Abstract
The red alga Chondracanthus chamissoi (Gigartinales) is endemic to the southern-central region of South America. In the Pacific Ocean, it is distributed from north-central Peru to Chiloe Island. This species is of economic importance because it is edible and used for carrageenan production. The tetrasporophyte phase was grown in the laboratory, obtaining male and female gametophytes that were incubated under different photoperiod, pH, salinity and temperature conditions. These gametophytes developed and generated reproductive structures that led to in vitro maturation. Subsequently, fertilisation occurred and formation of cystocarps was observed. Finally, carpospores were released and the formation of sporophytes completed the life history of this species under laboratory conditions. Reproductive phase growth rates were recorded for each of the different culture conditions used. Sporophytes reached the highest daily growth rate (22%), while gametophyte’s daily growth rate was slower (9%). This research confirms, in vitro, the assumption that C. chamissoi has a sexual triphasic life history Polysiphonia type with isomorphic gametophytes and tetrasporophytes. The development of the complete life history took 20 months in the laboratory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chondracanthus chamissoi (C Agardh) Kützing, is a marine red alga in the Gigartinales. This species is endemic to the Peruvian and Chilean coast and, in Chile, is distributed from Iquique (20°) to Chiloé (42°; Ramírez and Santelices 1991; Hoffmann and Santelices, 1997). This species is used in two ways, as raw material for the carrageenan industry and for human consumption (as salad) in Asian countries (Bulboa and Macchiavello, 2006). Infertile fronds and tetrasporic fronds are exported to Asian markets because of their soft texture. C. chamissoi gametophytes produce carrageenans of the kappa family, with a yield of 14.2% dry weight, while tetrasporophyte stages produce carrageenans of the lambda family, with a yield of 24.6% dry weight (Pereira et al., 2009).
In sheltered sites, this species can reach up to 50 cm in length and grows on hard substrates from the lower intertidal zone to 15-m depth (Hoffmann and Santelices, 1997). To date, studies on this species have addressed different aspects, such as reproductive phenology and population dynamics (González et al., 1997; Vásquez and Vega 2001; García et al. 1997; Ávila et al., 2008), growth phase development under laboratory conditions (González and Meneses 1996), vegetative propagation (Macchiavello et al., 2003; Sáez et al. 2008), cultivation under controlled conditions (Alveal et al., 1999) and field culture at an experimental level (Bulboa and Macchiavello, 2001; 2006; Bulboa et al., 2005).
Chondracanthus chamissoi reproductive behaviour is similar to other Gigartinales, where carpospore and tetraspore abundance at any time of the year is a function of: (1) the relative abundance of the corresponding reproductive phase, (2) the number of structures per frond and (3) the number of spores per reproductive structure (González and Meneses, 1996; Bulboa et al., 2010). González and Meneses (1996) report that for northern Chile (Puerto Aldea, 29º–30ºS), higher spore production, germination and recruitment of new seedlings occurred during summer; while Vásquez and Vega (2001) reported that near to Puerto Aldea, production of spores was higher in spring. On the other hand, in northern Chile, natural beds for this species show a positive relationship between temperature increase, growth and formation of reproductive structures. In this paper we report the life history of the species in vitro and the effect of abiotic factors on the growth and development of seedlings under controlled conditions.
Materials and methods
Life history
Cultures were started in October 2007, using fertile fronds bearing C. chamissoi cystocarps from Coliumo, Chile (36°31′S; 72°57′W). Reproductive plants in the cystocarpic phase were collected using hooka divers. Once collected, the plants were transported to the laboratory of the Institute of Science and Technology Arturo Prat University, in Puerto Montt. The samples were transported in a cooler with gelpack to keep the reproductive fronds cold and humid. Once in the laboratory, the seaweeds were separated (into reproductive and vegetative fronds); the mature ones selected and then washed under tap water. Finally, they were rinsed four times with filtered seawater to eliminate epiphytes.
Once the fronds were washed and dehydrated with paper towel, they were left for 5 h in darkness at ambient temperature. After this period, they were placed in a glass container (1,000 mL) with filtered and sterilised seawater, enriched with culture medium (PES) 10 mL L−1 (Provasoli, 1968). After a few hours, they started to sporulate and the first spores were observed. After reaching a high carpospore density, they were dispensed in Petri dishes (30 mL) and maintained under controlled conditions (temperature, irradiance and photoperiod).
In order to compare growth rates of gametophytes and tetrasporophytes from different sites, we developed spore cultures from four localities (Puerto Aldea, Coliumo, Cocholgue and Pilluco). Carpospores and tetraspores were cultivated in Petri dishes with Provasoli medium, replaced at weekly intervals, under a long photoperiod (16:08 L:D), a temperature of 13°C and a photon flux density of 10 μmol photons m−2 s−1. To evaluate growth, length was recorded every week in each culture, for a period of 10 weeks.
Effect of abiotic factors
The germlings obtained in the laboratory were cultivated with Provasoli medium replaced at weekly intervals, under the following conditions: pH (6.5, 8.5, 10.2), salinity (15‰, 25‰, 33‰) temperature (10°C, 13°C), and two photoperiods (12:12 hours and 16:08 hours LD). Vegetative thalli without reproductive structures were selected at random and cut into 4-mm pieces. Five pieces of thalli were placed in each Petri dish and four replicates were used for each condition. The seawater medium was renewed weekly and the experiment lasted 5 weeks.
Thalli were measured weekly to evaluate growth. Cultures were kept in controlled culture chambers with a long day photoperiod (16:08 LD) and temperatures of 10°C and 13°C. A two-way analysis of variance (ANOVA) was used to evaluate the growth difference.
Results
Life history
Once released, the carpospores (4,255 carpospores on average per cistocarp) settle on the bottom of the capsule (average16.6 μm) and begin the germination process, initially forming a thick transparent layer around the spore (Fig. 1a and b). Over the first 3 weeks, a Dumontia type division pattern was identified, observing individual discs with an average diameter of 49.7 μm (Fig. 1c). Subsequently, in some cases when spores germinate in close proximity to each other, coalescent discs were observed, which is that germinate fused, forming a larger disc (Fig. 1d). No rhizoids were observed in any of the discs. After 4 weeks of culture, small upright shoots began to appear in the centre of the individual discs (Fig. 1e). In the coalescent discs, the formation of more than one upright shoot per disc was observed (Fig. 1f). From the outset, carpospores were maintained in a culture chamber under controlled conditions with photoperiod (16:08 LD) and a temperature of 13°C. Carpospores settled on the Petri dish and germinated within 7 days of inoculation.
Carpospore development under culture. a Carpospore recently released and settled in the Petri dish; b Germinating carpospores; c Formation of individual tetrasporophytic discs; d Formation of coalescent discs from carpospores; e Formation of upright thalli in individual discs; and f Formation of thalli in coalescent discs
After 9 months of growing in the laboratory, thalli, 2.5 cm in length, began to form tetrasporangial sori (July 2008, Fig. 2a). Superficial sori matured and sporulated within a few days (Fig. 2b), releasing tetraspores. Tetraspores were inoculated into a culture medium and settled in Petri dishes, but culture was not viable. Nevertheless, after 3 months, tetrasporophytes became fertile and matured again and, this time, spores were viable and did germinate. At this stage, small discs formed uprights that later produced either male or female gametophytes.
The average number of tetrasporangial sori registered was 6.84 sori/lineal cm ± 0.82. Sori were small and located superficially very close to each other. They reached an average length of 0.57 mm. No protocol was applied for releasing the tetraspores, this occurred spontaneously in the Petri dishes.
Numbers of tetraspores released were counted, reaching an average of 1,100 spores/sorus. Tetraspores were separated from the reproductive fronds and incubated in Petri dishes with a Provasoli culture medium (half strength), under the same culture conditions of long day photoperiod (16:08 LD) and a temperature of 13°C.
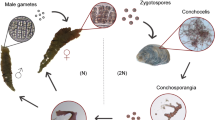
These tetraspores germinated and began to divide and, after 1 month, it was possible to observe microscopic gametophytic shoots. The growth of these shoots was multiaxial, by means of apical and marginal meristems. Thalli began to develop from the centre of the disc in a similar pattern to the tetrasporophytic phase. The gametophytes of approximately 10 to 15 mm were transferred to Erlenmeyer culture flasks (1 L).with aeration to accelerate growth. After 7 months, fertilisation occurred and formation of cystocarps was observed in culture flasks (Fig. 2c). Male gametophytes were evident since they did not have cystocarps. In the laboratory, the morphology of male gametophytes was a main axis with few branchlets, reaching sizes of up to 6 cm, while female gametophytes were smaller, with branchlets bearing cystocarps after fertilisation. Once matured, cystocarps without ostiole dehisced and carpospores were released into the culture flasks (Fig. 2d), completing the life history of C. chamissoi in vitro (Fig. 3). The cystocarps obtained in vitro are small and produce an average of 1,231 carpospores. Carpospores were dispensed in Petri dishes to restart the gametophytic phase cultivation again. During the development of the in vitro life history, tetrasporophytic and gametophytic thalli were used to evaluate the effect of abiotic factors upon the growth and development of the different phases.
The average daily growth rates for gametophytes ranged from 6.5%.d−1 to 9.0%.d−1, while for tetrasporophytes they spanned from 9.9 to 22.7%.d−1. The tetrasporophytes showed higher growth rates than the gametophytes (Table 1). Growth of the gametophytes between localities is similar, while growth of the tetrasporophytes differs between the localities with the exception of Cocholgue and Puerto Aldea.
Photoperiod
The results reveal that interaction between the factors studied is not significant (Table 2), possibly due to the fact that initial thalli sizes were similar throughout the first 6 weeks of the experiment. Nevertheless, each factor, in its own right, shows differences of considerable significance. Tetrasporophyte thalli sizes are significantly greater than those of the gametophyte thalli. This difference was significantly more evident in thalli maintained at a photoperiod of 16:8 LD (Table 2, Fig. 4a).
a Weekly growth (length) of C. chamissoi gametophyte and tetrasporophyte thalli from Puerto Aldea (30°17′S; 71°36′W), northern zone of Chile, under different photoperiod conditions (lozenge) tetrasporophytes 16:08 L:D; (black square) gametophytes 16:08 LD; (white circle) tetrasporophytes 12:12 L:D; (black up-pointing triangle) gametophytes 12:12 L:D; b weekly growth (length) of C. chamissoi tetrasporophyte thalli from Coliumo (36°41´ S; 72°57´ W), central zone of Chile, under different temperature conditions and constant photoperiod of 16:08 L:D (white square) tetrasporophyte 13°C; (black circle) tetraporophytes 10°C. Each data point represents the mean ± SE (n = 3)
Temperature
In the case of temperature, only the tetrasporophyte phase was tested. Statistical analysis showed significant differences in C. chamissoi thalli sizes at different temperatures. The tetrasporophyte thalli grew much faster at 13°C (Table 3, Fig. 4b)
Salinity
The results of experiments on salinity were similar to that observed in the case of photoperiod. The greatest effect (highest order interaction) was not significant (Table 4). Nevertheless, the factors (salinity and temperature) in their own right show significant differences. Thalli grew significantly more at a salinity of between 25 and 33 ppm, and at a temperature of 13°C (Fig. 5a). On the other hand, the tips that were cultured at a salinity of 15 ppm were smaller in size, both at 10°C and at 13°C. Furthermore, a considerable number of tips lost colouring and necrosis can be observed.
a Average size of C. chamissoi from Pilluco, Quetalmahue (41°52′S; 73°54′W), south of Chile, obtained at different temperature and salinity concentrations, after 5 weeks of culture under controlled conditions; dark bars represent salinity treatment of 33 ppm; white bars represent salinity treatment of 25 ppm; grey bars represent salinity treatment of 15 ppm. b Average size of C. chamissoi from Quetalmahue (41°52′S; 73°54′W), southern Chile, obtained at different temperature and pH concentrations, after 5 weeks of culture under controlled conditions; dark bars represent pH treatment of 6.54; white bars represent pH treatment of 8.52; grey bars represent pH treatment of 10.25. Each data point represents the mean ± SE (n = 30)
pH
Analysis revealed that all the effects are significant, given that thalli growth varied depending on the type of pH and temperature used in the experiments (Table 5). The thalli grew more quickly and reached larger sizes, in cultures with a pH of 6.5 and a temperature of 13°C (Fig. 5b). Thalli cultured at a pH of 10.2 were smaller under both temperature conditions. It is important to mention that, using nutrients with a basic pH, both at 10°C and at 13°C, the C. chamissoi tips lose pigmentation and, in other cases, they lose thalli tissue (necrosis).
Discussion
This study confirms in vitro the assumption that C. chamissoi has a sexual life history Polysiphonia- type (P-type) with isomorphic gametophytes and tetrasporophytes (González and Meneses 1996; Acleto, 1986). We were able to develop the complete life history in 20 months. Cultures were started with carpospores and, sporophytes matured under laboratory conditions, releasing viable tetraspores that gave rise to male and female gametophytes.
During the laboratory experiments, that included the effects of temperature, photoperiod, pH and salinity, it was found that the tetrasporophytic phase had higher growth rates than the gametophytic phase. These results agreed with those of González and Meneses (1996) for the early development stages of the same species. Our results demonstrated that long photoperiods (16:08 LD) clearly stimulate growth in both phases, gametophytic and sporophytic. However, significant differences were found between the growth values, being larger for the sporophytic phase. These results do not agree with those presented by Bulboa and Macchiavello (2001), which indicate that gametophytes have higher growth rates. Seasonal variation has been reported for carposporelings and tetrasporelings (Bulboa et al., 2010).
Even though both phases have similar requirements for the maturation process, the amount of spores released per reproductive structure is much greater during the gametophytic phase than during the sporophytic phase. The results from this study showed that sporophytes can have a growth rate of up to a 22%. This may explain, in part, the large presence of the sporophytic phase reported by González and Meneses (1997) for the natural seaweed beds of Puerto Aldea (30° S).
Maturation of the sporophytic phase was obtained in the laboratory after almost a year of culture and the formation of tetrasporangial sori only occurred under a long photoperiod (16:08 LD) and the highest temperature (13°C) conditions. These results partially agreed with field observations on natural beds undertaken by González and Meneses (1997) and Cáceres et al. (2008), where mature tetrasporic fronds were found all year round, although peaking during the summer period (from January to March in the southern hemisphere). Coincidentally, maturation of the cystocarpic phase occurred under the same conditions (long photoperiod and high temperature), since no cystocarps were observed under any other culture conditions.
Finally, if we extend our laboratory results to potential commercial field cultivations, they indicate that, for this species, cultivation should only be carried out in the marine environment. Furthermore, seedlings should be transferred to the sea during spring or summer, since the experiments clearly indicated that this is the period when optimal growing conditions will be present.
References
Acleto CO (1986) Algunos aspectos biológicos de Gigartina chamissoi (C. Ag.) J. Agardh (Rhodophyta, Gigartinales). Rev Ciencias UNMSM 74(1):38–47
Alveal K, Romo H, Werlinger C, Vallejos P, Alveal P (1999) Desarrollo inicial de Chondracanthus chamissoi sobre sustrato artificial. VII Congreso Latinoamericano sobre Ciencias del Mar. Lima, Perú, pp 14–15
Ávila M, Cáceres J, Romo H, Piel MI, Lobos P, Abades S (2008) Reproductive ecology of Chondracanthus chamissoi (Rhodophyta, Gigartinales) for management of wild population in central Chile. V Asian Pacific Phycological Forum. Wellington, New Zealand, p 104
Bulboa C, Macchiavello J (2001) The effects of light and temperature on different phases of the life cycle in the carrageenan producing alga Chondracanthus chamissoi (Rhodophyta, Gigartinales). Bot Mar 44:371–374
Bulboa C, Macchiavello J (2006) Cultivation of cystocarpic, tetrasporic and vegetative fronds of Chondracanthus chamissoi (Rhodophyta, Gigartinales) on ropes at two localities in the northern Chile. Inves Mar 34:109–112
Bulboa C, Macchiavello J, Oliveira E, Fonck E (2005) First attempt to cultivate the carrageenan- producing seaweed Chondracanthus chamissoi (C. Agardth) Kützing (Rhodophyta; Gigartinales) in Northern Chile. Aquat Res 36:1069–1074
Bulboa C, Macchiavello J, Veliz K, Oliveira E (2010) Germination rate and sporeling development of Chondracanthus chamissoi (Rhodophyta, Gigartinales) varies along a latitudinal gradient on the coast of Chile. Aquat Bot 92:137–141
Cáceres J, Ávila M, Piel M, Alveal K, Romo H, (2008) Dinámica poblacional y crecimiento de Chondracanthus chamissoi (“Chicoria de mar”) en dos praderas naturales de Chile central. VIII Congreso de Ficologia de America Latina y El Caribe. Reunión Iberoamericana de Ficología. Lima, Perú, p 152
García M, Ballesteros G, Zertuche J, Chee A (1997) Annual variation in size and reproductive phenology of the red alga Chondracanthus canaliculatus (Harvey) Guiry at Punta San Isidro, Baja California, Mexico. Cienc Mar 23(4):449–462
González J, Meneses I (1996) Differences in the early stages of development of gametophytes and tetrasporophytes of Chondracanthus chamissoi (C. Ag.) Kützing from Puerto Aldea, northern Chile. Aquaculture 143:91–107
González J, Meneses I, Vasquez J (1997) Field studies in Chondracanthus chamissoi (C. Agardh) Kützing: seasonal and spatial variations in life-cycle phases. Biol Pesq 26:3–12
Hoffmann A, Santelices B (1997) Flora marina de Chile central. Ediciones de la Universidad Católica de Chile, Santiago, p 434
Macchiavello J, Bulboa C, Edding M (2003) Vegetative propagation and spore recruitment in the carrageenophyte Chondracanthus chamissoi (Rhodophyta, Gigartinales) in northern Chile. Phycol Res 51:45–50
Pereira L, Critchley A, Amado AM, Ribeiro-Claro PJA (2009) A comparative analysis of phycocolloids produced by underutilized versus industrially utilized carrageenophytes (Gigartinales, Rhodophyta). J Appl Phycol 21:599–605
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and collection of algae. Proceedings of the US Japanese Society of Plant Physiologists Conference, Hakone, Japan. pp 63–75
Ramírez M, Santelices B (1991) Catálogo de las algas marinas bentónicas de la costa temperada del Pacífico de Sudamérica, Monografías Biológicas 5. Ediciones Pontificia Universidad Católica de Chile, Santiago, p 437
Sáez F, Macchiavello J, Fonck E, Bulboa C (2008) The role of the secondary attachment disc in vegetative propagation of Chondracanthus chamissoi (Gigartinales; Rhodophyta). Aquat Bot 89:63–65
Vásquez J, Alonso Vega J (2001) Chondracanthus chamissoi (Rhodophyta, Gigartinales) in northern Chile: ecological aspects for management of wild population. J Appl Phycol 13:267–277
Acknowledgements
This work was supported by Fondef Grant D06I1058 of the Comision Nacional de Ciencia y Tecnologia (Conicyt)—Chilean Government. Special thanks to Dr. Yanko Andrade for his valuable time and effort reviewing the manuscript. Thanks to Susan Angus for English version. We also thank valuable suggestions made by two anonymous reviewers who helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avila, M., Piel, M.I., Caceres, J.H. et al. Cultivation of the red alga Chondracanthus chamissoi: sexual reproduction and seedling production in culture under controlled conditions. J Appl Phycol 23, 529–536 (2011). https://doi.org/10.1007/s10811-010-9628-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9628-1