1.93, and 3.23 mM, respectively). Experiments were conducted in 1 L shake flasks.
The initial culture was harvested by centrifugation at 4000×g for 5 min before the day of experiment and washed twice with seawater without any nutrients, and then, the microalgal pellet was homogenized in 400 mL natural seawater as the inoculum. The inoculum was mixed in media of different metal ion concentrations during the experiments at an initial cell density of 2.5 × 106 cell mL−1. Control cultures without Ni were included. The experiments were carried out under the conditions described earlier in triplicate. The cell density in the cultures was measured using a hemocytometer. Dunaliella sp. were immobilized and stained by addition of 10 μL of Lugol’s solution to 50 μL of microalgae culture.
Determination of Ni removed and minerals
The Ni removed by the microalgae was determined by the protocol described by Stauber and Florence (1985).
Total Ni removed in cells was determined by filtration of 25 mL aliquots from each culture through two superposed 1.2 μm Millipore filters. The Ni levels were measured in both filters using the lower filter as blank.
For the determination of the Ni removed intracellularly, aliquots of 25 mL of each culture were centrifuged at 4000×g for 5 min and the pellet was re-suspended for 20 min in 25 mL of 0.02 M EDTA dissolved in seawater, thereby allowing only the intracellular Ni to be measured. After that, the culture was centrifuged and washed twice with seawater. The washed pellet was digested as in total Ni determination.
The determination of bioadsorbed Ni onto the cell surface was measured by subtracting the intracellular Ni concentration from the total Ni removed (bio-adsorbed Ni = total Ni − intracellular Ni).
Each one of the filters from the determination of total Ni removed, the pellets from the determination of Ni removed intracellularly, and the ash of Amphora subtropica and Dunaliella sp. were separately digested in a mixture of 5 mL of 1 M HNO3 and 0.5 mL of HCl (96%). Digested samples were brought to a final volume of 25 mL with distilled water. The Ni concentrations in the samples, potassium, calcium, iron, magnesium, zinc, and manganese were determined by atomic absorbance spectroscopy (Perkin Elmer analyst 200; Perkin Elmer, USA).
Biochemical analyses
The microalgal dry matter was determined by centrifuging 500 mL of the culture at 4000×g for 5 min. The pellet was washed twice with distilled water and dried at 105 °C until constant weight. After complete drying, the ash was obtained by incineration of a known weight of the dried sample in a muffle furnace at 550 °C for 6 h.
Protein content was determined according to the method of Lowry et al. (1951) using bovine serum albumin as a standard. Carbohydrates were determined using the phenol–sulfuric acid method of Dubois et al. (1956). Total lipid was extracted according to the modified protocol of Bligh and Dyer (1959). The fatty acid methyl ester (FAME) extraction and the analysis of FAME were performed as the protocol described by Dahmen Ben Moussa et al. (2016). Carotenoids were analyzed according to Hiscox and Israelstam (1979) using the dimethyl sulfoxide (DMSO), and the phenolic content was determined in the microalgae extracts according to Singleton and Rossi (1965).
Thiobarbituric acid-reactive substances (TBARS) and antioxidative enzyme assay
For enzyme assays, 1 g of wet biomass samples of A. subtropica and Dunaliella sp. were disrupted by alumina (1:1, w/w) in 1.5 mL of 0.05 M sodium phosphate buffer pH 7.8, at 4 °C. Mixtures were crushed in mortar and pestle, and the debris was removed from the algal extract by centrifugation at 10,000×g and 4 °C for 5 min (Dahmen Ben Moussa et al. 2016). The content of lipid peroxidation products, malondialdehyde (MDA), was determined according to the methods provided by Draper and Hadley (1990). The results were expressed as nmol mg−1 protein. Total superoxide dismutase activity (SOD) was determined by measuring its ability to inhibit the photoreduction of nitroblue tetrazolium (NBT) using riboflavin (Beyer and Fridovich 1987). The SOD activity was expressed as units mg−1 protein, at 25 °C. The catalase activity (CAT) was assayed spectrophotometrically by measuring the decrease of absorbance at 240 nm because of H2O2 decomposition. Glutathione peroxidase (GPx) activity was tested in the presence of added glutathione (Flohé and Günzler 1984).
Statistical analysis
The experiments were done in triplicate. Data are expressed as mean ± SD. One-way analysis of variance (ANOVA) was used to determine the differences among various experiments. Values were considered statistically significant when p < 0.05.
Results
Effect of Ni toxicity on the growth and morphological changes
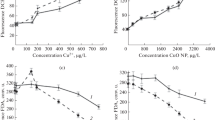
The growth of A. subtropica and Dunaliella sp. was significantly affected by Ni exposition. There was growth inhibition in cultures exposed to Ni (Fig. 1). ANOVA (p < 0.001) showed that this metal had a significant effect on growth of both A. subtropica (Fig. 1a) and Dunaliella sp. (Fig. 1b) after 3 days of exposure. The inhibition was proportional to Ni concentration: as the Ni concentration increased in the medium, growth decreased. By day 7, the growth of the cultures with Ni concentrations of 200 and 300 mg Ni L−1 decreased, and those with the highest Ni concentration assayed (500 mg L−1) showed 73 and 68% inhibition for A. subtropica and Dunaliella sp., respectively.
Observation of cell morphology confirmed that Ni exposure induced changes in cell structure (Fig. 2). The Ni-intoxicated microalgae showed a fragmentation of thylakoid membranes, cell damage with cell membrane lysis, and alterations in shape and size as compared to control cells.
Ni removal by the algae Amphora subtropica and Dunaliella sp.
These cells showed a high ability for Ni removal. Figure 3a, b shows the total Ni removed by the algal cells in terms of the Ni concentration present in the A. subtropica and Dunaliella sp. medium. The total Ni removed per cell was significant from the first day of culture. The highest amounts of metal removed were observed in cultures with the highest Ni concentration assayed (500 mg Ni L−1) and on the seventh day of culture (2.45 and 3.3 pg cell−1, respectively).
Part of the total Ni removed by cells was accumulated intracellularly and another part was adsorbed on the cell surface. As shown in Fig. 3c, d, the intracellular removal of Ni increased with the initial concentration of Ni in the culture media and the time of exposure. During the latency phase and for a concentration of 100 mg Ni L−1, Dunaliella sp. were able to remove 0.005 pg cell−1 which represents about 2.98% of the total removed Ni. However, A. subtropica were able to remove 0.07 pg cell−1 which represents about 61.84% of the total removed Ni. For the maximum concentration used (500 mg Ni L−1), the intracellular removal of metal increases and reaches 1.55 pg cell−1 (A. subtropica) and 0.03 pg cell−1 (Dunaliella sp.), representing 63.44 and 1.15% of the total removed Ni, respectively. The maximum amounts of Ni removed intracellularly were 1.98 and 0.21 pg cell−1 in cultures exposed to 500 mg Ni L−1 and after 7 days of culture, representing 80.82 and 6.37% of the total removed Ni, respectively. In cultures exposed to 100 mg Ni L−1, the uptake of Ni reached a value of 0.11 and 0.05 pg cell−1 for A. subtropica and Dunaliella sp., respectively, in 7 days. In the rest of the Ni-exposed culture assayed (200 and 300 mg L−1), the maximum amounts of Ni removed by bioaccumulation were 0.41 and 0.78 pg cell−1, respectively, into A. subtropica cells and 0.1 and 0.18 pg cell−1, respectively, into Dunaliella sp.
The determination of the Ni bioadsorbed to the cell surface of A. subtropica and Dunaliella sp. shows a kinetic similar to that observed for the total Ni removed (Fig. 3e, f). The quantity of Ni bioadsorbed increased rapidly in all the cultures during the latency phase (day 1) and then stabilized later, and thereafter, an increase was observed from the fifth day of microalgae exposure, for high Ni concentrations assayed.
In the cultures with the 300 mg L−1 applied, 23.6 and 88.0% of total Ni was removed by adsorption to the cell surface of A. subtropica and Dunaliella sp., respectively, at the seventh day of culture. For the highest Ni concentration used (500 mg L−1), the bioadsorbed fraction represents 36.55 and 98.85% of the total Ni removed during the latency phase and then decreases to 19.18 and 93.63% for A. subtropica and Dunaliella sp., respectively, during the decline phase.
The Ni appeared bound to the cell surface. The cell wall is a first barrier in the uptake of the metal and plays an important role in processes responsible for heavy metal tolerance.
Effect of Ni toxicity on biochemical composition
To assess the impact of Ni exposure on biochemical composition of microalgae A. subtropica and Dunaliella sp., its carbohydrate, protein, and lipid content was measured at the seventh day of culture (Table 1).
Similar to growth rate, protein and carbohydrate content of A. subtropica and Dunaliella sp. showed a significant decrease at 200–500 mg Ni L−1 concentrations (Table 1).
Increase in Ni concentration in growth medium significantly enhanced the total lipid in the algae (Table 1). For A. subtropica, the highest lipid content was found in cells cultivated at 300 mg Ni L−1, which was about 6.8-fold higher than in the control culture. However, for Dunaliella sp., the highest lipid content was found in cells cultivated at 500 mg Ni L−1, followed by 300 and 200 mg L−1 of Ni.
The phenolic content varied similarly to the lipid content and reached a maximum at 300 mg Ni L−1 (0.257 mg GAE g−1 fresh weight (Fw)) for A. subtropica and 500 mg Ni L−1 (0.289 mg GAE g−1 Fw) for Dunaliella sp. (Table 1).
Both A. subtropica and Dunaliella sp. showed significant reductions in total chlorophyll and chlorophyll a and b with increased metal concentrations (p < 0.05; Table 1). In contrast, the carotenoid contents significantly increased when exposed to high Ni concentration. Furthermore, the administration of 500 mg Ni L−1 in the growth medium significantly increased the ratio of Car/total Chl which is an indirect indication of Ni-induced oxidative stress and reduced PS II activity.
Amphora subtropica and Dunaliella sp. showed a different composition of fatty acids (Table 2). Amphora subtropica amassed fatty acid in the range of C14 to C20. Palmitic acid C16:0 was the major fatty acid in Ni stressful conditions. It represented 39.5 and 48.58% of total lipids of A. subtropica and Dunaliella sp., respectively. Similarly, the ratio of C18:1 and C18:0 was increased, while C20:4 and C20:5 decreased significantly when A. subtropica was grown under 300 mg L−1 of Ni. In the Dunaliella sp. culture grown under 500 mg Ni L−1, C16:1, C16:3, and C18:0 appeared and C18:1, C18:2, and C18:3 decreased.
The single-stage Ni stress significantly affected the mineral level in the microalgae. Increase in Ni concentration in the growth medium significantly enhanced the calcium, iron, magnesium, manganese, potassium, and zinc amount in the cells (Table 3).
Effect of Ni toxicity on stress biomarkers
The damage caused by ROS was estimated through the TBARS level and SOD, CAT, and GPx activities. The MDA content was enhanced with increasing Ni concentration (Table 4). The maximum MDA accumulation was observed at 300 mg Ni L−1 for A. subtropica and 500 mg Ni L−1 for Dunaliella sp., where the cells accumulated MDA four- and sixfold higher than the control. However, it declined with the Ni concentration up to 500 mg Ni L−1 in A. subtropica at the seventh day of culture.
Activities of antioxidant enzymes substantially changed when the cells were exposed to Ni. The antioxidant enzyme activities in the algae increased with Ni concentration. Significantly increased SOD, CAT, and GPx activities where observed at 300 mg L−1 of Ni for A. subtropica and 500 mg L−1 in Dunaliella sp.
Discussion
The negative effects of Ni on microalgae growth have been widely reported in many studies (Anantharaj et al. 2011; Monteiro et al. 2012). Indeed, the higher the stress intensity, the slower the growth rate of the algae and the higher the total amount of light absorbed by the cell during a dividing cycle.
The inhibition effect of this metal might be caused by impairment of photosynthetic mechanism, blockage of cell division, and inhibition of the cellular enzymatic systems (Monteiro et al. 2012). This is due to the substitution exercised by Ni on other metallic ions in metalloenzymes (principally, Zn, Cu, and Ca), as well as its great affinity for biological structures that contain –SH groups, like proteins and enzymes, causing the inhibition of growth, photosynthesis, respiration, and other cellular processes.
Under Ni exposure, a higher amount of ROS is accumulated in cells, which lowers photosynthetic efficiency of photosynthetic organisms by peroxidation of thylakoid lipids and inactivation of electron transport of PSII (Liu et al. 2012). Gong et al. (2011) have also reported that high Ni concentrations affect the PSII of several marine microalgae. Faced with these environmental challenges, microalgae elaborate a variety of protective strategies, especially those that protect against the oxidative stress generated by the presence of metals. Among these strategies cited “morphological changes,” the reduction in cell size limits the exchange with the external medium, which induces a reduction in Ni absorption. Similarly, Martinez Ruiz and Martinez Jeronimo (2015) also showed a morphological change in Ankistrodesmus falcatus after Ni exposure. Sacan et al. (2007) also reported that aluminum and lead caused cell membrane lysis on Dunaliella tertiolecta.
Ni uptake by A. subtropica and Dunaliella sp. followed an initial rapid phase of uptake during the first days of culture, reaching a maximum, and thereafter, there was a decrease or stabilization of the amount of total removed Ni. Jennings and Rainbow (1979), Torres et al. (1998), Pérez Rama et al. (2002), Belghith et al. (2016), and Winters et al. (2017) reported a similar pattern in Dunaliella tertiolecta, Phaeodactylum tricornutum, Tetraselmis suecica, Dunaliella salina, and Euglena gracilis.
The present results revealed that the metal removal was up to 37 and 98% in A. subtropica and Dunaliella sp., respectively, after 7 days of exposure. Travieso et al. (1999) reported 48 and 31% cadmium and chromium uptake by Chlorella and Scenedesmus, respectively. These cadmium and chromium fixation rates remain much lower than Ni results in the present study.
In our assay, the decrease in the Ni concentration is explained by both the adsorption and the accumulation of high amount of Ni into the cells. Heavy metal removal process generally occurs in two stages: a rapid step and a slow step (Adey et al. 2011; Monteiro et al. 2012). During the rapid stage, the metal adheres to cellular surfaces (bioadsorption). If the metal is going to cross the cell membrane and pass through the cytoplasm, this step (bioaccumulation) is much more complex and requires more energy and physiological activity.
Our results with Dunaliella sp. indicate that the bioadsorbed metal levels are higher than the intracellularly removed levels. Belghith et al. (2016) also reported that D. salina accumulated the metal mainly in a bioadsorbed form; the metal removed intracellularly represents a minor fraction of the total removed metal.
Different reports have shown that exclusion mechanisms and Ni adsorption to cell surfaces seem not to be the main tolerance mechanism to Ni. Chlorella vulgaris chelated and accumulated a higher amount of metal into the cells (Carr et al. 1998). Similarly, Pérez Rama et al. (2002) observed that Tetraselmis suecica accumulated the highest proportion of cadmium intracellularly.
There has been little commercial utilization of microalgal biosorption for metal removal or processes improvement. Both dead and living biomass can be used for metal removal. Non-living biomass has the benefit that it can be regenerated for multiple functions, but the metal is not up taken into cells, since metals are adsorbed only (Torres et al. 1998). This can be a disadvantage because different reports (Matsunaga et al. 1999, Pérez Rama et al. 2002) with Tetraselmis species and our work with A. subtropica indicate that intracellular metal levels may be higher than the bioadsorbed. This indicates that living biomass of these microalgal cells would be more effective for Ni removal than non-living biomass.
The lowest protein content was found in cells grown under 500 mg Ni L−1 which could be a consequence of protein breakdown during response to metal stress. Under stress, downregulation of genes involved in primary metabolism and protein synthesis, as well as activation of genes related to autophagy and protein degradation, has been observed in higher plants, macroalgae, and microalgae (Dittami et al. 2011, Pancha et al. 2015a). Lowest carbohydrate content was also found in cells grown under 500 mg Ni L−1 (1.15 and 0.73 pg cell−1 of A. subtropica and Dunaliella sp., respectively) against 4.5 and 2.9 pg cell−1, respectively, in the control culture. These results were in agreement with the observation by Belghith et al. (2016), who reported that metal stress resulted in decreases of carbohydrates.
Since results show that cell damage was mild, it is likely that the alterations observed in lipid levels were a reflection of Ni-induced response, suggesting that protective mechanisms were established and cells increased the amount of lipid in response to Ni damage. A common response of microalgae upon exposure to Ni as a stressful condition is the synthesis of intracellular lipid (Jiang et al. 2015; Dahmen Ben Moussa et al. 2016).
Whether the lipids are suitable for producing biodiesel has to be examined. Lipid oxidative stability is a crucial factor for biodiesel quality. The level of linolenic acid (C18:3) should not be > 12% according to the European Standard for biodiesel, which is also limited to 1%, for polyunsaturated fatty acids with four or more double bonds (Knothe 2006). In the lipids produced by A. subtropica and Dunaliella sp., C18:3, ω3 was 5.51 and 8.68%, respectively, and the ratios of SFA, MUFA, and PUFA were 58.49–68.93%, 31.5–12.54%, and 10.01–18.53%, respectively. Taken together, the lipid produced by this species under the Ni stressful conditions can be considered more suitable feedstock for biodiesel production, from the aspect of lipid quality, than for food applications. According to de la Vega et al. (2011), good biodiesel feedstock contains high ratios of saturated and monounsaturated fatty acids and low ratio of polyunsaturated fatty acids.
In this work, it was shown that Ni negatively affects chlorophyll content and positively affects the carotenoid content, as a result of the inhibitory effect of Ni on chlorophyll synthesis. Similar results have been reported on the decrease in Chl a (Sabatini et al. 2009) and carotenoid content (Pinto et al. 2003) in algae exposed to toxic metals. Moreover, change in Chl a content is a common stress response in plants and microalgae.
These results contrast to other studies that have reported that toxic metals did not affect either chlorophyll or carotenoid contents (Branco et al. 2010). On the other hand, some earlier studies have reported increased chlorophyll content induced by excess cobalt in mung bean plants grown in sand culture (Tewari et al. 2002). Authors suggested that increase in chlorophyll content is a result of enhanced accumulation of protoporphyrin XI, one of the precursors in chlorophyll biosynthesis.
Shariati and Hadi (2011) also reported that under stress conditions, Dunaliella species can accumulate significant amounts of valuable chemicals such as carotenoids, polyphenols, glycerol, vitamins, and proteins. The potential ability of carotenoids to act as immunomodulatory and antioxidant agents has led to more active research exploring their application in the prevention of human cancers (Murthy et al. 2005).
The present study showed the increased levels of minerals in the cells exposed to different concentrations of Ni, suggesting that A. subtropica and Dunaliella sp. were very sensitive to Ni. Recent study shows importance of signal transduction by minerals in higher accumulation of neutral lipids by Chlorella sp. (Chen et al. 2014).
The increased level of MDA, which is a biomarker of lipid peroxidation, in response to excess Ni is suggestive of strong induction of oxidative stress. The same response was observed in the freshwater diatom Nitzschia palea (Branco et al. 2010), the cyanobacterium Hapalosiphon fontinalis (Sabatini et al. 2009), and the green algae Scenedesmus vacuolatus (Zutshi et al. 2008) and Chlamydomonas reinhardtii (Elbaz et al. 2010) when exposed to toxic metals.
Our findings showed that the activity of antioxidant enzymes such as SOD, CAT, and GPx increased after Ni exposition. Increasing the ROS levels by Ni exposure can lead to severe cell injury or death. Thus, microalgae can protect themselves against metal toxicity. The highest ROS in cells are neutralized by several antioxidative enzymes such as superoxide dismutase (SOD) and catalase (CAT) (Pancha et al. 2015b). In addition, the induction of antioxidant enzymes is an important protective mechanism developed by various species to minimize cell oxidative damage (Apel and Hirt 2004). This result of the present work is consistent with the observation by Branco et al. (2010), who revealed that Ni stress significantly increased antioxidant enzymes in microalgae strains.
Within cells, SOD constitutes the first barrier of defense against O2 −· by quickly converting O2 −· to O2 and H2O2 (Alscher et al. 2002). The increased SOD activity might be attributed to the important production of superoxides, therefore resulting in activation of existing enzyme pools or upregulated expression of the gene. Cellular hydrogen peroxide (H2O2) generated by SOD-mediated reaction is highly toxic and must be controlled at low levels. CAT and GPx are considered the most dominant enzymes that catalyze the transformation of H2O2 into H2O (Luis et al. 2006).
In this study, CAT and GPx activities were increased in Ni-exposed algae, suggesting that CAT, GPx, and SOD can jointly eliminate H2O2. The similar result was obtained in Chlamydomonas reinhardtii with Hg-induced oxidative stress, suggesting that heavy metals induced an activation of these enzymes to decrease the reactive oxygen species (Elbaz et al. 2010).
In conclusion, the climatic conditions in Tunisia make it into one of the most suitable areas not only for mass culture of A. subtropica and Dunaliella sp. but also for that of other algae. We conclude that the A. subtropica and Dunaliella sp. possess a high metal absorption capacity, which confers to them a very significant role in water detoxification. On the other hand, the presence of Ni in the culture medium modified the physicochemical composition of the strain and exhibits a stressor for Amphora and Dunaliella, thereby increasing their capacity for lipid accumulation. Many mechanisms are responsible for the resistance to metal pollution such as antioxidant enzymes production, in addition to the secondary metabolites that protect cells against the oxidative stress.
References
Adey WH, Kangas PC, Mulbry W (2011) Algal turf scrubbing: cleaning surface waters with solar energy while producing a biofuel. Bioscience 61:434–441
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Anantharaj K, Govindasamy C, Natanamurugaraj G, Jeyachandran S (2011) Effect of heavy metals on marine diatom Amphora coffeaeformis (Agardh. Kutz). Glob J Environ Res 5:112–117
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Belghith T, Athmouni K, Bellassoued K, El Feki A, Ayadi H (2016) Physiological and biochemical response of Dunaliella salina to cadmium pollution. J Appl Phycol 28:991–999
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Branco D, Lima A, Almeida SF, Figueira E (2010) Sensitivity of biochemical markers to evaluate cadmium stress in the freshwater diatom Nitzschia palea (Kützing) W. Smith. Aquat Toxicol 99:109–117
Carr H, Carino F, Yang M, Wong M (1998) Characterization of the cadmium-binding capacity of Chlorella vulgaris. Bull Environ Contam Toxicol 60:433–440
Chekroun KB, Baghour M (2013) The role of algae in phytoremediation of heavy metals: a review. J Mater Environ Sci 4:873–880
Chen H, Zhang Y, He C, Wang Q (2014) Ca2+ signal transduction related to neutral lipid synthesis in an oil-producing green alga Chlorella sp. C2. Plant Cell Physiol 55:634–644
Dahmen Ben Moussa I, Chtourou H, Rezgui F, Sayadi S, Dhouib A (2016) Salinity stress increases lipid, secondary metabolites and enzyme activity in Amphora subtropica and Dunaliella sp. for biodiesel production. Bioresour Technol 218:816–825
Das K, Das S, Dhundasi S (2008) Nickel, its adverse health effects & oxidative stress. Indian J Med Res 128:412
de la Vega L, Fröbius K, Moreno R, Calzado MA, Geng H, Schmitz ML (2011) Control of nuclear HIPK2 localization and function by a SUMO interaction motif. Biochim Biophys Acta 1813:283–297
Dittami SM, Gravot A, Renault D, Goulitquer S, Eggert A, Bouchereau A, Boyen C, Tonon T (2011) Integrative analysis of metabolite and transcript abundance during the short-term response to saline and oxidative stress in the brown alga Ectocarpus siliculosus. Plant Cell Environ 34:629–642
Draper H, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Elbaz A, Wei YY, Meng Q, Zheng Q, Yang ZM (2010) Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 19:1285–1293
Ercal N, Gurer Orhan H, Aykin Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–120
Gong N, Shao K, Feng W, Lin Z, Liang C, Sun Y (2011) Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere 83:510–516
Hiscox JT, Israelstam G (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Jennings J, Rainbow P (1979) Accumulation of cadmium by Dunaliella tertiolecta Butcher. J Plankton Res 1:67–74
Jiang Y, Nunez M, Laverty KS, Quigg A (2015) Coupled effect of silicate and nickel on the growth and lipid production in the diatom Nitzschia perspicua. J Appl Phycol 27:1137–1148
Knothe G (2006) Analyzing biodiesel: standards and other methods. J Am Oil Chem Soc 83:823–833
Liu LN, Bryan SJ, Huang F, Yu J, Nixon PJ, Rich PR, Mullineaux CW (2012) Control of electron transport routes through redox-regulated redistribution of respiratory complexes. Proc Natl Acad Sci 109:11431–11436
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luis P, Behnke K, Toepel J, Wilhelm C (2006) Parallel analysis of transcript levels and physiological key parameters allows the identification of stress phase gene markers in Chlamydomonas reinhardtii under copper excess. Plant Cell Environ 29:2043–2054
Martinez Ruiz EB, Martinez Jeronimo F (2015) Nickel has biochemical, physiological, and structural effects on the green microalga Ankistrodesmus falcatus: an integrative study. Aquat Toxicol 169:27–36
Matagi S, Swai D, Mugabe R (1998) A review of heavy metal removal mechanisms in wetlands. Afr J Trop Hydrobiol Fish 8:13–25
Matsunaga T, Takeyama H, Nakao T, Yamazawa A (1999) Screening of marine microalgae for bioremediation of cadmium-polluted seawater. J Biotechnol 70:33–38
Monteiro CM, Castro PM, Malcata FX (2012) Metal uptake by microalgae: underlying mechanisms and practical applications. Biotechnol Prog 28:299–311
Moroney JV, Bartlett SG, Samuelsson G (2001) Carbonic anhydrases in plants and algae. Plant Cell Environ 24:141–153
Murthy KC, Vanitha A, Rajesha J, Swamy MM, Sowmya P, Ravishankar GA (2005) In vivo antioxidant activity of carotenoids from Dunaliella salina—a green microalga. Life Sci 76:1381–1390
Pancha I, Chokshi K, Mishra S (2015a) Enhanced biofuel production potential with nutritional stress amelioration through optimization of carbon source and light intensity in Scenedesmus sp. CCNM 1077. Bioresour Technol 179:565–572
Pancha I, Chokshi K, Maurya R, Trivedi K, Patidar SK, Ghosh A, Mishra S (2015b) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348
Perales Vela HV, Peña Castro JM, Cañizares Villanueva RO (2006) Heavy metal detoxification in eukaryotic microalgae. Chemosphere 64:1–10
Pérez Rama M, Alonso JA, Lopez CH, Vaamonde ET (2002) Cadmium removal by living cells of the marine microalga Tetraselmis suecica. Bioresour Technol 84:265–270
Pinto E, Sigaud-kutner T, Leitao MA, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal-induced oxidative stress in algae. J Phycol 39:1008–1018
Provasoli L, McLaughlin J, Droop M (1957) The development of artificial media for marine algae. Arch Mikrobiol 25:392–428
Sabatini SE, Juarez AB, Eppis MR, Bianchi L, Luquet CM, Reos de Molina MC (2009) Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol Environ Saf 72:1200–1206
Sacan MT, Oztay F, Bolkent S (2007) Exposure of Dunaliella tertiolecta to lead and aluminum: toxicity and effects on ultrastructure. Biol Trace Elem Res 120:264–272
Shariati M, Hadi MR (2011) Microalgal biotechnology and bioenergy in Dunaliella. In: Carpi A (ed) Progress in molecular and environmental bioengineering—from analysis and modeling to technology applications. InTech, Rijeka, pp 483–506
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Stauber J, Florence T (1985) The influence of iron on copper toxicity to the marine diatom, Nitzschia closterium (Ehrenberg) W. Smith. Aquat Toxicol 6:297–305
Tewari RK, Kumar P, Sharma PN, Bisht SS (2002) Modulation of oxidative stress responsive enzymes by excess cobalt. Plant Sci 162:381–388
Torres E, Cid A, Herrero C, Abalde J (1998) Removal of cadmium ions by the marine diatom Phaeodactylum tricornutum Bohlin accumulation and long-term kinetics of uptake. Bioresour Technol 63:213–220
Travieso L, Canizares R, Borja R, Benitez F, Dominguez A, Dupeyrón R, Valiente V (1999) Heavy metal removal by microalgae. Bull Environ Contam Toxicol 62:144–151
Winters C, Guéguen C, Noble A (2017) Equilibrium and kinetic studies of Cu(II) and Ni(II) sorption on living Euglena gracilis. J Appl Phycol 29:1391–1398
Zutshi S, Choudhary M, Bharat N, Abdin MZ, Fatma T (2008) Evaluation of antioxidant defense responses to lead stress in Hapalosiphon fontinalis-3391. J Phycol 44:889–896
Acknowledgements
This study was supported by the Ministry of Higher Education and Scientific Research of Tunisia under Contract Program of the Environmental Bioprocesses Laboratory.
Authors’ contributions
IDB designed and performed the experiments, analyzed the data, and wrote the paper. KA and HC participated in the design and execution of the experiments. HA, SS, and AD critically reviewed the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dahmen-Ben Moussa, I., Athmouni, K., Chtourou, H. et al. Phycoremediation potential, physiological, and biochemical response of Amphora subtropica and Dunaliella sp. to nickel pollution. J Appl Phycol 30, 931–941 (2018). https://doi.org/10.1007/s10811-017-1315-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1315-z