Abstract
The aim of this study was to describe the bimonthly variation in the proximate chemical composition, alginate, and fucoidan yield and ethanolic extract content, including the anticoagulant and antioxidant activity of the extracts and quality tests of alginates, from blades and stipes of Eisenia arborea collected in Bahía Magdalena, Baja California Sur, Mexico. Significant differences were found in the chemical composition between months and also between the alga structures. The major constituents in both blades (53.8%) and stipes (47.6%) were carbohydrates and ash (28.4 and 33.9%, respectively). The crude ethanolic extract yield and the antioxidant activity in blades were higher than in stipes throughout the period with a maximum in September (5.4% and EC50 = 82.7 μg mL−1). The highest yield of crude fucoidan was obtained in September (20%) for blades and March (8.7%) for stipes. The anticoagulant activity of fucoidan was higher in January for blades and May for stipes. The alginate yield showed significant difference (p > 0.05) between blades and stipes. The highest yield for blades was obtained in November (21.3%) and for stipes in January (24.5%). Our results suggest that the best period to harvest the alga is from September to March, considering the higher yields and better properties of the extracts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brown seaweeds are a diverse group of which the main use is human feed and alginate extraction, but also provide a rich resource of bioactive compounds, making them suitable for a wide variety of applications in the food, cosmetic, pharmaceutical, and chemical industries (Chapman and Chapman 1980; McHugh 2003; Jiao et al. 2011; Holdt and Kraan 2011). Despite the promising uses and applications of brown seaweeds, the main difficulty in selecting a species for an industrial process is that the chemical compounds and extracts from seaweed show variations in their yield and properties as the result of adaptive responses to the environmental conditions in which they live (Chapman and Chapman 1980; Lobban and Harrison 1994; Amsler 2008; Holdt and Kraan 2011; Skriptsova et al. 2012). Due to that, for over half a century (Black 1950) efforts have been made in research to describe the behavior of the chemical constituents in several species of seaweed, particularly in species which have a commercial interest, in order to optimize their use and select the best harvesting period (Rodríguez-Montesinos and Hernández-Carmona 1991; Usov et al. 2005; Hernández-Carmona et al. 2009; Holdt and Kraan 2011; Men'shova et al. 2013; Wu et al. 2014; Schiener et al. 2015; Skriptsova 2016).
In Mexico, most of the products derived from brown seaweed are imported and the country ceased its participation in the world market for brown seaweed more than a decade ago. The giant kelp Macrocystis pyrifera is the only brown seaweed hand-harvested for local trades, despite there being more species that could be used such as the kelp Eisenia arborea J.E. Areschoug (DOF 2012; Hernández-Carmona et al. 2012). Species of Eisenia are appreciated in Asian coastal areas for their use in human food and folk medicine and as a raw material for alginates, fucoidan, and several metabolites with interesting bioactivity, i.e., antioxidant, antitumoral, anti-inflammatory, and antiallergic (Sugiura et al. 2006, 2013; Ermakova et al. 2013; Men'shova et al. 2013; Shibata et al. 2015).
On the Mexican Pacific coast, E. arborea is commercially harvested in small amounts (26 wet tonnes per year) (Enrique Hernández, CRIP-Ensenada, personal communication), despite that it has been described as a potential food for animals including humans (Serviere-Zaragoza et al. 2002, 2003; Hernández-Carmona et al. 2009; Zertuche-González et al. 2014), as a raw material for alginates (Hernández-Carmona 1985; Arvizu et al. 2007; Murillo-Álvarez and Hernández-Carmona 2007), as a potential source of fucoidan (Muñoz-Ochoa et al. 2009), and as an alternative to feed abalone (Zertuche-González et al. 2014). Variations have been reported for proximate chemical composition and alginates, with remarkable geographical difference that pointed out that changes do not occur both between seasons and between structures at the southern limit of the species (Hernández-Carmona 1985; Serviere-Zaragoza et al. 2002; Arvizu et al. 2007; Hernández-Carmona et al. 2009). However, the variation of other extracts with commercial interest such as fucoidan or crude extracts is unknown.
We hypothesized that the chemical composition, biological activity, and properties of extracts from E. arborea do not change significantly over time and the changes do not depend on sampling frequency. Therefore, our objective was to describe the bimonthly variation in the proximate chemical composition, yield of alginate, fucoidan and ethanolic extract, the anticoagulant and antioxidant activity of the extracts, and quality of the properties of alginates, separately from blades and stipes from E. arborea collected in Bahía Magdalena (BM), Baja California Sur, Mexico. This information is important to develop a management plan for the species, including the best harvesting time and the potential uses of the algae.
Materials and methods
Eisenia arborea samples were collected at BM (Punta Arenas), Baja California Sur, Mexico (24° 16′ N and 25° 45′ N and 111° 20′ W and 112° 18′ W) (Fig. 1) by semiautonomous Hookah diving equipment, at 5–6 m depth, every 2 months, from September 2013 to July 2014. A minimum of 30 individuals were randomly selected and cut with a knife from the base of the stipes. Algae were divided into blades and stipes, sun-dried, and stored in plastic bags at room temperature. The algae were ground in a Pulvex 200 miller (Maquinaria Pulvex SA de CV, Mexico) to 30 mesh particle size.
Sea superficial temperature was estimated through satellite images obtained from the Group for High Resolution of Sea Surface Temperature (www.GHRSST.org). Images are at 1 day/1 km spatial resolution. We reported only the means and used the information to divide the sampling time into temperature periods.
Proximate chemical composition
The proximate chemical analyses (moisture, ash, crude fiber, proteins, total lipids, and carbohydrates) were carried out according to the AOAC (1995). Moisture was quantified by weight difference at 105 °C for 4 h, ash by calcination at 600 °C for 5 h, protein by the Dumas method with LECO FP-528 Equipment (USA), crude fiber by the successive hydrolysis method (acid/base), total lipids by a Soxtec Avanti 2050 Tecator (Foss Analytical AB, Sweden), and carbohydrates by calculating the difference: 100 − (% proteins + % lipids + % crude fiber + % ash).
Crude ethanolic extracts and antioxidant activity
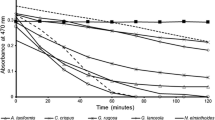
Samples of blades (300 g) and stipes (80 g) were extracted with 96% ethanol for 9 days, and replacement of the solvent every third day. The ethanolic solutions obtained were concentrated to dryness using a rotary evaporator (Yamato E-500) under reduced pressure at 40 °C. Ethanolic extract yield was calculated based on the dry weight of the algae. After extraction, the residual algae were dried at 30 °C for 12 h for the next aqueous extraction. For the antioxidant activity assay, the extracts were dissolved in ethanol at concentrations of 200, 100, 50 and 25 μg mL−1. The antioxidant activity was measured in terms of radical scavenging, using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method (Goupy et al. 1999). Activity was measured in test tubes, adding 1 mL of sample and 4 mL of the DPPH solution at 0.02%. Then, the mixed solution was stored in the dark for 30 min; finally, absorbance was measured on a spectrophotometer at 517 nm.
The scavenging effect was calculated using the following equation:
EC50 was calculated by the linear correlation graphical method, using ascorbic acid as a positive control.
Fucoidan: yield, anticoagulant activity, and characterization
A sample of 20 g of the algae previously extracted with ethanol was used for crude fucoidan extraction, according to Muñoz-Ochoa et al. (2009). The dried algae were extracted in distilled water (1 : 10 ratio, w/v) at 55 °C under continuous agitation for 2 h. The liquid was filtered and the process was repeated twice. The supernatant was clarified by centrifugation. A solution of 10% CaCl2 was added (1 : 1 ratio, v/v) and left for 24 h under refrigeration to promote precipitation of calcium alginates. The precipitate was separated by centrifugation, and the supernatant was mixed with distilled ethanol (1 : 3) and left to stand for 16 h to promote fucoidan precipitation. The precipitate was recovered by centrifugation and dried at 50 °C for 16 h. Fucoidan yield was calculated based on the initial dried weight of the algae.
The anticoagulant activity of each extract was assessed by prothrombin time (PT) and activated partial thromboplastin time (aPTT) assays. Both assays were carried out with human plasma treated with sodium citrate and a stock solution of 10 mg mL−1 of each sample. Each assay was performed by mixing 90 μL of human plasma with 10 μL of the extract in distilled water. The reagents were added according to the manufacturer’s instructions (Siemens Healthcare, Germany). The clot formation time was determined by visual inspection and reported in seconds. For samples that exceeded a coagulation time of 300 s, dose–response curves were performed at concentrations of 10, 5, 2.5, 1.25 and 0.625 mg mL−1 to determine the lowest concentration that doubled the control coagulation time (PT = 13 s; aPTT = 27 s) in each assay.
The crude fucoidan content was characterized partially by infrared (IR) spectroscopy. The spectra were recorded with a PerkinElmer Two with total reflectance attenuator (ATR) (USA). IR spectra were obtained after 28 scans with a resolution of 4 cm−1 in a range of 500–4000 cm−1. Total sugar and fucose content in the fucoidan extract was estimated by colorimetric assays according to Dubois et al. (1956) and Dische (1955).
Alginates: yield, properties, and characterization
The wet algae previously used to extract the crude fucoidan were used for alginate extraction following the method of Arvizu-Higuera et al. (2002). Briefly, wet tissue was put in 180 mL of a 0.1% formaldehyde solution for 16 h. Then, the formaldehyde solution was filtrated and a pre-acid extraction was carried out in 300 mL of distilled water and the pH was adjusted to 4 with 1 N hydrochloric acid (HCl) maintaining continuous stirring for 15 min. The algae were recovered by filtration and put in distilled water in a 1 : 25 ratio (w/v) and transferred to a water bath at 80 °C for 2 h with continuous stirring, and the pH was adjusted to 10 with sodium carbonate solution (10%). The solution was clarified by vacuum filtration with diatomaceous earth. Finally, the alginate was precipitated with ethanol at 96% in a 1 : 1 ratio (v/v). The fibers were recovered and dried for 12 h in an oven at 55 °C. Alginate yield was calculated based on the dry weight of the algae before being used for fucoidan extraction.
Viscosity was measured in 1% alginate solution at 22 °C using a Brookfield LTV viscometer (Brookfield, USA) (Hernández-Carmona et al. 1999). Color was estimated in 1% alginate solution, and transmittance was measured at 510 nm according to Hernández-Carmona et al. (1999). Gel strength was measured on calcium alginate gels using a TA.XTPlus texture analyzer (Stable Micro Systems, UK) according to Camacho and Hernández-Carmona (2012). The alginate IR spectrum was recorded in the same conditions as for fucoidan.
The mannuronic/guluronic acid (M/G) ratio was estimated semiquantitatively through the relationship between the absorption bands at 1125 and 1029 cm−1, from the alginate IR spectrum (Filippov and Kohn 1974).
Statistical analyses
All analysis was carried out in triplicate, except anticoagulant activity which was done in duplicate. Data were normal (Kolmogorov–Smirnov test, R Project 3.01). Significant differences between alga structures (blades or stipes) and between sampling times (months) and the yield of each component were determined using a multifactorial analysis of variance (MANOVA) test. Statistically significant interactions were analyzed using the Tukey post hoc test (Statgraphics Centurion XVII).
Results
Sea surface temperature ranged from 19 to 27 °C at the sampling site. Two periods are distinguished from the data obtained in BM, the warm period (between 23 and 26 °C), from June to November, and the cold period (between 19 and 22 °C), from December to May.
Proximate chemical composition
All constituents showed a significant difference between blades and stipes (p < 0.05) and between months (p < 0.05). The most abundant constituents of blades and stipes from E. arborea were ash and carbohydrates (Table 1). Moisture was less than 13% in all samples. Blades showed a higher content of ash (33.7%) and lipids (0.25%) in January, carbohydrates (55%) and crude fiber (5.6%) in July, and proteins (12.5%) in May. The stipes showed the highest content of ash in March (37.4%), carbohydrates (49.7%) and proteins (10.2%) in May, crude fiber (9.9%) in November, and lipids (0.03%) in July.
Yield of crude extracts and antioxidant activity
The ethanolic crude extract yield showed a significant variation (p < 0.05) between structures and months (Table 2). The yield and antioxidant activity were higher in blades than in stipes (p < 0.05). In blades, the highest yield and antioxidant activity were obtained in September (5.4%) and November (EC50 = 55 μg mL−1). Stipes showed the highest yield (2.07%) and antioxidant activity (EC50 = 352 μg mL−1) in May.
Fucoidan: yield, anticoagulant activity, and characterization
The fucoidan yield range was 7.8–20% for blades and 3.7–8.7% for stipes, with a significant difference between structures (Table 2). Only blades showed a significance difference between months. The highest yield in the blades was 20.6 ± 0.5% (September), while in stipes it was 7.8 ± 0.5% (March). All fucoidan samples analyzed by the PT and aPTT assays had high anticoagulant activity (>300 s) at a concentration of 10 mg mL−1. The samples from January (blades) and May (stipes) were the most active because they doubled the control time of the lowest concentration tested in the aPTT assay. The highest activity was observed in January for blades and May for stipes. For the PT assay, only samples from September to March were analyzed. The most active samples of fucoidan were from November (blades) and March (stipes) at 0.625 mg mL−1.
The fucoidan content of total sugars and fucose was significantly different between months (p < 0.05) and structures (p < 0.05) (Table 2). Fucoidan from blades had a higher content of total sugars and fucose than fucoidan from stipes. The IR spectrum showed the typical absorption bands for fucoidan, consistent with the pattern of bands of the fucoidan standard obtained from Fucus vesiculosus (F5631, Sigma). Absorption bands in the range 1680–1600 cm−1 show the presence of uronic acids. The bands at 1260–1200 cm−1 are attributed to vibrations of the S=O bond of the sulfate group; the presence of sulfates is corroborated with the band around 580 cm−1. The bands at 1100–1000 cm−1 correspond to hemiacetal rings, and those at 850–820 cm−1 are attributed to the substitutions of sulfate groups at the C2 or C3 and C4 positions of fucose residues (Fig. 2). These bands were consistent in both structures (blades and stipes) and were similar for samples of all months.
Alginates: yield, properties, and characterization
Alginate yield showed a significant difference (p < 0.05) between months and structures (Table 2). In November, January, and March, we obtained the highest yields in both blades (21.3, 21, and 18.5%) and stipes (21.2, 24.5, and 21.8%).
Viscosity and gel strength also showed a difference between months and structures (p < 0.05) (Table 2). Mean annual viscosity from stipes (538 mPa s−1) was twice as high as viscosity from blades (252 mPa s−1). Alginate viscosity from stipes ranged from 440 to 1009 mPa s−1, while from blades it was 140–464 mPa s−1. Alginate gel strength ranged from 1754 to 3239 g cm−2 for blades and 2503–3335 g cm−2 for stipes (Table 2). The highest viscosity and gel strength were obtained in January for blades (464 mPa s−1 and 3239 g cm−2) and stipes (1008 mPa s−1 and 3336 g cm−2). Alginate color was in the range 21–72 for transmittance at 510 nm for blades and 55–85 for stipes. Alginates were dark brown to amber in blades and amber in stipes.
The IR spectrum (Fig. 2) showed typical absorption bands for alginates at 950, 885–890, and 816 cm−1. Absorption bands corresponding to vibration of hemiacetal links from mannuronic and guluronic acid residues were observed at 1120 and 1027 cm−1, respectively. A proportion of these monosaccharides is shown in Table 2. Alginates from both blades and stipes showed an M/G ratio <1, meaning that both alginates had a higher proportion of guluronic acid in the chemical structures, and this was consistent in all samples.
Discussion
Proximate chemical composition
Carbohydrate content ranged from 44 to 46%, which is consistent with ranges reported for other brown seaweeds and for E. arborea (Chapman and Chapman 1980; Serviere-Zaragoza et al. 2002; Hernández-Carmona et al. 2009). This carbohydrate content is suitable to consider the species a resource for biofuel (van Hal et al. 2014). The high carbohydrate content in both structures is related to the large amount of polysaccharides in brown algae (Chapman and Chapman 1980; Lobban and Harrison 1994). In seaweeds, carbohydrate synthesis may be affected by high temperatures, the maximum growth period, and the increase of photosynthetic activity (Munda and Kremer 1977; Lobban and Harrison 1994; Marinho-Soriano et al. 2006; Gómez and Houvinen 2012) which prevails in BM in the warm period (Cervantes-Duarte et al. 2007; Zaitsev et al. 2014).
Ash content was similar to previous reports and confirmed the highest content in stipes (Arvizu et al. 2007; Hernández-Carmona et al. 2009). Ash content was also similar to other commercial species such as Undaria, Fucus, Ascophyllum, Costaria costata, and Macrocystis pyrifera (Carrillo-Domínguez et al. 2002; Holdt and Kraan 2011; Wu et al. 2014). Some authors have suggested that when salinity decreases in the sea, the ash content in algae also decreases (Kumar et al. 2015), but we observed the highest proportion of ash in January and March, which are months with low salinity in BM (Cervantes-Duarte et al. 2007, 2010; Zaitsev et al. 2014). Hence, it may indicate that the reduction of salinity is not indicative of the lower ash content found in E. arborea. This could be because some ash is made up of elements such as calcium, sodium, and potassium, which are linked with other constituents such as alginic acid (Kloareg and Quatrano 1988; Kraemer and Chapman 1991), and could have no relation to changes in salinity.
Protein content for both structures was similar to other brown algae, such as M. pyrifera (5–12%), Sargassum wightii (8–12%), Laminaria digitata (8–15%), and Ascophyllum nodosum (3–15%) (Rodríguez-Montesinos and Hernández-Carmona 1991; Fleurence 2004; Kumar et al. 2015). A higher proportion of protein was observed during the cold season (spring), when nitrate has higher bioavailability (Cervantes-Duarte et al. 2007, 2010). A positive correlation between nitrogen content and proteins in seaweed has been found in species such as Gracilaria cervicornis and Sargassum vulgare (Marinho-Soriano et al. 2006). During spring, E. arborea stores a high nitrogen content in its tissues (Hernández-Carmona et al. 2001), and that may have a relation to the higher level of protein synthesis in this season.
The moisture content of all samples indicated that E. arborea was properly dried and could be stored for a long time without decomposition (Arvizu et al. 2007).
Crude fiber content is low in algae. Despite that, they can be used as a food supplement since they help promote peristaltic movements and a reduction of intestinal transit time, and may reduce hypocholesterolemic and hypoglycemic effects (Gómez-Ordoñez et al. 2010). Eisenia arborea may have a high nutritional value because it contains important fatty acids such as omega 6 and 2 fatty acids (Hernández-Carmona et al. 2009).
Crude extracts and antioxidant activity
Ethanol is a solvent used for the extraction of polar compounds (Sarker et al. 2006); the high yields of crude extract in blades could be due to the presence of polar compounds in this part of the alga. The antioxidant activity may be due to the presence of major compounds that have that activity such as fucoxanthins, carotenoids, tocopherols, amino acids, polyphenols, terpenes, and flavonoids (Alstyne et al. 1999; Sugiura et al. 2006; Amsler 2008). With respect to antioxidant activity, the mean EC50 from blades (99.45 μg mL−1) was lower than that of other species of kelp such as Undaria sp. (420 μg mL−1) and Laminaria sp. (860 μg mL−1) (Ismail and Hong 2002), but the mean EC50 from stipes was higher (436.78 μg mL−1). This suggests that blades could be used to obtain antioxidant extracts or to find metabolites such as phlorotannins which have shown biological activity in species of Eisenia (Sugiura et al. 2006, 2013; Shibata et al. 2015).
Blades showed higher yield and antioxidant activity during the warm period, when the conditions in BM are characterized by high temperatures, low nutrient concentrations, and high irradiance (Cervantes-Duarte et al. 2007; Zaitsev et al. 2014). Most compounds which have antioxidant activity are molecules that protect the seaweeds against UV radiation, high oxygen concentrations, and pathogenic and epiphytic organisms (Amsler 2008). Therefore, the higher activity and yields in blades could be related to higher exposure to those harmful agents occurring during the warm period in BM and prevailing during the year (Zaitsev et al. 2014). However, it is necessary to conduct specific sampling to study more about this topic.
Fucoidan: yield, anticoagulant activity, and characterization
The fucoidan yield in E. arborea was in the same range as other commercial species such as F. vesiculosus (3.4–25.7%), Eisenia bicyclis (1.3, 1.4, and 3.1%), Saccharina japonica (3–25 and 0.87–4.26%), and Alaria fistulosa (2.9–14.5%) (Usui et al. 1980; Rupérez et al. 2002; Usov et al. 2005; Skriptsova et al. 2012; Ermakova et al. 2013; Men'shova et al. 2013; Skriptsova 2016).
The highest fucoidan yield in blades can be explained by the content of carbohydrates, because they are generally found in a higher proportion in the distal parts of seaweeds, from where they are translocated to meristematic zones (Küppers and Kremer 1978; Gómez and Houvinen 2012). In E. arborea, the distal parts are the blades and the meristematic zone is at the base of the blades (Setchell 1905). Nonetheless, the reproduction period of brown seaweeds is correlated with higher fucoidan content (Skriptsova et al. 2012; Skriptsova 2016). Although E. arborea is a perennial species, the maximum reproduction period is from July to November (McPeak 1981), which corresponds to the highest fucoidan content in blades during the sampling period.
Moreover, it was described that one of the functions of fucoidan in seaweeds is structural and the flow of water can influence the seasonal variation, especially with higher flow intensities (Kloareg and Quatrano 1988; Kraemer and Chapman 1991). This suggests that the singular fucoidan peak obtained in March may be related to the increased water flow in BM due to upwelling activity (Zaitsev et al. 2014).
The IR spectra of fucoidan from blades and stipes showed bands at 1606, 1620, and 1420 cm−1 attributable to carboxyl groups; bands at 1030 and 1089 cm−1 corresponding to the vibrations of hemiacetal links of sugar rings; bands at 1224, 1205, 1250, and 1220 cm−1 typically attributable to the vibration of sulfate ester groups; and bands at 820 and 824 cm−1 corresponding to vibrations of equatorial sulfate groups with a substitution pattern of fucose mainly at C4, with minor substitution at C3 and C2. The bands at 847 and 849 cm−1 are typical of axial sulfate groups, which is the pattern distribution in C2 or C3 of the fucose ring (Muñoz-Ochoa et al. 2009; Pereira et al. 2003; Seedevi et al. 2013; Shanthi et al. 2014). In all samples, the intensity bands of IR spectra for stipes were lower than in blades. This means that the fucoidan constituents of stipes were present in a lower proportion than in blades, which could be confirmed by quantitative determination of sugars and fucose, which were in present in a lower proportion during all months. Data for fucan absorption bands reported in the literature indicate that the bands around 840–845 cm−1 are typical of axial sulfate groups at position O4 of the fucose residue and a shoulder around 830–820 cm−1 corresponds to sulfate groups in the equatorial position of the C2 and C3 of fucose residues (Patankar et al. 1993; Chizhov et al. 1999; Marais and Joseleau 2001).
The anticoagulant activity of fucoidan extracts exceeded the blank 11-fold in the aPTT assay and 23-fold in the PT assay, throughout the sampling period and in both structures. This suggests that fucoidan from E. arborea could be used as an anticoagulant in extrinsic and intrinsic ways, and it could be obtained from seaweeds collected at any time of the year since high anticoagulant activity showed no pattern associated with its fucose content or total sugar content. It is possible that other factors such as sulfate content or molecular weight could explain the anticoagulant activity variation (Jiao et al. 2011) and should be studied for E. arborea.
In addition, it is important to describe more fully the bioactivity of fucoidan, since it has a wide variety of bioactivity such as antioxidant, antitumoral, and antiviral, which has been described in its congenial E. bicyclis (Jiao et al. 2011; Ermakova et al. 2013). In order to optimize their use, due to the high yield of fucoidan in blades, we suggest using this part of the algae as the main source to obtain it.
Alginates: yield, properties, and characterization
The variation in alginate yield from blades and stipes from E. arborea in BM is a novel discovery, because no significant variation has been reported before. Hernández-Carmona (1985) found no significant yield variation (2.8%), and Arvizu et al. (2007) found an even lower variation (0.09%). In this research, alginate yield varied in the order of 5% for blades and 13% for stipes. This suggests that studies with a low sample frequency may not show seasonal variations, and are inappropriate for selecting harvesting seasons for the algae.
In general, alginates from E. arborea showed low yields in comparison with other commercial species (Table 3) such as L. digitata (blades 51.8%, stipes 44.01 and 34.6%), L. hyperborea (33.2%), Alaria esculenta (37.4%), Saccharina latissima (28.5%), S. japonica (43%), and Undaria pinnatifida (51%) (Skriptsova et al. 2004, 2012; Men'shova et al. 2013; Fertah et al. 2014; Schiener et al. 2015). However, it was in the range of its congenial E. bicyclis (15.8%) (Men'shova et al. 2013). Nonetheless, it is an important marine resource along Mexican coasts, because according to our yield results only 5 kg of dry algae is necessary to obtain 1 kg of alginate.
Alginate color is attributable to the presence of phenolic compounds, which can be fixed with a formaldehyde solution (Hernández-Carmona et al. 1999). Because the samples came from a continuous extraction process, some of these compounds were also extracted and may explain the light coloration in alginates. The advantage of a continuous extraction process to produce different compounds is that fewer chemicals are used; for example, chlorine treatment to bleach alginates would not be necessary at an industrial level and the viscosity reduction could be avoided (Hernández-Carmona et al. 1999).
Alginate viscosity from blades (140–464 mPa s−1) and stipes (315–1009 mPa s−1) was lower than the viscosity previously reported by Arvizu et al. (2007) in both blades (1270–2210 mPa s−1) and stipes (793–1260 mPa s−1). Blade viscosity is classified as low-medium and stipe viscosity as medium-high (Reyes-Tisnado et al. 2000). So, alginates from blades may be used as an additive for textile printing on high-speed rollers (McHugh 2003), while stipe alginates may be used as an additive for thickeners, syrup preparation, and ice cream toppings (McHugh 1987).
There are few reports about alginate gel strength, and this is the first report specifically for E. arborea. The gel strength in blades (1755–3122 g cm−2) and stipes (2504–3516 g cm−2) was higher than the gel strength in many species, such as C. costata (980–1449 g cm−2) (Wu et al. 2014) and Sargassum cymosum (709 g cm−2) (Camacho and Hernández-Carmona 2012). According to Filippov and Kohn (1974), an M/G ratio <1 confirms a higher proportion of guluronic blocks than mannuronic blocks. The results are consistent with the premise that alginates with higher gel strength have a higher proportion of guluronic acid than mannuronic acid (Usov et al. 2005). Both alginates are suitable for use in cell encapsulation in biomedical and environmental applications (Reyes-Tisnado et al. 2000).
If the main interest is to produce alginates, we suggest algae harvesting from January to March. Although E. arborea is a perennial species, collection in this period would not affect the reproduction season, which is from July to November (McPeak 1981).
In conclusion, E. arborea shows changes in its chemical composition between seasons and structures in BM. These variations are detected only with high-frequency sampling (at least bimonthly). Blades can be considered for use as forage and human food, because they are rich in energy (carbohydrates) and minerals, with a low fat content. In addition, blades contain molecules with antioxidant activity, which give them added nutritional value. E. arborea stipes are a suitable resource for obtaining alginates that may compete in the market of food and pharmaceutical additives, and also have environmental and industrial applications. If alginates are the main interest to exploit this species, we recommend using both structures of the algae (blades and stipes). If the interest is food and bioactive molecules, we suggest using only blades, carrying out the harvest above the meristematic zone, as proposed by Mexican regulations (DOF 2012).
The best period to harvest E. arborea in BM is from September to March, in order to take advantage of the better properties of their compounds. According to our results, E. arborea is a potential resource to be exploited; however, it is important to quantify the available biomass and describe the impacts that resource extraction could represent to the ecosystem. We suggest assessing the harvesting methods and starting small-scale production at a pilot plant in order to begin commercializing the species.
References
Alstyne KL, McCarthy JJ III, Hustead CL, Duggins DO (1999) Geographic variation in polyphenolic levels of northeastern Pacific kelps and rockweeds. Mar Biol 133:371–379
Amsler CD (2008) Algal chemical ecology. Springer, NY
AOAC (1995) Official methods of analysis of the Association of Official Analytical Chemists, 16th edn. AOAC International, Arlington
Arvizu DL, Rodríguez YE, Hernández G, Murillo JI (2007) Chemical constituents of Eisenia arborea Areschoug from Baja California Sur, México. Inv Mar 35:63–69
Arvizu-Higuera DL, Hernández-Carmona G, Rodríguez-Montesinos YE (2002) Parámetros que afectan la conversión de ácido algínico en alginato de sodio. Cienc Mar 28:27–36
Black WAP (1950) Seasonal variation and chemical composition of the common British Laminariaceae. J Mar Biol Assoc UK 29:45–72
Camacho O, Hernández-Carmona G (2012) Phenology and alginates of two Sargassum species from the Caribbean coast of Colombia. Cienc Mar 38:381–393
Carrillo-Domínguez S, Casas-Valdez M, Ramos-Ramos F, Pérez-Gil F, Sánchez-Rodríguez I (2002) Algas marinas de Baja California Sur, México: valor nutrimental. Arch Latinoam Nutr 52:400–405
Cervantes-Duarte R, López-López S, González-Rodríguez E (2007) Características hidrológicas de Bahía Magdalena, B. C. S. México, en el periodo 2001-2003. CICIMAR Oceánides 22:1–11
Cervantes-Duarte R, López-López S, González-Rodríguez E, Futema-Jiménez S (2010) Ciclo estacional de nutrientes, temperatura, salinidad y clorofila a en Bahía Magdalena, BCS, México (2006-2007). CICIMAR Oceánides 25:111–120
Chapman VJ, Chapman DJ (1980) Seaweed and their uses. Chapman and Hall, London
Chizhov OA, Dell A, Morris HR, Haslam SM, McDowell RA, Shashkov AS, Nifant’ev NE, Khatuntseva EA, Usov AI (1999) A study of fucoidan from seaweed Chorda filum. Carbohydr Res 320:108–119
Dische Z (1955) New color reaction for determination of sugars in polysaccharides. Meth Biochem Anal 2:313–358
DOF. Diario Oficial de la Federación (2012) Acuerdo por el que se da a conocer el Plan de Manejo para la Pesquería de Macroalgas en Baja California, México. 30/11/2012
Dubois M, Gilles KA, Hamilton JK, Rebers PAJ, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Ermakova S, Men'shova R, Vishchuk O, Kim SM, Um BH, Isakov V, Zvyagintseva T (2013) Water-soluble polysaccharides from the brown alga Eisenia bicyclis: structural characteristics and antitumor activity. Algal Res 2:51–58
Fertah M, Belfkira A, Dahmane E, Taourirte M, Brouillette F (2014) Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab J Chem. doi:10.1016/j.arabjc.2014.05.003
Filippov MP, Kohn R (1974) Determination of composition of alginates by infrared spectroscopic method. Chemicke Zvesti 28:817–819
Fleurence J (2004) Seaweed proteins. In: Yada RY (ed) Proteins in food processing. Woodhead Publishing, London, pp 197–201
Gómez I, Houvinen P (2012) Morpho-funtionality of carbon metabolism in seaweeds. In: Wiencke C, Bischof K (eds) Seaweed biology. Springer, Berlin, pp 25–46
Gómez-Ordoñez E, Jiménez-Escrig A, Rupérez P (2010) Dietary and physicochemical properties of several edible seaweed from the northwestern Spanish coast. Food Res Int 43:2289–2294
Goupy P, Hugues M, Boivin P, Amiot MJ (1999) Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J Sci Food Agr 79:1625–1634
Hernández-Carmona G (1985) Variación estacional del contenido de alginatos en tres especies de feofitas de Baja California Sur, México. Inv Mar 2:29–45
Hernández-Carmona G, McHugh DJ, Arvizu-Higuera DL, Rodríguez-Montesinos YE (1999) Pilot plant scale extraction of alginate from Macrocystis pyrifera. 1: effect of pre-extraction treatments on yield and quality of alginate. J Appl Phycol 10:507–513
Hernández-Carmona G, Robledo D, Serviere-Zaragoza E (2001) Effect of nutrient availability on Macrocystis pyrifera recruitment and survival near its southern limit of Baja California. Bot Mar 44:221–229
Hernández-Carmona G, Carrillo-Domínguez S, Arvizu-Higuera DL, Rodríguez-Montesinos YE, Murillo-Álvarez JL, Muñoz-Ochoa M, Castillo-Domínguez RM (2009) Monthly variation in the chemical composition of Eisenia arborea J. E. Areschoug. J Appl Phycol 21:607–616
Hernández-Carmona G, Rodríguez-Montesinos YE, Arvizu-Higuera DL, Reyes-Tisnado R, Murillo-Álvarez JL, Muñoz-Ochoa M (2012) Avances tecnológicos en la producción de alginatos en México. Ing Invest Tecnol 13:155–168
Holdt LS, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Ismail A, Hong TS (2002) Antioxidant activity of selected commercial seaweeds. Mal J Nutr 8:167–177
Jiao G, Yu G, Zhang J, Stephen H (2011) Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs 9:196–233
Kloareg B, Quatrano RS (1988) Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr Mar Biol Annu Rev 26:259–315
Kraemer GP, Chapman J (1991) Effects of tensile force and nutrient availability on carbon uptake and cell wall synthesis in blades of juvenile Egregia menziessi (Turn.) Aresch. (Phaeophyta). J Phycol 27:47–53
Kumar S, Saboo D, Levine I (2015) Assessment of nutritional value in a brown seaweed Sargassum wightii and their seasonal variations. Algal Res 9:117–125
Küppers U, Kremer BP (1978) Longitudinal profiles of carbon dioxide fixation capacities in marine macroalgae. Plant Physiol 62:49–53
Lobban CS, Harrison PJ (1994) Seaweed ecology and physiology. Cambridge University Press, London
Marais MF, Joseleau JP (2001) A fucoidan fraction from Ascophyllum nodosum. Carbohydr Res 336:155–159
Marinho-Soriano EP, Fonseca C, Moreira WSC (2006) Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour Technol 97:2402–2406
McHugh DJ (1987) Production, properties and uses of alginates. In: McHugh DJ (ed) Production and utilization of products from seaweeds. Food and Agriculture Organization of UN, Rome, pp 58–115
McHugh DJ (2003) A guide to the seaweed industry. FAO Fisheries Technical Paper 441. FAO Publications, Rome
McPeak RH (1981) Fruiting in several species of Laminariales from Southern California. Proc Int Seaweed Symp 8:405–409
Men'shova RV, Ermakova SP, Um BH, Zvyagintseva TN (2013) The composition and structural characteristics of polysaccharides of the brown alga Eisenia bicyclis. Russ J Mar Biol 39:208–213
Munda IM, Kremer BP (1977) Chemical composition and physiological properties of fucoids under condition of reduced salinity. Mar Biol 42:9–15
Muñoz-Ochoa M, Murillo-Álvarez JI, Rodríguez-Montesinos YE, Hernández-Carmona G, Arvizu-Higuera DL (2009) Anticoagulant screening of marine alga from Mexico, and partial characterization of the active sulfated polysaccharide from Eisenia arborea. CICIMAR Oceánides 24:41–51
Murillo-Álvarez JI, Hernández-Carmona G (2007) Monomer composition and sequence of sodium alginate extracted at pilot plant scale from three commercially important seaweeds from Mexico. J Appl Phycol 19:545–548
Patankar MS, Oehninger S, Barnett T, Williams RL, Clark GF (1993) A revised structure for fucoidan may explain some of its biological activities. J Biol Chem 268:21770–21776
Pereira L, Sousa A, Coelho H, Amado AM, Ribeiro-Claro PJA (2003) Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol Eng 20:223–228
Reyes-Tisnado R, López-Gutiérrez F, Hernández-Carmona G, Castro-Moroyoqui P (2000) Propiedades fisicoquímicas y aplicaciones de los alginatos, polisacáridos de las algas phaeophytas. Secretaría del Medio Ambiente, Recursos Naturales y Pesca, México
Rodríguez-Montesinos YE, Hernández-Carmona G (1991) Variación estacional y geográfica de la composición química de Macrocystis pyrifera en la costa Occidental de Baja California. Cienc Mar 17:91–107
Rodríguez-Montesinos YE, Arvizu-Higuera DL, Hernández-Carmona G (2008) Seasonal variation on size and chemical constituents of Sargassum sinicola Setchell et Gardner from Bahía de La Paz, Baja California Sur, Mexico. Phycol Res 56:33–38
Rupérez P, Ahrazem O, Leal JA (2002) Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J Agric Food Chem 50:840–845
Sarker SD, Latif Z, Gray AI (2006) Methods in biotechnology: natural product isolation. Human Press, USA
Schiener P, Black KD, Stanley MS, Green DH (2015) The seasonal variation in the chemical composition of the kelp species Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J Appl Phycol 27:363–373
Seedevi P, Sudharsan S, Kumar SV, Srinivasan A, Vairamani S, Shanmugan A (2013) Isolation and characterization of sulphated polysaccharides from Codium tomentosum (J. Stackhouse, 1797) collected from southeast coast of India. Adv Appl Sci Res 4:72–77
Serviere-Zaragoza E, Gómez-López D, Ponce-Díaz G (2002) Gross chemical composition of three common macroalgae and sea grass on the pacific coast of Baja California, Mexico. Hidrobiológica 12:113–117
Serviere-Zaragoza E, García-Hernández VC, Siqueiros-Beltrones DA (2003) Diversity and distribution of macroalgae associated with abalone (Haliotis spp.) habitats in Baja California Sur, Mexico. Bull Mar Sci 72:725–739
Setchell WA (1905) Post-embryonal stages of the Laminariaceae. Univ Calif Publ Bot 2:115–138
Shanthi N, Eluvakkal T, Arunkumar K (2014) Characterization of galactose rich fucoidan with anticoagulation potential isolated from Turbinaria decurrens Bory de Saint-Vincent occurring along the Coast of Gulf of Mannar (Pamban), India. J Pharmacog Phytochem 3:132–137
Shibata T, Nagayama K, Sugiura S, Makino S, Ueda M, Tamaru Y (2015) Analysis on composition and antioxidative properties of phlorotannins isolated from Japanese Eisenia and Ecklonia species. Am J Plant Sci 6:2510–2521
Skriptsova AV (2016) Seasonal variations in the fucoidan content of brown algae from Peter the Great Bay, sea of Japan. Russ J Mar Biol 42:351–356
Skriptsova A, Khomenko V, Isakov V (2004) Seasonal changes in growth rate, morphology and alginate content in Undaria pinnatifida at the northern limit in the Sea of Japan (Russia). J Appl Phycol 16:17–21
Skriptsova AV, Sherchenko NM, Torbeera DV, Zvyagintseva TN (2012) Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Mar Biotechnol 14:304–311
Sugiura Y, Matsuda K, Yamada Y et al (2006) Isolation of a new anti-allergic phlorotannin, phlorofuroeckol-B from edible brown algae Eisenia arborea. Biosci Biotechnol Biochem 70:2807–2811
Sugiura Y, Tanaka R, Katsuzaki H, Imai K, Matsushita T (2013) The anti-inflammatory effects of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. J Funct Foods 5:2019–2023
Usov AI, Smirnova GP, Klochkora G (2005) Polysaccharides of algae: the polysaccharide composition of the pacific brown alga Alaria fistulosa (Alariaceae, Laminariales). Russ Chem Bull 54:1282–1286
Usui T, Asari K, Mizuno T (1980) Isolation of highly purified fucoidan from Eisenia bicyclis and its anticoagulant and antitumor activities. Agric Biol Chem 44:1965–1966
van Hal JP, Huijgen WJJ, López-Contreras AM (2014) Opportunities and challenges for seaweed in the biobased economy. Trends Biotech 32:231–233
Wu X, Wang G, Fu X (2014) Variation in the chemical composition of Costaria costata during harvest. J Appl Phycol 26:2389–2396
Zaitsev O, Trasviña-Castro A, Linero-Cueto J et al (2014) Oceanographic conditions over the continental shelf off Magdalena Bay (Mexico) in 2011-2012. Cienc Mar 40:89–112
Zertuche-González JA, Sánchez-Barredo M, Guzmán-Calderón JM, Altamirano-Gómez Z (2014) Eisenia arborea J. E. Areschoug as abalone diet on an IMTA farm in Baja California, México. J Appl Phycol 26:957–960
Acknowledgements
We are grateful for financial support from the projects SIP 20131446, SIP 20131204, and CONACyT 224896, to CONAPESCA for the fishery permit PPF/DGOPA-025/14 and to Alejandro Ramos Rodríguez M.Sc., Amaru Márquez Artavia M.Sc., and Olinda Soriano Santiago M.Sc. for their help with satellite data. We appreciate the logistical support from Antelmo Morales Castillejos and Jorge Luis Jiménez and thank Ing. Armando Hernández López for his help in editing images. CLC thanks CONACyT (no. 369133) and BEIFI-IPN for scholarships. The authors are grateful for the fellowship provided by COFAA and EDI from the Instituto Politécnico Nacional and CONACyT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Landa-Cansigno, C., Hernández-Carmona, G., Arvizu-Higuera, D.L. et al. Bimonthly variation in the chemical composition and biological activity of the brown seaweed Eisenia arborea (Laminariales: Ochrophyta) from Bahía Magdalena, Baja California Sur, Mexico. J Appl Phycol 29, 2605–2615 (2017). https://doi.org/10.1007/s10811-017-1195-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1195-2