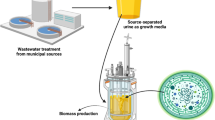
Abstract
In this study, for the first time, a microalga was grown on non-diluted human urine. The essential growth requirements for the species Chlorella sorokiniana were determined for different types of human urine (fresh, hydrolysed, male and female). Batch experimental results using microtiter plates showed that both fresh and synthetic urine supported rapid growth of this species, provided additional trace elements (Cu, Fe, Mn, and Zn) were added. When using hydrolysed urine instead of fresh urine, additional magnesium had to be added as it precipitates during hydrolysis of urea. C. sorokiniana was able to grow on non-diluted urine with a specific growth rate as high as 0.104 h−1 under light-limited conditions (105 μmol photons m−2 s−1), and the growth was not inhibited by ammonium up to a concentration of 1,400 mg NH4 +-N L−1. The highest growth rate on human urine was as high as 0.158 h−1. Because it was demonstrated that concentrated urine is a rich and good nutrient source for the production of microalgae, its application for a large-scale economical and sustainable microalgae production for biochemicals, biofuels and biofertilizers becomes feasible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional municipal wastewater treatment is costly and inefficient in nutrient and energy recovery because the wastewater is highly diluted. New sanitation concepts, in which separate wastewaters are collected and treated at their source, have been proposed and are considered more efficient for this purpose (Zeeman et al. 2008). In domestic wastewater, more than half of the nutrient load originates from urine. Within a volume of only 1 % of the total wastewater volume, urine contains 40 % of the phosphorus load, 69 % of the nitrogen load, and 60 % of the potassium load (Zeeman et al. 2008; Zeeman and Kujawa-Roeleveld 2011). Clearly this indicates that a large fraction of the nutrients may be directly recovered from source-separated urine.
Methods for nutrient recovery from source-separated urine were discussed by Maurer et al. (2006). Recently, a new bio-electrochemical method based on a microbial fuel cell for energy production combined with nitrogen recovery from urine was proposed by Kuntke et al. (2012). So far, only struvite precipitation and ammonia stripping have been applied at full scale. Struvite precipitation is usually used for simultaneous recovery of phosphorus and nitrogen. The end product can be used as a fertilizer. However, the fraction of nitrogen that can be recovered is very small. Ammonia stripping requires a large energy input (Maurer et al. 2003) and only recovers nitrogen.
Another option for nutrient recovery from human urine is microalgae cultivation. Microalgae are photoautotrophic microorganisms that take up and accumulate nutrients. Chlorella species, for example, consist of 6–8 % nitrogen and 1–2 % phosphorus on a dry weight basis (Oh-Hama and Miyachi 1988). In line with these numbers, cultivation of microalgae on urine could maximize phosphorus recovery along with nitrogen recovery compared to other methods such as struvite precipitation. Large-scale cultivation of microalgae would then create a sink for these nutrients.
Microalgae biomass can be used as a biofertilizer and as a potential source of biochemicals and biofuels. Large-scale microalgae production for biofuels is not yet economically feasible (Kovacevic and Wesseler 2010). The algal culture medium usually is made up of fertilizers, and this accounts for 7–11 % of the total microalgae production costs (Norsker et al. 2011). This can become even higher due to depletion of natural resources, in particular phosphate rock. Coupling microalgae cultivation for biofuels to urine treatment therefore is considered a realistic option to reduce the costs of microalgae production (Pittman et al. 2011) although it is appreciated that several logistic problems need to be solved to achieve this. Eventually, to close nutrient cycles, microalgae biomass or the remaining nutrients from processed biomass can be used as fertiliser.
Using human urine for cultivation of microalgae and cyanobacteria (blue-green algae) was first studied for life support systems for missions in outer space. Several studies demonstrated that cyanobacteria and microalgae can grow on highly diluted human urine (Adamsson 2000; Feng and Wu 2006; Yang et al. 2008). The urine sources used in these studies were either diluted synthetic urine, or urine which was diluted more than 100 times. No information is available at all about microalgae growth on concentrated urine and about the specific needs of microalgae growing on concentrated human urine. Moreover, the composition of urine changes due to hydrolysis and precipitation while it is transported and stored (Udert et al. 2003). This could have an effect on microalgae growth because of the presence of adverse concentrations of free ammonia or the precipitation of essential nutrients.
In this study, for the first time, microalgae were grown on pure urine. In addition, the essential requirements for microalgae growth on different types of urine (fresh, hydrolysed, male, female) were determined.
Materials and methods
Microalgae
The selected species, Chlorella sorokiniana, demonstrated better growth than Chlorella vulgaris, Scenedesmus obliquus, and mixed algae culture in preliminary tests on human urine (unpublished results) and therefore was selected for further experiments. C. sorokiniana CCAP211/8K was obtained from the Culture Collection of Algae and Protozoa, Oban, UK. Pre-cultivation was done with M8a medium (Kliphuis et al. 2010) in 250-mL enclosed shake flasks with 100 mL of liquid culture at 2 % CO2 (v/v), 25 °C, an irradiance of 20–40 μmol photons m−2 s−1, and a 16/8-h day/night cycle. A culture in its linear growth phase was used as an inoculum for an experiment on the requirement of trace elements (Experiment I). The inoculum in a linear growth phase used for growth experiments on diluted and concentrated urine (Experiment II and III) was first concentrated by centrifuging for 3 min at 13,800 relative centrifugal force and resuspending the pellet in demineralized water.
Collection of urine
Two batches of male and female urine were collected without dilution from healthy employees of the Sub-department of Environmental Technology, Wageningen University, The Netherlands. Male and female urine were tested separately to see whether or not differences in gender-related compositions, e.g., sex hormones, would affect microalgae growth. The urine was kept at 4 °C prior to the experiments. Urine hydrolysis, a decomposition of urea into ammonia and bicarbonate, was done by letting urine bottles stand with continuous mixing on a shaker at 30 °C. The ammonia concentration was regularly analysed and urine taken at different incubation times represented different degrees of hydrolysis.
Urine-based growth media
Experiment I: the requirement of trace elements
The first batch of male and female urine was used in this experiment. The composition of male urine was as follows: chemical oxygen demand (COD) of 7,480 mg-O2 L−1, total nitrogen (TN) of 6,340 mg L−1, ammonium nitrogen (NH4 +-N) of 442 mg L−1, and total phosphorus (TP) of 401 mg L−1. The composition of female urine was as follows: COD of 6,305 mg-O2 L−1, TN of 6,500 mg L−1, NH4 +-N of 361 mg L−1, and TP of 510 mg L−1. These male and female urine were diluted 20 times with demineralised water (1 urine volume to the total volume of 20, named MU 1/20 and FU 1/20). With addition of iron and micronutrients (B, Cu, Mn, and Zn) that are referred to as trace elements in this study, final concentrations of added compounds in 20 times diluted urine were equal to those on M8a medium: 320 μM EDTA ferric sodium salt, 100 μM Na2EDTA.2H2O, 1 μM H3BO3, 66 μM MnCl2.4H2O, 11 μM ZnSO4.7H2O, and 7.3 μM CuSO4.5H2O. NaHCO3 was added to all diluted media with a final concentration of 10 mM and the pH was adjusted to 7 with 3 M HCl.
Experiment II: growth experiment on diluted urine
The second batch of male and female urine was used in this experiment. Fresh and hydrolysed urine with different degrees of hydrolysis were diluted five and ten times with demineralised water (1 urine volume to the total volume of 5 and 10, named 1/5 and 1/10). For the experiments with additional magnesium, the urine pH was adjusted to 7 prior to addition of MgCl2.H2O and diluting with demineralised water. Magnesium concentration in undiluted urine was set to 0.02 mM. Trace elements were added while diluting urine with demineralised water resulting in the following concentrations in five and ten times diluted urine: 32 μM EDTA ferric sodium salt, 10 μM Na2EDTA.2H2O, 0.1 μM H3BO3, 6.6 μM MnCl2.4H2O, 1.1 μM ZnSO4.7H2O, and 0.73 μM CuSO4.5H2O.
Synthetic urine modified from Feng and Wu (2006) consisted of (in 1 L) 10.72 g urea, 4.12 g K2HPO4, 4.83 g NaCl, 2.37 g Na2SO4, 1 g creatine, 0.65 g tri-sodium citrate dihydrate (C6H5Na3O7.2H2O), 0.5 g CaCl2.2H2O, 0.47 g MgCl2.H2O, and 0.29 g KCl. To mimic hydrolysed urine, 28.8 g NH4HCO3 was added to serve as a nitrogen source instead of urea. Trace elements were added to synthetic urine resulting in the following concentrations: 320 μM EDTA ferric sodium salt, 100 μM Na2EDTA.2H2O, 1 μM H3BO3, 66 μM MnCl2.4H2O, 11 μM ZnSO4.7H2O, and 7.3 μM CuSO4.5H2O. Synthetic urine with either urea or ammonium as a nitrogen source was then diluted five and ten times with demineralised water and the pH was adjusted to 7.
Table 1 shows the composition of different stocks of non-diluted urine used in this experiment, as well as the composition of synthetic urine and M8a medium.
Experiment III: growth experiment on concentrated urine
The same batch of male and female urine as for Experiment II was used. For non-diluted urine and two times diluted urine (1 urine volume with 1 volume of demineralised water). The concentration of trace elements was doubled from that used in five and ten times diluted urine. NaHCO3 was added to all diluted media with a final concentration of 10 mM and the pH was adjusted to 7.
Experimental set-up
The experiments were done in 24-well microtiter plates under non-axenic conditions, and all growth media were not sterilised. For Experiment I and II, each well was filled with 1 mL of growth medium. After filling the growth media, each well was inoculated with 20–50 μL of microalgae culture to obtain an optical density at 750 nm (OD750) of 0.05–0.10. For Experiment III, each well was filled with only 0.35 mL of growth medium and inoculated with 10 μL of microalgae culture. The smaller volume was used to simulate a short optical path and to increase biomass concentration and volumetric productivity while still working within the linear measuring range of the well plate reader.
Each medium was tested in triplicate. The plates were incubated in an orbital shaking incubator (Infors HT Multitron, Switzerland) operated at 100 rpm, 35 °C, and 80 % humidity. The cultures were continuously illuminated with fluorescence lamps at an average irradiance of 105 μmol photons PAR m−2 s−1. 4 % CO2 (v/v) was supplied to prevent CO2 limitation.
A repeated batch mode was applied. After incubating for 24 h, the culture was diluted by first re-suspending the cultures in each well, transferring them to clean 1.5-mL Eppendorf tubes, re-filling each well with 1 mL of fresh growth medium (with the same composition as the previous day), and re-inoculating each well with 40–80 μL of the cultures from the Eppendorf tubes. The amount of inoculum depended on the OD750 of the culture in order to maintain the OD750 at the start of each consecutive day between 0.05 and 0.1. This repetitive procedure allowed to test whether or not the microalgae would be able to maintain their growth in a continuous cultivation system. For the experiment on the trace element requirement, after 48 h of growth the trace elements were added to diluted urine media with no initial trace elements added.
The OD750 nm was measured five times a day with a BIOTEK (EL800) spectrophotometer (i.e., a well plate reader). Prior to optical density measurement, cultures were re-suspended to detach precipitated algae from plate surface. The pH was measured daily before and after daily diluting of algal cultures by a Sentron pH meter. Because the urine has colour, the OD750 of the urine media without microalgae was subtracted from the OD750 of samples containing microalgae. Reported values were averaged over triplicated samples, and error bars in all figures indicated standard deviation of three replicates.
Analytical methods
NH4 +-N and PO4 3--P concentrations were determined according to standard methods (APHA 1998) using the continuous flow analyser (SKALAR). Chemical oxygen demand (CODsol) and total nitrogen (TN) were measured photometrically according to standard methods (APHA 1998) using Dr Lange® test kits. Prior to analyses, urine samples were filtered (0.45 μm) for solid removal. The ICP-OES (VISTA-MPX) was used to determine B, Ca, Fe, K, Mg, Na, and Zn concentrations. Prior to an ICP analysis, samples were acidified with nitric acid (HNO3) to the final concentration of 1 % acid.
Calculations
To be able to compare microalgae growth under different conditions, the specific growth rate of the microalgae was calculated although there was no clear exponential growth under the light-limiting conditions applied. The reported specific growth rate was calculated by a linear regression of the natural logarithm of OD750 versus culturing time and specific growth rate at a certain time was calculated according to the following equation:
where μ is the specific growth rate, OD750-t1 and OD750-t2 are the optical density at 750 nm at time t 1 and t 2, respectively. Calculated growth rates were analysed using a multivariate analysis in the IBM SPSS Statistics 19 software. Significant differences in growth rate were tested using Sidak (multiple comparison) with 95 % confidence interval.
Results
Experiment I: the need for trace elements for microalgae growth on urine
Figure 1 shows growth as OD750 of C. sorokiniana on 20 times diluted fresh male and female urine with and without additional trace elements (B, Cu, Fe, Mn, and Zn); MU1/20, MU1/20+trace, FU1/20, and FU1/20+trace. Growth of C. sorokiniana on both male and female urine, with and without addition of trace elements, was similar during the first 24 h (p = 1.0 for all paired samples). When the cultures were diluted, growth on the urine without additional trace elements decreased drastically to about half of that on the medium with trace elements. This suggests that the small amount of trace elements contained in the inoculum could support microalgae growth during the first 24 h but after dilution insufficient trace elements were available to support substantial growth on the second day.
During the second daily dilution, trace elements were added to the urine media lacking these elements and this resulted in similar growth on both urine media on day 3. Re-growth after adding trace elements confirmed that decreasing growth was a result of low trace element concentrations. The specific growth rate of these algae ranged from 0.06 to 0.07 h−1 at low concentrations of trace elements on day 2 to 0.10–0.16 h−1 with addition of trace elements on day 3 (Table 2). Although similar growth was observed, the highest specific growth rate took place on 20 times diluted male urine with trace elements (MU1/20+trace) on day 3. There is no significant difference between the specific growth rates on male and female urine (p = 1.0), except for the growth rate on MU1/20+trace on day 3, which is higher than growth rates on any other media.
Experiment II: microalgae growth on diluted urine
Growth on synthetic urine
Most of the nitrogen in fresh urine is present as urea. When urine is stored, urea is hydrolysed into free ammonia (NH3), ammonium (NH4 +), and bicarbonate (HCO3 −). Consequently, urea and ammonium are the major forms of nitrogen in urine and both are known to support growth of microalgae (Birdsey and Lynch 1962). However, high free ammonia concentrations can inhibit microalgae growth. Therefore, synthetic urine with either urea or ammonium was tested.
Figure 2 shows growth as an increase of OD750 of C. sorokiniana on five and ten times diluted synthetic urine, with urea and ammonium as a nitrogen source, and on M8a medium as a reference: SYNur1/5, SYNur1/10, SYNam1/5, SYNam1/10, and M8a. The experimental results showed similar growth on synthetic urine and M8a medium, indicating that urea and ammonium support microalgae growth as well as nitrate, the nitrogen source in M8a medium. On day 1, growth on all synthetic urine media was similar (p > 0.05) and ranged from 0.100 to 0.123 h−1. After daily dilution, re-growth could be observed on all synthetic urine media with a specific growth rate between 0.083 and 0.113 h−1. Calculated specific growth rates of C. sorokiniana on different dilutions of synthetic urine are given in Table 2. The highest growth rate of 0.123 h−1 was observed on SYNam1/10 on day 1.
During cultivation, the pH of diluted synthetic urine with urea was between 6.8 and 7.3 and the pH of diluted synthetic urine with ammonium was between 7.5 and 8.2 (Table 2). At a pH higher than 8, the fraction of free ammonia in synthetic urine with ammonium may have affected growth of the microalgae. For SYNam1/5, the ammonium concentration was around 940 mg-N L−1. At a pH of 8.2 and temperature of 35 °C, the free ammonia (NH3) concentration could reach 140 mg-N L−1, which is much higher than the reported free ammonia level causing inhibition of photoassimilation (1.2 mM) (Azov and Goldman 1982). C. sorokiniana apparently could tolerate this ammonia level as demonstrated by a specific growth rate as high as 0.113 h−1 on day 2. In addition, it was calculated that the specific growth rate on this medium is not statistically different from that on M8a medium.
Growth on fresh urine
On fresh or slightly hydrolysed urine, the microalgae were able to grow on all media. Figure 3 shows the increase of OD750 representing microalgae growth on five and ten times diluted male and female urine: MU1/5, MU1/10, FU1/5, and FU1/10. On day 1, specific growth rates on all urine media ranged between 0.101 and 0.114 h−1 and no significant difference was observed among these media and on M8a (p > 0.05). After the cultures were daily diluted on day 2, re-growth could be maintained and specific growth rates on all urine media were similar (p > 0.05). However, the specific growth rate on day 2 was slightly lower than that of day 1 (0.08–0.10 h−1). Again after daily dilution on day 3, rapid growth was observed on all media with a slight increase in specific growth rate in comparison to day 2 except for MU1/5. Calculated specific growth rates of C. sorokiniana on different dilutions of male and female urine are shown in Table 2. The highest growth rate on real urine media was observed on MU1/10 on day 1 (0.116 h−1).
When comparing growth rates on male and female urine with synthetic urine, specific growth rates on both male and female urine were similar to that on synthetic urine with either urea or ammonia. Similar growth on real and fresh urine in comparison to synthetic urine indicated no inhibitory effect that could possibly result from organic compounds or other compounds present in real urine. Moreover, similar growth on male and female urine from both Experiment I and II showed that differences in gender related urine composition (e.g., presence of different hormones) did not significantly affect microalgae growth in this study.
During the experiments, the pH of male urine media ranged between 5.7 and 8.0, whereas the pH of female urine media ranged between 7.1 and 7.9 (Table 2). The lowest pH was observed on male urine on day 1. However, this low pH did not seem to affect microalgal growth because there was no significant difference among growth rates on male urine, female urine, and M8a medium (p > 0.05). These results show that it is feasible to cultivate C. sorokiniana on fresh urine with minimal dilution.
Growth on hydrolysed urine
When urine is collected and stored, hydrolysis can readily occur resulting in increasing ammonia concentrations and this could be toxic to microalgae. Figure 4a and b show growth of C. sorokiniana as the increase of OD750 on five and ten times dilution of fully hydrolysed male and female urine: HMU1/5, HMU1/10, HFU1/5, and HFU1/10. In addition, Fig. 4c and d show growth on five and ten times dilution of partially hydrolysed male and female urine: 40 %HMU, 60 %HMU, 20 %HFU, and 70 %HFU. On fully hydrolysed male and female urine, the specific growth rate was significantly lower than that on synthetic and fresh urine.
Specific growth rate on urine media with a degree of hydrolysis of 60 % and 70 % was similar to that on fully hydrolysed urine media. Slightly higher growth rate was observed on urine with lower degrees of hydrolysis: 40 % and 20 %. The specific growth rates calculated over 24 h for microalgae grown on fully hydrolysed male and female urine and 60 and 70 % hydrolysis of male and female urine were within a range of 0.064–0.075 h−1. Specific growth rates on 40 %HMU and 20 %HFU, respectively were 0.088 and 0.092 h−1. The culture pH ranged between 6.8 and 7.9. After 24 h, the microalgae stopped growing. This poor growth could have been caused by either high ammonia concentrations, which are known to be toxic to microalgae, or by specific nutrient limitations.
When comparing growth on hydrolysed urine with synthetic urine with ammonium, higher growth was observed on synthetic urine even though synthetic urine contains a higher ammonium concentration: 4,700 mg NH4 +-N L−1 compared to 3,260 and 2,150 mg NH4 +-N L−1, respectively for undiluted hydrolysed male and female urine. The ammonium concentrations in 40 and 20 % hydrolysed male and female urine were even lower but still growth on these media was poor. Moreover, during the experiments, the pH of the hydrolysed urine was within a range of pH 6.6 to 7.9 (Table 2). This pH range was slightly lower than the pH of synthetic urine. Consequently, the fraction of free ammonia in hydrolysed urine must be lower than that of synthetic urine. Apparently free ammonia was not the major factor limiting microalgae growth on hydrolysed urine.
Table 1 shows that hydrolysed urine contains less magnesium (Mg2+) than fresh urine. The reported magnesium content of Chlorella sp. ranges between 0.36 and 0.8 % on dry weight basis (Oh-Hama and Miyachi 1988). The Mg2+ concentration of diluted hydrolysed urine never was higher than 0.15 mg L−1 which could support only 40 mg dry biomass L−1 at 0.36 % magnesium content. Hence, this could explain the poor growth observed in this study.
The effect of magnesium addition on growth on hydrolysed urine
To check the hypothesis of magnesium (Mg2+) limited growth, we tested the effect of Mg2+ addition to hydrolysed urine. Figure 5 shows growth of C. sorokiniana on five and ten times diluted fully hydrolysed male and female urine with additional Mg2+. When Mg2+ was added to hydrolysed urine, the microalgae were able to grow and maintain growth after repeated daily dilution. Specific growth rates on hydrolysed male and female urine with additional Mg2+ ranged between 0.095 and 0.111 h−1 (Table 2). There was no significant difference of the specific growth rate on hydrolysed urine with additional Mg2+ as compared to that on synthetic and fresh urine confirming that Mg2+ limited growth of microalgae on hydrolysed urine.
Experiment III: microalgae growth on concentrated urine
The previous results showed the possibility to cultivate microalgae on minimally diluted urine. On concentrated urine, microalgae might not be able to maintain growth. In Experiment III, it was therefore attempted to grow C. sorokiniana on concentrated urine. At high nutrient concentrations, as those in concentrated urine, high biomass densities are needed to remove all nutrients. To maintain a high productivity in such dense cultures, however, it is needed to increase the light supply to the cultures. This can be done by increasing the ratio between the light-exposed surface area and the culture volume (S/V ratio). To test this strategy, the wells of the microtiter plates were filled with a smaller volume of culture medium. Consequently, the S/V ratio was increased resulting in a higher light input to the cultures.
In Fig. 6a and b, growth of C. sorokiniana is shown on non-diluted and two times diluted fresh male and female urine: MU, MU1/2, FU, and FU1/2. In addition, growth on two times diluted hydrolysed female urine with additional Mg2+, synthetic urine with urea, and M8a are shown in Fig. 6c. The results demonstrate that microalgae can grow on concentrated urine. The corresponding specific growth rates are shown in Table 3. Although there was almost no growth on non-diluted male urine (MU), growth could still be observed on two times diluted male urine (MU1/2). On the contrary, growth rate on non-diluted female urine (FU) was better than that of male urine. On day 2 and 3, the specific growth rate on FU had increased to the same level as observed on FU1/2, MU1/2, and M8a. Apparently the microalgae adapt to this concentrated medium. The pH of the urine media in this experiment was within the range of pH 7.4–8.4, indicating the occurrence of hydrolysis.
OD750 in time of C. sorokiniana grown on a concentrated male urine with additional trace elements, b female urine with additional trace elements, and c hydrolysed female urine with magnesium and trace elements, synthetic urine with urea, and M8a. Error bars show standard deviation (n = 3). In this experiment, 0.35 mL of growth media was used to mimic a short light-path system
On non-diluted synthetic urine with urea (SYNur), good growth was observed with the specific growth rate ranging between 0.072 and 0.116 h−1. The specific growth rate was relatively low on day 1 but on days 2 and 3 it was equal to that of diluted fresh and synthetic urine. The pH of SYNur was maintained at pH 7.2–7.7 (Table 2). As expected, based on the high ammonium concentration, no growth was observed on non-diluted synthetic urine containing ammonium instead of urea.
C. sorokiniana was also successfully grown on hydrolysed urine with only a dilution factor of two with additional Mg2+. This urine medium had an ammonium concentration of about 1,400 mg NH4 +-N L−1 and the pH ranged between 7.6 and 8.0. During the cultivation, the highest specific growth rate on this medium was up to 0.149 h−1. This shows that C. sorokiniana can tolerate ammonium levels as high as 1,400 mg NH4 +-N L−1.
As compared to results from Experiment II, an OD750 obtained in this experiment was somewhat lower but relatively high specific growth rates calculated according to the “Calculations” section were still obtained. Due to the fact that only one third of the culture media was used for simulating a short light-path system, 1 OD750 unit in this experiment would correspond to a three times higher biomass density if 1 mL of growth medium was used. With a factor of 3, biomass density on concentrated urine in this experiment would be comparable to that from Experiment I and II. Results of this experiment clearly show the feasibility to cultivate microalgae on concentrated human urine provided that trace elements and (if needed) magnesium were supplemented.
Discussion
Chlorella sorokiniana is a fast-growing microalga with reported maximum specific growth rates up to 0.25 h−1 (Sorokin 1967; Cuaresma et al. 2009) under autotrophic and light saturating conditions. In this study, the highest specific growth rate of C. sorokiniana achieved on real urine was 0.158 h−1 (MU1/20+trace elements, day 3). Nevertheless, this is still lower than the reported maximum specific growth rate. This is most likely related to the relatively irradiance of 105 μmol photons m−2 s−1 considering that the growth of C. sorokiniana only saturates above 300 μmol photons m−2 s−1 (Janssen et al. 1999). Moreover, mutual shading would further decrease the average light supply per microalgae cell during batch cultivation.
In our study, we minimized urine dilution and achieved relatively high growth rate which was maintained for several days when daily diluting the cultures. Although the tested period lasted for only 3 days, rapid growth can be observed already on the first day for most experiments. As can be seen in almost all figures even in the first day, a clear lag phase cannot be distinguished. In most cases, microalgae growth increased further during day 2 and day 3 showing adaptation of the microalgae to the growth media used.
In this study, microalgae biomass density was measured and expressed as the optical density measured at 750 nm. In addition, the OD at 680 nm was measured as well. At 680 nm, the absorbance of light by chlorophylls adds to the OD measured (OD680). At 750 nm, it is only scattering of light that determines the OD (OD750). If the bacterial population increases, this would be reflected in a change in the OD680/OD750 ratio. This ratio would become lower and approach 1 in case the bacteria would dominate. The OD680/OD750 ratio of microalgae grown on fresh urine and on hydrolysed urine with additional magnesium ranged between 1.8 ± 0.2 and 2.2 ± 0.1. This was comparable to the OD680/OD750 ratio on M8a medium (2 ± 0.2), which did not contain any organic compounds. Consequently, microalgae dominated all the cultures and the optical density is a reliable measure for microalgae biomass density.
In this study, under light-limited conditions, the highest growth rate on five and ten times diluted fresh urine and synthetic urine respectively were 0.116 and 0.123 h−1. This growth is comparable to growth on a standard medium, M8a, showing that urine with additional trace elements is a good growth medium for this microalga. Fresh urine well supports growth of microalgae with supplemental iron and micronutrients. Additional magnesium is needed for growing microalgae on hydrolysed urine. The required amount of these compounds depends upon the initial concentration in the urine, which can vary from person to person according to dietary differences.
Re-growth after trace element addition to fresh urine clearly showed the need for these elements. The trace elements Fe, Cu, Mn, and Zn that were added have been shown to play an important role in microalgal metabolism, whereas B is only required for some algal species (Oh-Hama and Miyachi 1988; O’Kelley 1968). The minimum concentrations of trace elements required for growth of C. sorokiniana reported by Eyster (1978) were 1 μM of Fe, 10−6 μM of Cu, 0.3 μM of Mn, and 1 μM of Zn. The requirement of B was not indicated as neither positive nor negative effect was observed at a concentration of 0.001–10 mg L−1 (McBride et al. 1971).
In urine, these trace elements are present mostly at low concentrations. The measured Fe concentration was lower than the detection limit (0.9 μM or 0.05 mg L−1), whereas the measured concentration of Cu and Zn of the urine used in this study were within the range of 3–9 and 1–11 μM, respectively. Reported concentrations of trace elements in urine in other studies are respectively 1.2–7.2 μM of Fe (Chang et al. 2013; Ronteltap et al. 2007a), 0.04–2.25 μM of Cu (Ronteltap et al. 2007a, b), 0.001–0.05 μM of Mn (Tsalev 1984), and 1.1–8.2 μM of Zn (Ronteltap et al. 2007a). Initial concentrations of these elements are relatively low except for Cu that is present at higher concentration than the minimum concentration needed for C. sorokiniana (Eyster 1978). However, when the urine is hydrolysed, Cu potentially precipitates (Ronteltap et al. 2007b), and this could result in a deficiency condition. Among these trace elements, manganese (Mn) seems to be the most critical element lacking in urine but also Fe, Cu, and Zn are present at vary concentrations and can potentially precipitate.
Magnesium is needed for production of chlorophyll, and it is also believed to play a role in algae metabolism (Sydney et al. 2010). The results of this study confirm that Mg2+ is important for microalgae growth, and this finding agrees with other studies that showed enhancement of microalgae growth with additional Mg2+ (Adamsson 2000; Mandalam and Palsson 1998). The Mg2+ requirement can be estimated from the growth results on fresh urine. Fresh male urine diluted ten times (MU1/10) contained 0.42 mg-Mg2+ L−1 (Table 1) and resulted in a biomass increase to an OD750 of 0.7, which is comparable to an algae density of 200 mg-dry biomass L−1. Assuming that Mg2+ was fully utilized, the calculated Mg2+ content of microalgae would then be 2 mg g-biomass−1. This mass fraction can be used to assess the maximum amount of Mg2+ required to support microalgae growth up to a given biomass density.
A major loss of Mg2+ could be due to precipitation of struvite (MgNH4PO4.6H2O) during hydrolysis of urea. Udert et al. (2003) simulated precipitation of struvite and octacalcium phosphate in urine and reported 87 % precipitation potential at only 11 % hydrolysis. At 24 % hydrolysis, the precipitation potential increased to 97 %. This phenomenon could explain the significant decrease in Mg2+ concentration in the urine with 20 % and 40 % hydrolysis. In addition to Mg2+, Chlorella biomass contains 0.005–0.08 % Calcium (Ca). Precipitation of hydroxyapatite (Ca10(PO4)6(OH)2) causes a reduction of Ca2+ when the Mg2+ concentration decreases (Udert et al. 2003). However, the Ca2+ content of microalgae is relatively low and urine contains higher Ca2+ than Mg2+ concentration. This explains why we did not observe Ca2+ deficiency affecting microalgae growth.
In most cases, the urine N/P atomic ratio is relatively higher (up to 28:1) than a balance value (16:1) for microalgae known as the Redfield ratio (Redfield 1958). This ratio increases when urine is hydrolysed because a significant amount of phosphate precipitates as struvite, hydroxyapatite, or other forms (Udert et al. 2003). However, in this study the effect of the high N/P ratio is minimal because of light being the real growth limiting factor in the short cultivation period applied. In practice, phosphorus, light, or both can limit growth and that has to be taken into account when designing microalgae-based treatment systems.
As mentioned earlier, urea hydrolysis immediately occurs leading to struvite precipitation and depletion of Mg2+ and a decrease in availability. Struvite has low solubility (Ronteltap et al. 2007b). Nevertheless, dissolution of struvite in hydrolysed urine is possible with addition of chemicals. To prevent urine hydrolysis, there are several methods that can be applied including acidification, microfiltration, ultrafiltration, and the use of urease inhibitors which are aiming at removing or inactivating bacterial growth (Maurer et al. 2006). But some of these techniques are costly and energy consuming. Acidification needs acid to stabilise urine but later the pH needs to be neutralised again in order to grow microalgae. Using urease inhibitors is not reliable and has potential negative effects on humans and the environment (Benini et al. 1999). Unless the urine is freshly collected and used for microalgae growth, addition of Mg2+ directly to the microalgae culture would then be a less complicated and more cost-effective option. The estimated Mg2+ requirement for C. sorokiniana observed in this study is about 2 mg g-biomass−1.
In conclusion, C. sorokiniana is able to grow on concentrated human urine up to 1.4 g NH4 +-N L−1 at a pH lower than 8.0. Maximum growth was obtained on 20 times diluted urine with additional trace elements. Microalgae growth on pure urine is possible at slightly reduced growth rates. Human urine supports rapid microalgae growth with additional trace elements and/or magnesium, depending on the urine composition. Urine can serve as a rich source of major nutrients for the large-scale sustainable production of microalgae biomass.
References
Adamsson M (2000) Potential use of human urine by greenhouse culturing of microalgae (Scenedesmus acuminatus), zooplankton (Daphnia magna) and tomatoes (Lycopersicon). Ecol Eng 16:243–254
APHA (1998) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington D.C
Azov Y, Goldman JC (1982) Free ammonia inhibition of algal photosynthesis in intensive cultures. Appl Environ Microb 43:735–739
Benini S, Rypniewski WR, Wilson KS, Miletti S, Ciurli S, Mangani S (1999) A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure 7:205–216
Birdsey EC, Lynch VH (1962) Utilization of nitrogen compounds by unicellular algae. Science 137:763–764
Chang Y, Wu Z, Bian L, Feng D, Leung DYC (2013) Cultivation of Spirulina platensis for biomass production and nutrient removal from synthetic human urine. Appl Energ 102:427–431
Cuaresma M, Janssen M, Vílchez C, Wijffels RH (2009) Productivity of Chlorella sorokiniana in a short light-path (SLP) panel photobioreactor under high irradiance. Biotechnol Bioeng 104:352–359
Eyster C (1978) Nutrient concentration requirements for Chlorella Sorokiniana. Ohio J Sci 78:79–81
Feng D, Wu Z (2006) Culture of Spirulina platensis in human urine for biomass production and O2 evolution. J Zhejiang Univ Sci B 7:34–37
Janssen M, Kuijpers TC, Veldhoen B, Ternbach MB, Tramper J, Mur LR, Wijffels RH, Osinga JTJGB, Wijffels RH (1999) Specific growth rate of Chlamydomonas reinhardtii and Chlorella sorokiniana under medium duration light/dark cycles: 13–87 s. J Biotech 70:323–333
Kliphuis AMJ, Winter L, Vejrazka C, Martens DE, Janssen M, Wijffels RH (2010) Photosynthetic efficiency of Chlorella sorokiniana in a turbulently mixed short light-path photobioreactor. Biotechnol Progr 26:687–696
Kovacevic V, Wesseler J (2010) Cost-effectiveness analysis of algae energy production in the EU. Energ Policy 38:5749–5757
Kuntke P, Śmiech KM, Bruning H, Zeeman G, Saakes M, Sleutels THJA, Hamelers HVM, Buisman CJN (2012) Ammonium recovery and energy production from urine by a microbial fuel cell. Water Res 46:2627–2636
Mandalam RK, Palsson B (1998) Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnol Bioeng 59:605–611
Maurer M, Pronk W, Larsen TA (2006) Treatment processes for source-separated urine. Water Res 40:3151–3166
Maurer M, Schwegler P, Larsen TA (2003) Nutrients in urine: energetic aspects of removal and recovery. Water Sci Technol 48:37–46
McBride L, Chorney W, Skok J (1971) Growth of Chlorella in relation to boron supply. Bot Gaz 132:10–13
Norsker N-H, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production - a close look at the economics. Biotechnol Adv 29:24–27
O'Kelley JC (1968) Mineral nutrition of algae. Annu Rev Plant Phys 19:89–112
Oh-Hama T, Miyachi S (1988) Chlorella. In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal biotechnology. Cambridge University Press, Cambridge, pp 3–26
Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Biores Technol 102:17–25
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 230A:205–221
Ronteltap M, Maurer M, Gujer W (2007a) The behaviour of pharmaceuticals and heavy metals during struvite precipitation in urine. Water Res 41:1859–1868
Ronteltap M, Maurer M, Gujer W (2007b) Struvite precipitation thermodynamics in source-separated urine. Water Res 41:977–984
Sorokin C (1967) New high-temperature Chlorella. Science 158:1204–1205
Sydney EB, Sturm W, de Carvalho JC, Thomaz-Soccol V, Larroche C, Pandey A, Soccol CR (2010) Potential carbon dioxide fixation by industrially important microalgae. Biores Technol 101:5892–5896
Tsalev DL (1984) Atomic absorption spectrometry in occupational and environmental health practice. Volume II: Determination of individual elements. CRC Press Inc, Boca Raton
Udert KM, Larsen TA, Biebow M, Gujer W (2003) Urea hydrolysis and precipitation dynamics in a urine-collecting system. Water Res 37:2571–2582
Yang C, Liu H, Li M, Yu C, Yu G (2008) Treating urine by Spirulina platensis. Acta Astronaut 63(7–10):1049–1054
Zeeman G, Kujawa-Roeleveld K (2011) Resource recovery from source separated domestic waste(water) streams; full scale results. Water Sci Technol 64:1987–1992
Zeeman G, Kujawa K, Mes T, Hernandez H, Graaff M, Abu-Ghunmi L, Mels A, Meulman B, Temmink H, Buisman C, Van Lier J, Lettinga G (2008) Anaerobic treatment as a core technology for energy, nutrients and water recovery from source-separated domestic waste(water). Water Sci Technol 57:1207–1212
Acknowledgments
The project is financially supported by Innowater funding provided by the Dutch Department of Economic Affairs. The Ph.D. student is financially granted by the Ministry of Science and Technology, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tuantet, K., Janssen, M., Temmink, H. et al. Microalgae growth on concentrated human urine. J Appl Phycol 26, 287–297 (2014). https://doi.org/10.1007/s10811-013-0108-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0108-2