Abstract
Shortages in nutrients and freshwater for a growing population are critical global issues. Source separation of waste streams combined with decentralized resource recovery is a promising approach to address this problem. Urine contains within 1% of household wastewater up to 80% of nitrogen (N) and 50% of phosphorus (P). Since microalgae are efficient at nutrient uptake, growing them in urine is a promising technology to clean urine and produce biomass as fertilizer. The aim of this study was to develop a process for nutrient recovery from minimally diluted human urine using immobilized cultivation of microalgae on porous substrate photobioreactors (PSBRs). Treatment of urine, unamended except for a 1:1 dilution with tap water, was performed with the green alga Desmodesmus abundans, chosen among 96 algal strains derived from urine-specific enrichments and culture collections. A growth rate of 7.2 g dry weight m−2 day−1 and removal efficiencies for N and P of 13.1 and 94.1% were determined. Pre-treatment of urine with activated carbon was found to eliminate potentially detrimental effects of pharmaceuticals. In combination with other technologies, PSBRs could be applied in decentralized resource recovery systems, helping to close the link between sanitation and food production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The availability of freshwater and nutrients to supply basic human needs and ensure food security is among the major future challenges facing humanity as a whole. In order to produce food for a rapidly growing human population, industrial agriculture is in demand of large quantities of mineral fertilizer, mainly composed of nitrogen (N) and phosphorus (P). In contrast to N, which can be acquired from the atmosphere via the Haber-Bosch process, P is a finite resource which is mined from mineral rock deposits. Projections predict the depletion of land-based P reserves within 50–250 years (Isherwood 2000; Smil 2000; Cordell et al. 2009). However, the P present in human urine and feces could account for up to 22% of global P demand if it were recovered as fertilizer (Glibert et al. 2006). While the treatment of human excreta thus presents a valuable opportunity to close the link between sanitation and food, current technology is not designed for this purpose (Verstraete et al. 2009; Ashley et al. 2011). Centralized municipal wastewater treatment plants are using large amounts of (drinking) water to transport small amounts of waste and due to this dilution are not effective at recovery of resources (Dockhorn 2016).
A relatively new idea in applied research is the source separation of household waste streams combined with decentralized sanitation and resource recovery (Langergraber and Muellegger 2005; Larsen and Lienert 2007; Zeeman and Kujawa-Roeleveld 2011). The unifying principle is to separately collect waste streams and to treat them on-site, aiming for maximal resource recovery and water savings. Since urine contains in less than 1% of the total volume of household wastewater up to 80% of the N and 40–50% of the P (Wilsenach and van Loosdrecht 2006), it is an attractive stream for the recovery of nutrients. Urine can be directly applied to agricultural plots for fertilization, but several problems are associated with this practice such as runoff to the environment, as well as volatilization of N depending on climate conditions (Rodhe et al. 2004). Furthermore large volumes of liquid need to be stored and transported, since constant application would oversupply the fields (Winker et al. 2009) and detrimentally increase soil salinity (Jönsson 2004; Mnkeni et al. 2008). A technique to uncouple the application of fertilizer from urine excretion makes use of bacterial hydrolysis of urea, by intentional precipitation of struvite, a mineral containing NH4 + and PO4 2− in an equimolar ratio (Ban and Dave 2004; Ronteltap et al. 2007). The process can be realized with simple means and has been demonstrated at pilot scale in various studies (Lind et al. 2000; Adnan et al. 2003; Etter et al. 2011). The major shortcoming of this technology is the limited N recovery potential, dictated by the 1:1 molar ratio of N and P in struvite. Since N:P ratios in human urine can range between 30:1 and 50:1 (Putnam 1971), struvite precipitation leaves the largest fraction of N unused.
An alternative can be the incorporation of nutrients into microalgae, phototrophic organisms which are very efficient at taking up these compounds. Although practical experience is still scarce, microalgal biomass grown on wastewater might be used as a slow-release fertilizer, due to its elemental composition being similar to that of plants (Kebede-Westhead et al. 2004; Mulbry et al. 2005). Alternatively, high-value compounds (e.g., pigments) could be extracted from biomass, enhancing the economic feasibility of this approach (Cai et al. 2013). While there is a growing body of research dealing with growing microalgae on urine for nutrient recovery, previous studies have used a limited number of strains at high urine dilutions (Adamsson 2000; Feng and Wu 2006; Yang et al. 2008; Chang et al. 2013; Jaatinen et al. 2016) and/or have supplemented the urine with trace elements and additional nutrients (Tuantet et al. 2014a; Tuantet et al. 2014b). In the context of source separation for resource recovery, the use of low dilution without the addition of chemicals should be imperative, in order to maximize the water and resource efficiency of the process. Storage of urine under non-sterile conditions leads to bacterial hydrolysis of urea into ammonium (NH4 +)/ammonia (NH3), increasing the pH of the solution and shifting the equilibrium between NH4 + and NH3 towards the uncharged ammonia (Warner 1942), which is toxic to all life, including microalgae (Azov and Goldman 1982). The high pH further causes volatilization of NH3, as well as precipitation of phosphate-containing minerals (e.g., struvite or calcium phosphate), making urine an unstable liquid in storage (Maurer et al. 2006). In order to prevent these detrimental effects, using fresh urine to grow an alga that can metabolize urea while exhibiting tight pH control is of utmost importance. The selection of an algal strain that is adapted to growth on urine might further circumvent the need for addition of nutrients.
Previously employed suspension-based systems for microalgal cultivation have major shortcomings when practically applied, most notably the high energy consumption for separating biomass and water (harvesting) (Hoffmann 1998) and high capital expenses CAPEX for reactors, which can be preventive of application in the wastewater field (Acién et al. 2012). Submerged biofilm systems solve some of these issues by growing a productive and dense biomass which is simple to harvest (Kesaano and Sims 2014). Nevertheless, washout of cells is commonly observed in such systems (Boelee et al. 2011; Posadas et al. 2013), leading to problems especially in regions were the concentration of suspended particles in discharge water is strictly controlled (Mallick 2002). A relatively new approach to the technical cultivation of algal biofilms is the Twin-Layer porous substrate bioreactor (TL-PSBR). Here, cells are immobilized on a sheet-like porous substrate impermeable to them, but permeable to the liquid (e.g., wastewater) and nutrients therein. The medium is applied to an inner sheet and is transported down this layer by gravity. The low energy demand for water circulation as well as the low water content of the biomass and associated ease of harvesting and processing of biomass make these systems attractive (Podola et al. 2016). The low shear forces to which cells are exposed further enable the cultivation of a large diversity of microalgal species (Nowack et al. 2005; Naumann et al. 2013; Benstein et al. 2014; Kiperstok et al. 2016). The system has previously been employed for nutrient recovery from various municipal wastewaters, both at the laboratory (Shi et al. 2007) and prototype scale (Shi et al. 2014), but so far, not for the treatment of source-separated human urine.
The aim of this study was to establish a robust process in which PSBR-immobilized microalgae recover nutrients from minimally diluted, unamended human urine at laboratory scale. It was hypothesized that this could be achieved by identifying a suitable algal strain in a bioprospecting approach and by the subsequent determination of optimal process parameters in a technical environment.
Materials and methods
Nutrient determination
Nutrient measurements were based on spectrophotometric analyses using an infinite PRO 200 96-well multiplate reader (Tecan, Switzerland). Nitrate (NO3 −) was determined according to Miranda et al. (2001), while ortho-phosphate (PO4 −) was determined according to Murphy and Riley (1962). Total N and total P were measured as NO3 − and PO4 − after a persulfate digestion based on Cabrera and Beare (1993).
Urine batches
Three batches of human urine were used in the course of this study. The details of the collections are summarized in Table 1. Batch 0 was collected in 2015 from three male members of the laboratory. Batches A and B were collected in 2015 and 2016, from student volunteers of both genders. Collections lasted 3–5 days. During collection, urine was stored in 25 L plastic containers at 4 °C. Once the collection of one batch was complete, the volume was mixed and stored frozen in plastic bottles of 1 L at − 20 °C until use. Electrical conductivity and pH of urine as well as nutrient contents were measured routinely before use of a batch.
When using urine of batch B, an inhibitory effect on algal growth was detected. To alleviate this effect, urine was treated with activated carbon in the following way: 10 g of activated carbon type “Carbopal MB4” (Donau Carbon, Frankfurt, Germany) per 1 L of 1:1 (tap water) diluted urine was suspended in the liquid and mixed thoroughly on a magnetic stirrer for 10 min at room temperature. The particles were filtered out by a series of decantations over coffee filters of type “Aromata No 4” (Lidl, Neckarsulm, Germany).
Cultures and isolations
To find an algal strain optimally suited for growth on human urine, two different strategies were employed: (a) isolation of new strains from the environment using urine as growth medium and (b) testing of established strains, isolated from wastewater sources. The complete the list of strains which were isolated and acquired from culture collections and used in the screening can be found in the appendix (Table S1). Isolations were performed by placing plastic containers with urine of batch 0, diluted 1:1, 1:5, and 1:10 with ultrapure water, in the garden of the Biocenter, University of Cologne. These “traps” were left for 6–8 weeks in April and May of 2015, until algal growth was visible. The establishment of clonal cultures was achieved through enrichment cultures, set up by placing a subsample of urine in artificial growth medium and incubating it at 23 °C and ~ 100 μmol photons m−2 s−1 light intensity using a 14:10 h light/dark cycle. The media used were Waris-H (McFadden and Melkonian 1986) and BG-11 (Stanier et al. 1971) as modified by Naumann et al. (2013). Isolation of single cells was performed using the microcapillary technique, as described by Pringsheim (1946). Once unialgal cultures were established, they were made axenic by spraying onto an agar dish and subsequent picking of a single colony, as described by Surek and Melkonian (2004).
Suspension screening
The initial screening of algal strains for growth on urine was performed in 96-well microtiter plates using undiluted, 1:1, 1:5, and 1:10 (MQ) diluted urine, resulting in triplicate cultures of 200 μL each. Absorbance at 750 nm was used as a proxy for growth. Inoculation was performed from log-phase cultures, which were harvested and washed by centrifugation and threefold replacement of the liquid with urine. Cultures were inoculated at an absorbance of 0.1 and grown at 23 °C and 80 μmol photons m−2 s−1 with 14:10 h light/dark cycle on a LED table for 6 days. A negative control of non-inoculated urine was used in each experiment, to account for bacterial growth and other sources of background turbidity.
Experiments on Twin-Layer PSBR
Laboratory scale Twin-Layer experiments were conducted as described by Schultze et al. (2015), based on Shi et al. (2007). These publications can be consulted for a visual representation of the experimental system. The presented results are the outcome of three independent replications. Following a screening to find the most suitable strain, physicochemical conditions were optimized. Six hundred-micromole photons m−2 s−1 light intensity and a pH of 6.5 were then used to determine the potential of nutrient recovery. A dry weight inoculation density of 2.5 g m−2 and a flow of CO2-enriched air (2.5% (v:v) at 1 L min−1) were used throughout. Liquid cultures used for inoculation were maintained axenic, which was validated by microscopic visual inspection and subculturing in bacterial standard medium (BSM), as described by Surek and Melkonian (2004). Polycarbonate filter disks (Whatman, Germany) with 0.2-μm pore size with an inoculation area of 0.254 cm2 were used for determination of growth, while a nitrocellulose membrane of type “Zeta-Probe” (BIO RAD, USA) with 0.45-μm pore size and an inoculation area of 0.3 m2 was used as a substrate layer for the nutrient recovery experiment. All Twin-Layer experiments were conducted at 21 °C and dilutions of urine and replacement of evaporated water were done using tap water, unless otherwise stated. Medium volume was 0.5 L and it was exchanged after 4 days, unless otherwise stated.
To determine the potential for nutrient recovery, the pH was kept constant by means of on-demand CO2 addition to the medium, using a PH-803 system (Analytical Instruments, Colombo, Sri Lanka) controlling a solenoid valve type 356 3/2NC G1/8 (ASCO-Numatics, Michigan, USA), which regulated a flow of 5% (v:v) CO2-enriched air when the set pH value was exceeded. Concentrations of N and P were measured daily, and the experiment was conducted until no more reduction in P was detectable. Algal biomass was scraped off with a rubber spatula from the surface and washed in ultrapure water by repeated centrifugation and exchange of the liquid. This suspension was used for dry weight determination on polycarbonate filters. Afterwards, biomass was ground with a mortar and pestle and ~ 5 mg was weighed into tin cups for analysis of nitrogen content with a Flash 2000 elemental analyzer (Thermo Scientific, USA). P content of the biomass was determined by acid digestion according to Hu and Barker (1999) and subsequent measurement of dissolved ortho-phosphate as described above.
Statistics
All statistical analyses were performed with GraphPad Prism software for Windows, version 6.01 (GraphPad Software, USA). Comparison of replicate measurements was analyzed by one-way ANOVA with multiple comparisons and Tukey’s post hoc test. Rates were calculated as linear regressions over a certain time period. Comparison of rates was performed by analysis of covariance with multiple comparisons.
Results
Of the 24 isolates recovered from the urine traps, most belonged to the green algal classes Chlorophyceae and Trebouxiophyceae, while a smaller number was made up of Cyanobacteria and Bacillariophyceae. The most abundant species were Chlorella spp. and Chlamydomonas spp. (see Table S1 for a full listing and designations of the strains isolated and used for screening). In a first set of experiments with strains CCAC 3496, CCAC 0126, and U5.5, satisfactory growth was observed for all dilutions but not for undiluted urine. To optimize water efficiency of the system, a 1:1 dilution was chosen for all further trials. Of the 96 strains, which were screened, 9 displayed an increase in absorbance between day 0 and day 6 which was higher than that of the negative control and thus unequivocally associated with algal growth (Fig. 1).
Summary of suspension screening. Increase in absorbance at 750 nm between day 0 and day 6 was used as measure of growth. Mean absorbance of negative controls was subtracted and only positive values are shown. a Desmodesmus sp. (U 2.4). b Desmodesmus abundans (CCAC 3496). c Chlamydomonas sp. (U 5.5). d Chlorella sp. (U 2.1). e Halochlorella rubescens (CCAC 0126). f Chlorella sp. (U 10.10). g Monoraphidium cf. litorale (VZ 246). h Chlamydomonas sp. (BI 11). i Chlorella sp. (VZ 392). Full strain designations are found in the supporting information (Table S1). Values represent the mean ± SD (n = 3)
These strains were used for comparing their growth on urine using the Twin-Layer PSBR system (Fig. 2). Between days 6 and 9, all strains, except for Desmodesmus abundans CCAC 3496, showed a decline or stagnation of biomass. Only this strain showed linear growth for a period of 9 days. To validate this result, the test of growth with this strain was repeated. The slopes (growth rates) of the two runs were found to be statistically not different (P = 0.2906). The pooled growth rate can be expressed as 10.33 ± 0.354 g m−2 day−1. This strain was selected for further experiments.
Summary of immobilized screening. Biomass growth of algal strains on 1:1 (tap water) diluted urine of batch A. a Desmodesmus sp. (U 2.4). b Desmodesmus abundans (CCAC 3496). c Chlamydomonas sp. (U 5.5). d Chlorella sp. (U 2.1). e Halochlorella rubescens (CCAC 0126). f Chlorella sp. (U 10.10). g Monoraphidium cf. litorale (VZ 246). h Chlamydomonas sp. (BI 11). i Chlorella sp. (VZ 392). Full strain designations are found in the supporting information (Table S1). Values represent the mean ± SD (n = 3). The experiment with D. abundans (CCAC 3496) was repeated (triangles). Lines represent linear regressions
When urine of batch B was used to grow D. abundans CCAC 3496, an unexpected decrease in biomass growth, concurrent with a gradual bleaching, was observed (Fig. 3a). This effect could be alleviated when urine was treated with activated carbon (Fig. 3b), although this treatment resulted in 17.19 and 10.11% removal of total N and total P, respectively. After activated carbon treatment, a growth rate of 7.216 ± 0.2319 g m−2 day−1 was achieved.
Effect of activated carbon on algal growth on urine of batch B. a Untreated urine. b Activated carbon-treated urine. Left panels show biomass over time and right panels show photographs of filters with algal biomass on day 12. Scale bar is identical for both images. Line represents linear regression. Values represent the mean ± SD (n = 3)
To assess the potential of recovering N and P with immobilized D. abundans CCAC 3496, a surface of 0.3 m2 was inoculated and urine was circulated for 5 days, until no more change in P concentration was observable. The final biomass on the reactor surface was 36.19 ± 2.945 g m−2, which can be converted into a growth rate of 7.238 g m−2 day−1, assuming linear growth. The N and P content of this biomass was 5.36% (w/w) and 2.1% (w/w), respectively. Thus, it can be calculated that the portion of the removed elements, which was recovered in the form of biomass, was 87.1 and 87.5% for N and P, respectively (Table 2). The removal efficiency of N was calculated to be 13.1%; hence, there were still significant quantities of nitrogen present after 5 days. The efficiency of removal for P was 94.1%; hence, almost all phosphorus had been removed from urine at the end of the treatment.
Discussion
Comparison to other studies
This study used bioprospecting, which resulted in finding a strain with good immobilized growth on minimally diluted urine without the need for addition of trace elements or other nutrients. The achieved growth rates between 7.216 and 10.33 g m−2 day−1 are comparable, yet somewhat lower, than those found in the PSBR model green alga Halochlorella rubescens on standard growth medium (Schultze et al. 2015). However, light intensities above 1000 μmol m−2 s−1 were used in that study, while the optimal light intensity for D. abundans was 600 μmol photons m−2 s−1. When the biomass productivity in this study is related to the volume of urine, a productivity of 14.5 g L−1 day−1 can be calculated for the growth surface of 0.3 m2. This compares favorably with the 9.3 g L−1 day−1 reported for a continuously operated flat-plate photobioreactor treating urine augmented with trace elements, in the only published study using urine at the same low dilution (Tuantet et al. 2014b). When the authors enriched urine with Mg and P and shortened the light path, productivity increased to 14.8 g L−1 day−1. In the same study, nutrient removal of 87 and 76% was achieved for N and P, respectively. This N removal efficiency is much higher than the efficiency obtained in this study, which can be explained by the addition of P, optimizing the N:P ratio. The higher P removal achieved in the present study might be due to the addition of P by Tuantet, et al. (2014b). It has to be stated that the operation in continuous culture mode can compromise on performance as opposed to batch mode. Furthermore, since operational parameters such as light intensity and temperature differed between studies, comparisons are inherently only approximations.
The present study shows a discrepancy between the amount of nutrients which were removed from the liquid phase and the quantities recovered in the form of biomass from the Twin-Layer surface. Essentially, 16.9% of N and 16.5% of P remained unaccounted for. Although this has not been quantified presently, this portion of nutrients was most likely bound in the form of heterotrophic bacterial biomass which had visibly developed in the tubes and liquid reservoirs of the system. A loss of N might also have been caused by bacterial transformation of ammonium to nitrate, during daytime aerobic conditions, and subsequent denitrification during anaerobic conditions in the algal biofilm at night. While nitrogen transformation is hypothetical, the possibility of anoxia in Twin-Layer algal biofilms has been shown by Li et al. 2016. Whatever the cause, this missing nutrient fraction shows the importance of the bacterial population developing in any open treatment system, even if the inoculum culture is sterile. It should be stressed that the effect of bacterial communities must be taken into account when using algae for wastewater treatment.
Inhibitory effects
Urine batch B contained a soluble dissolved compound or element with an inhibitory effect, which could be excluded from the liquid by activated carbon treatment. The most plausible explanation appears to be a pharmaceutical, or residue thereof, in this batch of urine. Urine is known to be the major excretion route of drugs leaving the human body (Jjemba 2006; Bester et al. 2008). Among those most commonly used are non-steroidal anti-inflammatory drugs (NSAIDs; e.g., ibuprofen and diclofenac). NSAIDs have recently been tested for their effect on axenic laboratory cultures of eukaryotic algae (Bácsi et al. 2016). In this study, the growth of Desmodesmus communis, a relative to D. abundans CCAC 3496, was inhibited by various NSAIDs at concentrations of 100 mg L−1. Cleuvers (2004) performed inhibition experiments with Desmodesmus subspicatus and found that the combination of four different compounds increased the inhibition up to 75-fold. Activated carbon is known as an effective adsorbent for a variety of organic and inorganic molecules, mainly due to its large surface area, its internal porosity structure and presence of surface-binding groups (Yin et al. 2007). Indeed, activated carbon treatment has been proposed as a viable option for reducing the risk of the enrichment of pharmaceuticals (e.g., antibiotics, beta blockers, and NSAIDs) in decentralized sanitation and resource reuse systems (Udert et al. 2015). In the case of the algal treatment studied here, it appears that the use of activated carbon is a necessary safety measure, ensuring that algal growth is not inhibited in the presence of pharmaceuticals in urine.
Integration into a treatment system
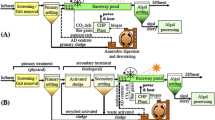
To illustrate the application of this algal system in a decentralized resource recovery situation, a scenario analysis is presented here (Fig. 4), based on the following assumptions: The basis is a three-person household, with an average daily urine excretion of 1.4 L per person (Tortora and Derrickson 2006). Averages from all collections in this study are used for concentrations of nutrients in urine. Long-term nutrient uptake rates are calculated using algal growth rate and content of P in biomass, in order to level out fluctuations, e.g., due to luxury uptake mechanisms (Shi et al. 2007, 2014). Based on a growth rate of 7.25 g m−2 day−1, an uptake rate of 0.15 g P m−2 day−1 is thus estimated. A minimum of 7.5 m2 growth surface, based on a single Twin-Layer PSBR module of 1.5-m height and 3-m width (inoculated on both sides) is proposed. Assuming year-round operation, the reactor would produce ~ 18.5 kg dry algal biomass, available as fertilizer. An estimation of the potential agricultural yield is based on available data for a crop rotation system in India: Wheat (Triticum aestivum) and soybean (Glycine max) are cultivated on the same plot of land (Damodar Reddy et al. 1999; Aulakh et al. 2003). Considering P as the limiting nutrient, an optimal dosage/yield relation was found for an application rate of 2.62 g P m−2 acre−1 (Aulakh et al. 2003). Assuming a short-term P availability from algal biomass of 50% in soil (based on Mulbry et al. 2005), an agricultural plot of approximately 66.5 m2 could be optimally supplied with P. Based on Damodar Reddy et al. (1999) and Aulakh et al. (2003), it can be estimated that about 31 kg of wheat grain and 16 kg of soybean could be produced per year.
Flow diagram of nutrients and materials in a proposed decentralized treatment and resource recovery system based on nutrient recovery from source-separated urine microalgae immobilized on Twin Layers. Nutrient excretion values are based on the estimates for a household of three persons over 1 year, while plant growth parameters are based on a P-limited soil, utilizing year-round crop rotation in a semi-arid subtropical climate, according to Aulakh et al. (2003) and Damodar Reddy et al. (1999)
To eliminate all remaining N, the algal treatment should be followed up by other steps such as zeolites (Ban and Dave 2004; Beler-Baykal et al. 2004), volatilization and re-suspension via a microbial fuel cell (Kuntke et al. 2012), or the completely autotrophic nitrogen removal via nitrate process (CANON) (Ahn 2006). Final polishing of the effluent could be performed in a sand or reed bed filtration (Ellis 1987; Green and Upton 1994), after which the purified water might be used for irrigation of plants, toilet flushing, or be discharged.
Due to the lower material input and potentially lower energy requirements for harvesting (Podola et al. 2016), the use of Twin-Layer PSBR systems could make large-scale operations more feasible than suspension-type PBRs. Should the stability and safety of the process be validated at pilot scale, it could be integrated into a decentralized sanitation and resource recovery framework, providing slow-release microalgal fertilizer for food production, partially closing local nutrient cycles and decreasing pressures on freshwater reserves.
References
Acién FG, Fernández JM, Magán JJ, Molina E (2012) Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol Adv 30:1344–1353
Adamsson M (2000) Potential use of human urine by greenhouse culturing of microalgae (Scenedesmus acuminatus), zooplankton (Daphnia magna) and tomatoes (Lycopersicon). Ecol Eng 16:243–254
Adnan A, Mavinic DS, Koch FA (2003) Pilot-scale study of phosphorus recovery through struvite crystallization—examining the process feasibility. J Environ Eng Sci 2:315–324
Ahn Y-H (2006) Sustainable nitrogen elimination biotechnologies: a review. Process Biochem 41:1709–1721
Ashley K, Cordell D, Mavinic D (2011) A brief history of phosphorus: from the philosopher’s stone to nutrient recovery and reuse. Chemosphere 84:737–746
Aulakh MS, Pasricha NS, Bahl GS (2003) Phosphorus fertilizer response in an irrigated soybean–wheat production system on a subtropical, semiarid soil. Field Crop Res 80:99–109
Azov Y, Goldman JC (1982) Free ammonia inhibition of algal photosynthesis in intensive cultures. Appl Environ Microbiol 43:735–739
Bácsi I, B-Béres V, Kókai Z et al (2016) Effects of non-steroidal anti-inflammatory drugs on cyanobacteria and algae in laboratory strains and in natural algal assemblages. Environ Pollut 212:508–518
Ban ZS, Dave G (2004) Laboratory studies on recovery of N and P from human urine through struvite crystallisation and zeolite adsorption. Environ Technol 25:111–121
Beler-Baykal B, Bayram S, Akkaymak E, Cinar S (2004) Removal of ammonium from human urine through ion exchange with clinoptilolite and its recovery for further reuse. Water Sci Technol 50:149–156
Benstein RM, Çebi Z, Podola B, Melkonian M (2014) Immobilized growth of the peridinin-producing marine dinoflagellate Symbiodinium in a simple biofilm photobioreactor. Mar Biotech 16:621-628
Bester K, Scholes L, Wahlberg C, McArdell CS (2008) Sources and mass flows of xenobiotics in urban water cycles—an overview on current knowledge and data gaps. Water Air Soil Pollut Focus 8:407–423
Boelee NC, Temmink H, Janssen M, Buisman CJ, Wijffels RH (2011) Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res 45:5925–5933
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sust Energ Rev 19:360–369
Chang Y, Wu Z, Bian L, Feng D, Leung DYC (2013) Cultivation of Spirulina platensis for biomass production and nutrient removal from synthetic human urine. Appl Energy 102:427–431
Cleuvers M (2004) Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol Environ Saf 59:309–315
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19:292–305
Damodar Reddy D, Subba Rao A, Sammi Reddy K, Takkar P (1999) Yield sustainability and phosphorus utilization in soybean–wheat system on vertisols in response to integrated use of manure and fertilizer phosphorus. Field Crop Res 62:181–190
Dockhorn T (2016) The resource economic dimension of wastewater treatment vs. green technologies. In: Ngo HH, Guo W, Surampalli RY, Zhang TC (eds) Green Technologies for Sustainable Water Management. American Society of Civil Engineers, Reston, p 755-756
Ellis K (1987) Slow sand filtration as a technique for the tertiary treatment of municipal sewages. Water Res 21:403–410
Etter B, Tilley E, Khadka R, Udert KM (2011) Low-cost struvite production using source-separated urine in Nepal. Water Res 45:852–862
Feng D, Wu Z (2006) Culture of Spirulina platensis in human urine for biomass production and O2 evolution. J Zhejiang Univ Sci B 7:34–37
Glibert PM, Harrison J, Heil C, Seitzinger S (2006) Escalating worldwide use of urea—a global change contributing to coastal eutrophication. Biogeochemistry 77:441–463
Green MB, Upton J (1994) Constructed reed beds: a cost-effective way to polish wastewater effluents for small communities. Water Environ Res 66:188–192
Hoffmann JP (1998) Wastewater treatment with suspended and non-suspended algae. J Phycol 34:757–763
Hu Y, Barker AV (1999) A single plant tissue digestion for macronutrient analysis. Commun Soil Sci Plant Anal 30:677–687
Isherwood K (2000) Mineral fertilizer use and the environment. United Nations Environment Programme, Paris, p 40
Jaatinen S, Lakaniemi A-M, Rintala J (2016) Use of diluted urine for cultivation of Chlorella vulgaris. Environ Technol 37:1159–1170
Jjemba PK (2006) Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol Environ Saf 63:113–130
Jönsson H (2004) EcoSanRes Programme: guidelines on the use of urine and faeces in crop production. Stockholm Environment Institute, Stockholm, p 17–20
Kebede-Westhead E, Pizarro C, Mulbry WW (2004) Treatment of dairy manure effluent using freshwater algae: elemental composition of algal biomass at different manure loading rates. J Agric Food Chem 52:7293–7296
Kesaano M, Sims RC (2014) Algal biofilm based technology for wastewater treatment. Algal Res 5:231–240
Kiperstok AC, Sebestyén P, Podola B, Melkonian M (2016) Biofilm cultivation of Haematococcus pluvialis enables a highly productive one-phase process for astaxanthin production using high light intensities. Algal Res 21:213–222
Kuntke P, Śmiech KM, Bruning H, Zeeman G, Saakes M, Sleutels THJA, Hamelers HVM, Buisman CJN (2012) Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res 46:2627–2636
Langergraber G, Muellegger E (2005) Ecological sanitation—a way to solve global sanitation problems? Environ Int 31:433–444
Larsen TA, Lienert J (2007) Novaquatis final report. NoMix - A new approach to urban water management. Swiss Federal Institute for Environmental Science (EAWAG), Duebendorf, p 4–5
Li T, Piltz B, Podola B, Dron A, de Beer D, Melkonian M (2016) Microscale profiling of photosynthesis-related variables in a highly productive biofilm photobioreactor. Biotechnol Bioeng 113:1046–1055
Lind B-B, Ban Z, Bydén S (2000) Nutrient recovery from human urine by struvite crystallization with ammonia adsorption on zeolite and wollastonite. Bioresour Technol 73:169–174
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals 15:377–390
Maurer M, Pronk W, Larsen TA (2006) Treatment processes for source-separated urine. Water Res 40:3151–3166
McFadden GI, Melkonian M (1986) Use of Hepes buffer for microalgal culture media and fixation for electron microscopy. Phycologia 25:551–557
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Mnkeni PNS, Kutu FRF, Muchaonyerwa P, Austin LM (2008) Evaluation of human urine as a source of nutrients for selected vegetables and maize under tunnel house conditions in the Eastern Cape, South Africa. Waste Manag Res 26:132–139
Mulbry W, Westhead EK, Pizarro C, Sikora L (2005) Recycling of manure nutrients: use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresour Technol 96:451–458
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Naumann T, Çebi Z, Podola B, Melkonian M (2013) Growing microalgae as aquaculture feeds on twin-layers: a novel solid-state photobioreactor. J Appl Phycol 25:1413–1420
Nowack ECM, Podola B, Melkonian M (2005) The 96-well Twin-Layer system: a novel approach in the cultivation of microalgae. Protist 156:239–251
Podola B, Li T, Melkonian M (2016) Porous substrate bioreactors: a paradigm shift in microalgal biotechnology? Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2016.06.004
Posadas E, García-Encina P-A, Soltau A et al (2013) Carbon and nutrient removal from centrates and domestic wastewater using algal–bacterial biofilm bioreactors. Bioresour Technol 139:50–58
Pringsheim E (1946) Pure cultures of algae. Hafner Publ Co, London, p 23–25
Putnam DF (1971) Composition and concentrative properties of human urine. Washington D.C, National Aeronautics and Space Administration (NASA), p 38
Rodhe L, Richert Stintzing A, Steineck S (2004) Ammonia emissions after application of human urine to a clay soil for barley growth. Nutr Cycl Agroecosyst 68:191–198
Ronteltap M, Maurer M, Gujer W (2007) Struvite precipitation thermodynamics in source-separated urine. Water Res 41:977–984
Schultze LKP, Simon MV, Li T, Langenbach D, Podola B, Melkonian M (2015) High light and carbon dioxide optimize surface productivity in a twin-layer biofilm photobioreactor. Algal Res 8:37–44
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423
Shi J, Podola B, Melkonian M (2014) Application of a prototype-scale Twin-Layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour Technol 154:260–266
Smil V (2000) Phosphorus in the environment: natural flows and human interferences. Annu Rev Energy Environ 25:53–88
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Surek B, Melkonian M (2004) CCAC—Culture Collection of Algae at the University of Cologne: a new collection of axenic algae with emphasis on flagellates. Nova Hedwigia 79:77–92
Tortora GJ, Derrickson B (2006) The urinary system. In: Principles of anatomy and physiology, 11th edn. John Wiley & Sons, Hoboken, pp 992–1035
Tuantet K, Janssen M, Temmink H, Zeeman G, Wijffels RH, Buisma CJN (2014a) Microalgae growth on concentrated human urine. J Appl Phycol 26:287–297
Tuantet K, Temmink H, Zeeman G, Janssen M, Wijffels RH, Buisman CJN (2014b) Nutrient removal and microalgal biomass production on urine in a short light-path photobioreactor. Water Res 55:162–174
Udert KM, Buckley CA, Wächter M, McArdell CS, Kohn T, Strande L, Zöllig H, Fumasoli A, Oberson A, Etter B (2015) Technologies for the treatment of source-separated urine in the eThekwini Municipality. Water SA 41:212–221
Verstraete W, Van de Caveye P, Diamantis V (2009) Maximum use of resources present in domestic “used water”. Bioresour Technol 100:5537–5545
Warner R (1942) The kinetics of the hydrolysis of urea and of arginine. J Biol Chem 142:705–723
Wilsenach JA, van Loosdrecht MC (2006) Integration of processes to treat wastewater and source-separated urine. J Environ Eng 132:331–341
Winker M, Vinnerås B, Muskolus A, Arnold U, Clemens J (2009) Fertiliser products from new sanitation systems: their potential values and risks. Bioresour Technol 100:4090–4096
Yang C, Liu H, Li M, Yu C, Yu G (2008) Treating urine by Spirulina platensis. Acta Astronaut 63:1049–1054
Yin CY, Aroua MK, Daud WMAW (2007) Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep Purif Technol 52:403–415
Zeeman G, Kujawa-Roeleveld K (2011) Resource recovery from source separated domestic waste(water) streams; full scale results. Water Sci Technol 64:1987–1992
Acknowledgements
This study was supported by the University of Cologne (KST 158901001). The authors would like to thank Dr. Tong Li and Dr. Björn Podola (both from the University of Cologne) for helpful discussions and practical support in the course of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 30 kb)
Rights and permissions
About this article
Cite this article
Piltz, B., Melkonian, M. Immobilized microalgae for nutrient recovery from source-separated human urine. J Appl Phycol 30, 421–429 (2018). https://doi.org/10.1007/s10811-017-1266-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1266-4