Abstract
Vegetative propagation of Chondracanthus chamissoi by means of secondary attachment discs (SAD) has been an effective strategy for maintaining the species biomass in natural beds. Suspended culture was used in the present study. Biomass, epiphytes, length of new thalli, and number of secondary attachment discs of C. chamissoi were measured during different seasons as well as different depths (2, 4, and 6 m), and cultivation times (1, 2, 3, and 4 months). The greatest accumulation of biomass, biofouling, and SAD formation occurred during spring cultivation, while maximum lengths were observed in winter. Maximum values of each variable were observed at 2 m depth in most cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chondracanthus chamissoi (C Agardh) Kützing is a benthic marine red alga found in the lower intertidal zone up to 15 m depth and reaches lengths of 50 cm (Hoffman and Santelices 1997). Its distribution is from Paita, Peru (5°S) to Ancud, Chile (42°S) (Ramírez and Santelices 1991), but it has been also reported on the coasts of Korea, Japan, and France, suggesting that it is not necessarily endemic to the Southeast Pacific (Yeon et al. 2015). According to records from ProChile (2016), worldwide total available biomass of this algae varied between 1000 and 1500 t (wet weight) between 2005 and 2012. Total landings of C. chamissoi currently reach the order of 1600 t wet weight, of the order of 900 and 700 t from Chile and Peru respectively, which is equivalent to about 354 t of dry product.
This species has become important because of its commercial value, principally due to its use for carrageenan extraction and for human consumption in Asian countries (Bulboa and Macchiavello 2006; Avila et al. 2011). Its most common destinations are Japan and Taiwan (ProChile 2016). Chondracanthus chamissoi carrageenan content reached up to 24.6% dry weight (Pereira et al. 2009). There is currently a growing demand for its direct consumption as food given its exceptional nutritional qualities, among which stand out: a high concentration of monounsaturated fatty acids with a total of 47.07%, composed mainly of oleic acid (Sánchez-Machado et al. 2004; Pohl and Zurheide 1979), followed by palmitoleic acid, 8.11%, and essential linoleic and linolenic fatty acids, which are powerful fat-soluble antioxidant agents (Ortiz 2011).
This red alga has been widely exploited along the Chilean and Peruvian coasts, leading to 90% decreases in stocks in the last 15 years, with landings of 24,000 t in 2000, followed by 4600 t in 2004, and 900 t in 2010 (SERNAPESCA 2014). These declines in extracted volumes are related to the collapse of natural beds as well as to the appearance of opportunistic species such as Ulva spp. and Codium fragile, which can colonize hard substrata, thus displacing C. chamissoi. This has been observed repeatedly in beds located in Caldera and Calderilla in northern Chile (Pers. Obs.).
Due to the deterioration of natural beds several authors have developed management strategies based on ecological studies (González and Meneses 1996; González et al. 1997; Vásquez and Vega 2001; Macchiavello et al. 2003). Moreover, various controlled propagation techniques have been developed (Bulboa and Macchiavello 2001; Bulboa et al. 2008; Sáez et al. 2008). Of these, spore propagation (Bulboa et al. 2010; Ávila et al. 2011; Barrientos and Otaíza 2014) and vegetative propagation have been promising, because C. chamissoi develop secondary attachment discs, which allow it adhere to substrate and generate new plants (Bulboa et al. 2005, 2013; Bulboa and Macchiavello 2006). This ability also has been observed in Chondracanthus squarrulosus (Pacheco-Ruiz et al. 2005) and in species in the genera Dictyota, Bryopsis, Sphacelaria and Nereocystis (Sarabhai and Arora 2002). Under controlled conditions, secondary attachment discs facilitate vegetative propagation from thalli fragments, in contrast to spore propagation which requires more rigorous and costly maintenance (Hernández et al. 2007; Bulboa et al. 2013).
In Chile, C. chamissoi prices range from US $ 12.5 per dry kg to about US $ 35 per kg for an elaborated, colored product of good quality (ProChile 2016). These high prices come with strict quality standards on the market. The product must be clean, be free of impurities and epibionts, and have a specific texture and color (Bulboa and Macchiavello 2006). These specifications are difficult to obtain from natural beds throughout the year because of variations in morphology (Acleto 1986), growth (Bulboa and Macchiavello 2001; Bulboa et al. 2008), fluctuation in seasonal abundance across life cycle stages (González et al. 1997) and susceptibility to epiphytes (Bulboa et al. 2005, 2007). Culture of C. chamissoi is an alternative production method that could lessen the burden on collapsed natural beds, satisfy current unmet demand, move away from the seasonality of natural populations, and create a higher quality product.
We evaluated the growth of C. chamissoi during suspended vegetative culture in the sea under varying seasons, depths, and culture times, with the purpose of validating the technology for the productive scaling and the production of high quality biomass.
Materials and methods
Collection of specimens
Chondracanthus chamissoi fronds were obtained by scuba diving in natural beds located in Puerto Aldea Bay (30° 17′ 31″ S; 71° 36′ 32″ W), during the months of July and October 2015, as well in January and April 2016. Fronds were transported in coolers (isothermal boxes made of plastic or styrofoam), with two or three ice packs to keep low temperatures (10–15 °C), to the Marine Botany Laboratory at the Universidad Católica del Norte. Vegetative thalli (without reproductive structures) and with similar morphology (not very branched individuals with natural reddish tone, and thalli of ± 1 cm width) were selected, discarding specimens in reproductive stages or poor health. Finally were rinsed with filtered seawater and cleaned of sediment and epiphytes as suggested by Bulboa and Macchiavello (2001) and Bulboa et al. (2005).
Fifteen kilogram of clean thalli was transferred into a 1000 L outdoor tank. The tank was a fiberglass container (1.80 × 0.70 × 0.85 m deep; ± 1000 L) for aquaculture industrial use (Ocean Teach S.A, Chile). All pipes, connectors and valves were made of PVC. The thalli were maintained with a continuous flow of filtered seawater (150 L h−1), and air was supplied by a blower until the inoculation started. The seawater filtration system consisted of a mechanical cartridge filters set (1, 10, and 25 μm) and a 40 W ultraviolet lamp (model GPH793T5L, Biolight SA).
Temperature, salinity, and pH inside the tanks were recorded using a HOBO Pendant model UA-002-08 sensor.
Substrate inoculation
Vegetative thalli fragments (2 to 5 cm) were inoculated in polypropylene mesh for horticulture products; these inoculation units are 1 m length, 4 cm width, with 0.5 cm mesh openings. Inoculation units were inoculated with 9 g of seaweed biomass per linear meter (g m−1), then were placed in the PVC structure frame (10 mesh each) and kept in the hatchery for 20 days. A total of 800 inoculation units were made for each season. Once this period was finished, the inoculation units were placed in the sea.
The inoculation was carried out in the months of August and November 2015, as well in February and May 2016, with a total of one inoculation process for each evaluated season.
Seasonal culture in the ocean
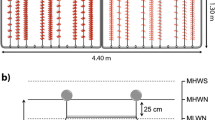
At the start of each season, inoculation units were fixed to 6 mm lines (culture units) with 20 inoculation units per meter of line and placed vertically in the water column at 2, 4 and 6 m depth. A concrete weight of 500 g was placed at every unit base to provide stability. Units were tied to a line (20 mm diameter, 100 m length) which was kept horizontal at 1 m depth with flotation buoys (long line).
Thirty-six culture units (replicates) were installed at each depth and for each season in a Benthic Resources Exploitation Management Area (AMERB for its acronym in Spanish), located in Caleta Hornos (29° 38′ 28″ S; 71° 18′ 23″ W).
Four cultures were carried out: winter (August 2015), spring (November 2015), summer (February 2016), and autumn (May 2016). After each culture, successive destructive sampling was done every 30 days for a total of 120 days for each seasonal culture. This was done by randomly removing three culture units (replicates) per depth, evaluating a total of 18 inoculation units. Biomass in gram wet seaweed per linear meter (g m−1), length of new thalli (cm), number of secondary attachment discs per linear meter of substrate (SAD m−1) and amount of biofouling (g m−1) were recorded.
Measurement of biofouling was carried out by removing organisms adhered to newly cultivated fronds. Organisms were analyzed using a stereo microscope, the classification was carried out reaching the lowest taxonomic level, using appropriate literature on marine invertebrates (Zagal et al. 2001) and seaweeds (Hoffman and Santelices 1997). Light and temperature were recorded at various depths in the water column using a HOBO Pendant model UA-002-08 sensor.
Data analysis
A three-way ANOVA was done to evaluate effects of depth, culture times, and seasonality of the variables mentioned above, as suggested by Conover (1980). A Tukey test was also carried out for those treatments that presented significant differences and the homogenous subgroups were identified separately for each depth. Spearman correlations were done between biomass, number of SADs, and epiphyte biomass. All statistics were done to a 95% significance level using SPSS (IBM version 22).
Results
Abiotic parameters
Variations in temperature (°C), salinity (PSU), and pH in the hatchery (outdoor tank) recorded in the experimental period during winter, spring, summer, and autumn inoculations are shown in Table 1. Table 2 shows variations in irradiance (μmol photons m−2 s−1) and sea temperature (°C) at various propagation depths, recorded during the experimental period (August 2015 to August 2016) in Caleta Hornos, Chile.
Biomass
Chondracanthus chamissoi had actively grown in all of the evaluated conditions, with significant differences (P < 0.05) between seasonal culture, depths, and culture times (Table 3). Biomass increased progressively with culture time (months), with the highest values after 4 months for winter, spring, and autumn, reaching average values of 156.6 ± 31.8, 217.0 ± 17.7 and 64.7 ± 11.7 g m−1, respectively. During summer, maximum accumulation of biomass was 38.2 ± 10.2 g m−1 after 2 months of culture, with a decrease in the following months (Fig. 1).
Biomass and epiphyte levels observed in Chondracanthus chamissoi culture at sea, for 4 months, at depths of 2, 4, and 6 m in different seasons of the year. a, b Winter. c, d Spring. e, f Summer. g, h Autumn culture. The letters over the columns indicate significant differences between months of culture (Tukey test). Data expressed as the mean ± SD (n = 9)
There was a decrease in biomass with greater depth, the greatest accumulation of biomass was found at the shallowest depth evaluated (2 m), which was observed in the culture during winter, spring, and autumn, while the peak in summer was at 4 m depth. Different culture times produced significant differences in biomass in most cases (P < 0.05 Tukey test), with the exception of the winter culture at 4 m, were no differences between accumulated biomass at months 3 and 4 was observed. This was similar to biomass observed in autumn sowing at 2 and 6 m depth, while at 4 m no differences were observed between the second and fourth month of culture.
Epiphyte level
Epiphytes were present throughout the culture period, with significant differences between seasons, depths, and culture times (P < 0.05). The organisms recorded as epiphytes on C. chamissoi were Ceramium sp. (Rhodophyta) and Bugula spp. (Bryozoa). Epiphyte level showed similar tendencies as C. chamissoi biomass with respect to seasonality and depth, with a positive correlation of ρ = 0.73 (P < 0.001). The amount of epiphytes registered did not exceed the harvested biomass of C. chamissoi at any point during the culture period (Fig. 1).
A progressive increase in epiphyte levels during propagation was observed. The highest levels were 79.5 ± 9.5 g m−1 during the spring, followed by 54.5 ± 20.0 and 17.3 ± 6.2 g m−1 for winter and summer cultures, respectively. The autumn culture had the lowest levels of epiphytes at less than 2 g m−1 at all depths and propagation times evaluated. A peak was reached at 4 months of propagation for winter and spring culture. In summer, the peak occurred at the second month and in autumn, it occurred during the first month. Like biomass, the highest values of epiphytes were recorded at 2 m depth for the winter, spring and autumn cultures, while in summer the peak was observed at month 4.
Thalli length
Similar to biomass, measurements of thalli length showed a progressive increase with culture time in all the seasons except for summer. With respect to depth, greater lengths were observed at 2 m depth only for spring, summer, and autumn, while in winter the maximum length was obtained at 4 m depth. Thallus length also showed significant differences between the evaluated factors and their combination (P < 0.05) (Table 3).
Winter culture measurements exceeded 10 cm in thallus length for depths of 4 and 6 m. In spring, values ranged from 4.1 ± 1.1 to 7.6 ± 2.4 cm, followed by the autumn culture which presented values between 4.0 ± 1.0 and 6.2 ± 1.2 cm. All these lengths were reached after 4 months of culture, while in the summer culture, values of 4.2 ± 1.7 and 3.3 ± 1.5 cm were observed for depths of 2 and 4 m after 4 months, unlike for the 6 m depth where the maximum recorded length was 3.4 ± 1.0 cm at the second month of culture.
Secondary attachment discs
SADs per linear meter of substrate showed a similar tendency as biomass do, with respect to depth and culture time, showing significant differences between seasons, depths, and culture times (P < 0.05). The highest values were observed at 2 m depth, after 4 months of culture for winter, spring, and autumn, with values of 454.6 ± 77.9, 759 ± 51.1 and 265.2 ± 45.6 SAD m−1, respectively, whereas in summer a decrease was observed after the second month at all evaluated depths, registering a maximum value of 224.0 ± 28.7 SAD m−1 at 2 m depth. This variable was positively correlated with variations in recorded biomass, with a value of ρ = 0.82 (P < 0.001).
In conclusion we can emphasize that all the evaluated factors generated significant differences for the study variables, and all of them presented a progressive increase in relation to culture time with some exceptions for the summer season. The biomass, SAD, and epiphyte level presented a similar trend, with maximum values being observed at depth of 2 m in all cases, despite the differences generated by the seasonal effect.
The thalli length did not present a pattern similar to that of the other variables, since their maximum values did not coincide with the season of greater biomass production and SAD. In general, the spring season was the most productive in terms of biomass and SAD. The greatest lengths were obtained in winter culture and the lowest levels of epiphytes were recorded in autumn.
Discussion
Highest biomass values were always obtained at lowest evaluated depth, independent of the season in which the culture was carried out, as observed by Bulboa et al. (2005), in suspended culture of C. chamissoi on lines, with higher production at 1 m depth, as opposed to 3 and 5 m.
Several experimental studies have shown that C. chamissoi is highly adaptable to light and temperature variation, obtaining higher growth rates between 20 and 25 °C, and irradiances of 70 to 120 μmol photons m−2 s−1 (Bulboa and Macchiavello 2001; Bulboa et al. 2008). These values are in the highest range evaluated in this study and agree in part with our current results, due to greater accumulation of biomass in the first meters of depth, where temperature and light irradiance levels were the highest throughout the experimental period.
Most productive periods were the winter and spring culture, and the least productive were in summer and autumn. This is similar to the results obtained by Bulboa et al. (2005), who reported a higher amount of biomass for gametophytic and sporophytic thalli in autumn, with values between 158 and 160 g m−1, respectively, after 2 months of propagation and winter with production between 102 and 120 g m−1, respectively, after 3 months of culture. In contrast, lowest levels of biomass were reported in summer, with a range value of 44 to 57 g m−1 after 1 month of suspended cultivation of C. chamissoi in Herradura Bay (Coquimbo, Chile). On the other hand, Bulboa and Macchiavello (2006) reported maximum levels of biomass for C. chamissoi vegetative thalli between 44 and 93 g m−1, after 1 and 2 months of cultivation, for the localities of Coquimbo and Calderilla, respectively. These reported values do not exceed the maximum value obtained in this study in the winter and spring seasons, which are the most productive seasons of the year, for cultivation and natural beds (Vásquez and Vega 2001).
The period of greatest biomass accumulation in this study is similar to the period of greatest biomass production previously observed in natural beds in northern Chile between late winter, spring, and early summer (Vásquez and Vega 2001). On the other hand, low C. chamissoi biomass in warmer months may be associated with herbivore proliferation or the low availability of nutrients and high light irradiances (Vásquez and Vega 2001). Additionally, Bulboa et al. (2005) associate the low yield of C. chamissoi in warm months with thalli detachment and increases in epiphytes. We suggest that harvest should be carried out after 4 months of cultivation for cultures done in winter and spring, after 2 months of culture in summer, and after 3 months of culture in autumn.
Epiphytes are one of the major problems associated with the culture of macroalgae (Lüning and Pang 2003). This problem was also observed in this study, with epiphytes present throughout the experimental period. Epiphyte levels were positively correlated with C. chamissoi biomass production. However, although overall epiphytic levels did not exceed C. chamissoi biomass in the autumn season, they were less than 2 g m−1, meaning that C. chamissoi remained mostly free of epiphytes. Autumn was not the best season in terms of biomass produced, but the low presence of epiphytes is important for C. chamissoi commercial uses, especially when is used in human consumption.
Though thallus length did not show the same pattern with respect to depth in all of the seasonal cultures, maximum lengths were reached in winter culture at all tested depths after 4 months of culture at sea. Bulboa et al. (2005) reported maximum lengths between 15 and 20 cm in a period of 4 months, on the other hand, Bulboa and Macchiavello (2006) reported maximum lengths between 10 and 20 cm in C. chamissoi thalli cultured at sea after a period of 2 months. These values exceed the maximum range reported in this study; however, it must be taken into consideration that in this study, propagation was started with the inoculation of small thallus fragments, favoring the formation of SAD before the culture at sea and not with inoculation of whole plants as in the case of the works cited above. On the other hand, Bulboa et al. (2013) reported higher lengths reached by C. chamissoi thalli during the summer season in comparison to the winter season, with inoculations similar to those of the present study, but these propagations were done in tanks. It should be noted that the maximum length reached in the present study was recorded in the winter culture, which begins with the lowest light and temperature levels of the year.
In the present study, SAD showed a similar trend as biomass, with a positive correlation that can be interpreted as greater development of new thalli. This would imply a higher formation of SAD and consequently more new individuals via this method of vegetative reproduction, leading to a greater amount of biomass. Bulboa et al. (2013) report higher formation of SADs in winter than summer. This observation agrees with this study, in which lowest amount of SAD were recorded during summer culture. However, Macchiavello et al. (2003) reported greater re-adhesion of drifting thalli in natural C. chamissoi beds during the summer period.
The results obtained in this study indicate that C. chamissoi possesses adequate biological attributes to be cultivated because it presented active growth in all experimental treatments, even considering seasonal and bathymetric variations in all of the evaluated variables.
References
Acleto C (1986) Algunos aspectos biológicos de Gigartina chamissoi (C. Ag.) J. Agardh (Rhodophyta, Gigartinales). Revista de Ciencias UNMSM 74(1):38–47
Avila M, Piel M, Cáceres J, Alveal K (2011) Cultivation of the red alga Chondracanthus chamissoi: sexual reproduction and seedling production in culture under controlled conditions. J Appl Phycol 23:529–536
Barrientos E, Otaíza R (2014) Juveniles generados a partir de esporas no asentadas de Chondracanthus chamissoi (Rhodophyta: Gigartinales) presentan capacidad de adhesión al sustrato. Rev Biol Mar Oceanogr 49:135–140
Bulboa C, Macchiavello J (2001) The effects of light and temperature on different phases of the life cycle in the carrageenan producing alga Chondracanthus chamissoi (Rhodophyta, Gigartinales). Bot Mar 44:371–374
Bulboa C, Macchiavello J (2006) Cultivation of cystocarpic, tetrasporic and vegetative fronds of Chondracanthus chamissoi (Rhodophyta, Gigartinales) on ropes at two localities in northern Chile. Investig Mar 34(1):109–112
Bulboa C, Macchiavello J, Oliveira E, Fonk E (2005) First attempt to cultivate the carrageenan-producing seaweed Chondracanthus chamissoi (C. Agardh) Kutzing (Rhodophyta; Gigartinales) in northern Chile. Aquac Res 36:1069–1074
Bulboa C, Macchiavello J, Véliz K, Macaya E, Oliveira E (2007) In vitro recruitment of Ulva sp. and Enteromorpha sp. on gametophytic and teraesporophytic thalli of four populations of Chondracanthus chamissoi from Chile. J Appl Phycol 19:247–254
Bulboa C, Macchiavello J, Oliveira E, Véliz K (2008) Growth rate differences between four Chilean populations of edible seaweed Chondracanthus chamissoi (Rhodophyta, Gigartinales). Aquac Res 39:1550–1555
Bulboa C, Macchiavello J, Véliz K, Oliveira E (2010) Germination rate and sporeling development of Chondracanthus chamissoi (Rhodophyta, Gigartinales) varies along a latitudinal gradient on the coast of Chile. Aquat Bot 92:137–141
Bulboa C, Véliz K, Sáez F, Sepúlveda C, Vega L, Macchiavello J (2013) A new method for cultivation of the carragenophyte and edible red seaweed Chondracanthus chamissoi based on secondary attachment disc: development in outdoor tanks. Aquaculture 410-411:86–94
Conover W (1980) Practical nonparametric statistics. John Wiley and Sons Inc, New York
González J, Meneses I (1996) Differences in the early stages of development of gametophytes and tetrasporophytes of Chondracanthus chamissoi (C. Ag.) Kutzing from Puerto Aldea, northern Chile. Aquaculture 143:91–107
González J, Meneses I, Vásquez J (1997) Field studies in Chondracanthus chamissoi (C. Agardh) Kutzing: seasonal and spatial variations in life-cycle phases. Biología Pesquera 26:3–12
Hernández M, Buschmann A, Cifuentes M, Correa J, Westermeier R (2007) Vegetative propagation of the carrageenophytic red alga Gigartina skottsbergii Sechell et Gardner: indoor and field experiments. Aquaculture 262:120–128
Hoffmann A, Santelices B (1997) Flora Marina de Chile central, Ediciones Universidad Católica de Chile Santiago, p 434
Lüning K, Pang S (2003) Mass cultivation of seaweeds: current aspects and approaches. J Appl Phycol 15:115–119
Macchiavello J, Bulboa C, Edding M (2003) Vegetative propagation and spore-based recruitment in the carrageenophyte Chondracanthus chamissoi (Gigartinales, Rhodophyta) in northern Chile. Phycol Res 51:45–50
Ortiz J (2011) Composición Nutricional y Funcional de Algas Rodofíceas. Project DI 02–2002. Universidad de Chile, Monografía, p 32
Pacheco-Ruiz I, Zertuche J, Espinoza J (2005) The role of secondary attachment disc in the survival of Chondracanthus squarrulosus (Rhodophyta, Gigartinales). Phycologia 36:120–127
Pereira L, Critchley A, Amado A, Ribeiro-Claro P (2009) A comparative analysis of phycocolloids produced by underutilized versus industrially utilized carragenophytes (Gigartinales, Rhodophyta). J Appl Phycol 21:599–605
Pohl P, Zurheide F (1979) Fatty acids and lipids of marine algae and the control of their biosynthesis by environmental factors. In: Hoppe HA, Levring T, Tanaka Y (eds) Marine algae in pharmaceutical science. Walter de Gruyter, Berlin, pp 473–523
ProChile (2016) Chicorea de mar (Chondracanthus chamissoi): Situación y perspectivas. Innova Chile CORFO. COD:13 BRP2-22126. Chile
Ramírez M, Santelices B (1991) Catálogo de las algas marinas bentónicas de la costa temperada del Pacífico Sudamericano. Monografías Biológicas. Facultad de Ciencias Biológicas. Pontificia Universidad Católica del Norte. Publicaciones Periódicas Vicerrectoría Académica, Santiago, 5: 1–499
Sáez F, Macchiavello J, Fonk E, Bulboa C (2008) The role of the secondary attachment disc in the vegetative propagation of Chondracanthus chamissoi (Gigartinales; Rhodophyta). Aquat Bot 89:63–65
Sanchez-Machado D, Lopez-Cervantes J, Lopez-Hernandez J, Paseiro-Losada P, Simal-Lozano J (2004) Determination of the uronic acid composition of seaweed dietary fibre by HPLC. Biomed Chromatogr 18:90–97
Sarabhai B, Arora C (2002) Textbook of algae. Anmol Publications, New Delhi. 368pp
SERNAPESCA (2014) Anuarios estadísticos de pesca, Servicio Nacional de Pesca y Acuicultura, Chile
Vásquez J, Vega A (2001) Chondracanthus chamissoi (Rhodophyta, Gigartinales) in northern Chile: ecological aspects for management of wild populations. J Appl Phycol 13:267–277
Yeon M, Macaya E, Sook M (2015) Molecular evidence for verifying the distribution of Chondracanthus chamissoi and C. teedei (Gigartinaceae, Rhodophyta). Bot Mar 58:103–113
Zagal C, Hermosilla C, Riedemann A (2001) Guía de invertebrados marinos del litoral Valdiviano, Edit. Universidad Austral de Chile, 217 pp
Acknowledgements
We gratefully appreciate the efforts of the staff of the Marine Botany Laboratory of Universidad Católica del Norte. We also thank Dr. Mario Edding Villablanca for reviewing an earlier draft of this manuscript.
Funding
This work was supported by Fondef Grant ID14I10343.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Macchiavello, J., Sepúlveda, C., Basaure, H. et al. Suspended culture of Chondracanthus chamissoi (Rhodophyta: Gigartinales) in Caleta Hornos (northern Chile) via vegetative propagation with secondary attachment discs. J Appl Phycol 30, 1149–1155 (2018). https://doi.org/10.1007/s10811-017-1307-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1307-z