Abstract
Fucoidans are sulfated polysaccharides with proven pharmacological effects localized in the cell wall of marine brown algae. The majority of studies have been performed with temperate brown algal species, but in recent years, the evaluation of species from tropical areas has been growing. The aim of this study was to determine the protective effect of fucoidans extracted from the tropical brown seaweeds Dictyota ciliolata, Padina sanctae-crucis, and Sargassum fluitans, against oxidative stress (OS). The D. ciliolata fucoidan (FDc) exhibited the highest reactive oxygen species (ROS) scavenging activity (26%), followed by P. sanctae-crucis fucoidan (FPs) (22%) and S. fluitans fucoidan (FSf) (14%). No cytotoxic effect was detected for any of the extracted fucoidans at a concentration of 2 mg mL−1. Not only did the fucoidans tested show protective effect against OS by reducing ROS generation, but they also increased the glutathione (GSH) level and restored catalase (CAT) activity. Fucoidans obtained from tropical seaweeds could be used as a potential natural ingredient for functional foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) are highly reactive molecules that are continuously produced within the cell during normal physiological metabolism; they include hydrogen peroxide (H2O2), hydroxyl radical (OH·), superoxide anion (O2 −), and nitric oxide (NO) (Birben et al. 2012). At low levels, ROS have diverse positive effects on the cell, such as signal transduction and cytotoxicity against pathogens (Dickinson and Chang 2012). However, at high levels, ROS induce damage to various biomolecules such as proteins, lipids, and nucleic acids, which leads to the development of several age-related and chronic diseases such as cancer, diabetes, and neurodegenerative, cardiovascular and liver diseases (Brieger et al. 2012). It has been described that antioxidants can contribute to ameliorate damage induced by oxidative stress (OS), improving the intracellular antioxidant, enzymatic, and non-enzymatic systems (Birben et al. 2012; Brieger et al. 2012). The majority of antioxidants have been isolated from vegetables, fruits, and plants and recently from several marine organisms, particularly seaweeds (Ngo et al. 2011; Freile-Pelegrín and Robledo 2013). Diverse compounds have been isolated from seaweeds with potential antioxidant activity such as fucoxanthins, phlorotannins and fucoidans (Balboa et al. 2013). Fucoidan is a sulfated polysaccharide found exclusively in brown seaweeds (Phaeophyceae). It consists primarily of l-fucose units and sulfate groups, along with small quantities of d-galactose, d-mannose, d-xylose, uronic acids, proteins and phenolic compounds (Li et al. 2008). Extensive research on fucoidans from brown seaweeds has shown many beneficial pharmacological effects in vitro and in vivo, such as anti-inflammatory, antioxidant and hepatoprotective activities (Ananthi et al. 2010; Ross et al., 2012; Vijayavazkar and Vaseela 2012; Xu et al. 2017) and as possible anticancer agents (Lowenthal and Fitton 2015). Clinical studies have also their anticoagulant and antiviral effects (Irhimeh et al. 2009; Negishi et al. 2013), and more recently, their potential as an adjuvant during chemotherapy in patients with gastric cancer is being used as a nutritional supplement that protects gastric mucosa (Ikeguchi et al. 2015). Due to all of these beneficial effects, fucoidans are considered as a source of bioactive ingredients for functional foods (Vo and Kim 2013).
Many of these studies have been done using fucoidans obtained from temperate brown seaweeds and not until recent years those species from tropical zones (Ananthi et al. 2010; Vasquez et al. 2012; Castro et al. 2015). In this regard, Mexico is considered a country with a vast coast, and in particular at the Caribbean coast of the Yucatan Peninsula where a tropical climate prevails, diverse species of Phaeophyceae, particularly of the order Dictyotales and Fucales, have been described (Robledo 1998). Despite of the above, very few studies have evaluated the pharmacological effect of their fucoidans, with the exception of those reported by Chale-Dzul et al. (2015), who described the hepato-protective effects of Turbinaria tricostata fucoidans. Therefore, the aim of this study was to determine whether fucoidans isolated from Dictyota ciliolata, Padina sanctae-crucis, and Sargassum fluitans have antioxidant properties that can protect against OS induced by different oxidants. We used the HepG2 cell line in vitro as a model to evaluate their protective effects. The partial characterization of the extracted fucoidans was used to discuss the difference between their activities.
Materials and methods
Algal material
Dictyota ciliolata, Padina sanctae-crucis, and Sargassum fluitans were collected in Puerto Morelos, Mexico (20° 46′ 07″ N, 86° 57′ 14″ W) during February 2012. The collected biomass was centrifuged on-site to remove excess seawater using a commercial portable centrifuge and was stored in plastic bags and maintained on ice during transport to the laboratory. The seaweeds were washed thoroughly with freshwater to remove salts, sand, and epiphytes, and were stored at −20 °C. Voucher specimens were identified and deposited at the Mexican National Herbarium (MEXU) Institute of Biology-National Autonomous University of Mexico (IB-UNAM).
Fucoidan extraction
Fucoidans were extracted according to the procedure described by Foley et al. (2011) with modification by Chale-Dzul et al. (2015). Briefly, seaweeds previously stored at −20 °C were thawed and washed with freshwater, and up to 100 g of each seaweed was milled using a commercial blender to obtain a homogeneous paste. The paste was pretreated with 500 mL of ethanol (EtOH) (80% v/v) at room temperature for 12 h. The mixture was filtered, and the solid fraction was extracted with EtOH (80% v/v) at 70 °C for 12 h. Successive extraction of the solid residue with Milli-Q H2O was done first at room temperature for 7 h (S1), at 70 °C for 7 h (S2), and at 70 °C for 4 h (S3). The resultant water fractions (a combination of S1, S2, and S3) were treated with 2 M CaCl2 at room temperature in order to precipitate alginates; after alginate removal by centrifugation at 10,000 rpm for 30 min, the resulting extract was fucoidan-rich. Dialysis was carried out over a 48-h period to decrease salinity employing Milli-Q H2O with changes every 12 h. Fucoidans obtained were freeze-dried and stored until required.
Chemical analysis
Total carbohydrates and the sulfate content of extracts were quantified utilizing the phenol sulfuric acid method described by Dubois et al. (1956) and the turbidimetric method of Jackson and McCandless (1978), respectively. Uronic acid content was measured with a modification of the carbazole method employing a combination of sulfamate to suppress browning and carbazole to color the uronic acids using d-glucuronic acid as a standard (Blumenkrantz and Asboe-Hanse 1973; Filisetti-Cozzi and Carpita 1991). The protein content was determined according to Bradford (1976) with some modifications. Briefly, 10 mg of each fucoidan was dissolved in 1 mL of deionized water, and then, 10 μL of this solution was placed in a 96-well microplate and mixed with 90 μL of Bradford reagent (Sigma-Aldrich, USA); the plate was incubated at room temperature for 10 min, and finally, the absorbance was measured at 595 nm.
Total phenol content (TPC) was determined using the Folin–Ciocalteau reagent according to Attard (2013) with some modifications. Briefly, 10 mg of each fucoidan was dissolved in 1 mL of deionized water, and then, 10 μL of this solution was mixed with 90 μL of Folin–Ciocalteau reagent (1:10) in a 96-well microplate, followed by the addition of 80 μL of 1 M sodium carbonate (Sigma-Aldrich, USA). The mixture was incubated at room temperature for 20 min in the absence of light, and the absorbance was recorded at 620 nm. The TPC (expressed as % of dry weight) was calculated using a standard curve of phloroglucinol.
The Fourier transform infrared spectroscopy (FTIR) spectra of each one of fucoidans (15 mg) in KB pellets were recorded using the Thermo Nicolet Nexus 670 spectrometer with a DTGS-KBr detector.
Cell culture
The cell lines of human hepatoma (HepG2, ATCC HB-8065) and human embryonic kidney (Hek-293, ATCC-CRL-1573) were purchased from the American Type Culture Collection (ATCC). The cell lines were cultured in sterile Costar 75-cm2 flasks containing minimal essential medium (MEN, Gibco) supplemented with fetal bovine serum (FBS) (10% v/v), 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin for 48 h at 37 °C in an atmosphere of 5% CO2 and 95% humidity. Cells were subcultured every 5 days by trypsinization with 0.05% trypsine–EDTA solution.
Cytotoxicity assay
The toxicity of D. ciliolata fucoidan (FDc), P. sanctae-crucis fucoidan (FPs), and S. fluitans fucoidan (FSf) was evaluated in HepG2 and Hek-293 cell lines. The cells were plated at a density of 5 × 103 cells well−1 in a flat-bottom, 96-well microtiter plate and were incubated under conditions described in cell culture section. After 48 h of incubation, the cells were treated with appropriate dilutions of FDc, FPs, and FSf (0.125, 0.25, 0.5, 1, and 2 mg mL−1) and incubated for 48 h. At the end of the incubation time, the medium was replaced with fresh new medium and a 4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg mL−1 in phosphate-buffered saline (PBS)) and 10 μL of phenazine methosulfate (PMS) solution (5 mg mL−1 in PBS) were added and the plates were incubated at 37 °C for 4 h (Mosmann 1983). After the incubation time, the medium was carefully removed and the formazan crystals generated were solubilized by addition of 100 μL of acidified isopropanol (0.4 N HCl). The plates were read at 540 nm in a microplate reader (GloMax-Multi Detection System with Instinct Software). Dimethyl sulfoxide (DMSO) 0.005% and docetaxel were employed as negative and positive controls, respectively. The concentration of the fucoidans that killed the 50% of the cells (CC50) was calculated using the GraphPad Prism 4.0 software (USA).
Evaluation of the protective effect against H2O2-induced OS
Hydrogen peroxide (H2O2) can react with transition metals such as Fe2+ or Cu+; as a result of this, the extremely reactive hydroxyl radical (·OH) is generated, leading to oxidative stress (OS) and cell damage. The HepG2 cell line was plated at a density of 5 × 103 cells well−1 in a flat-bottom, 96-well microtiter plate and was incubated under conditions described in cell culture section. After 48 h of incubation, the cells were treated with the appropriate dilutions of the FDc, FPs, and FSf (0.125, 0.25, 0.5, and 1 mg mL−1) and incubated for 24 h. At the end of the incubation time, hepatic cytotoxicity was induced with 1 mM H2O2 for 3 h. Cell survival was determined using the CellTiter-Glo luminescent cell viability assay kit. Each well was treated with a volume of CellTiter-Glo reagent equal to the volume of cell culture (50 μL), mixed for 2 min on an orbital shaker to induce cell lysis, and incubated at room temperature for 10 min, after which the stable luminescent signal was recorded in a microplate reader (GloMax-Multi Detection System with Instinct Software). Non-treated cells, 1 mM H2O2 and 1 mM N-acetyl cysteine (NACT) were used as negative, damage and protective controls, respectively. The viability of the cell was calculated using the GraphPad Prism 4.0 software (USA).
In vitro antioxidant activity
DPPH radical scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) is a stable, free radical compound that is frequently used to measured free radical scavenging capacity of antioxidants. DPPH radical scavenging activity of the fucoidans was determined according to the method of Sharma and Bhat (2009) with modifications described by Chale-Dzul et al. (2015). Briefly, 100 μL of fucoidans at various concentrations (0.125, 0.25, 0.5 1, and 2 mg mL−1) was mixed with 100 μL of a fresh DPPH solution (300 μM) and then was gently mixed by pipetting and incubated at room temperature for 30 min in the dark. The absorbance recorded at 517 nm ascorbic acid (AA) was used as positive control.
The radical scavenging activity (in percentage) was measured as the decrease in the absorbance of DPPH and was calculated by using the following equation:
where A Control is the absorbance of the control (DPPH solution without sample), A Sample is the absorbance of the test sample (DPPH solution + test sample) and A Sample blank is the absorbance of the sample only (sample without DPPH solution).
Measurement of ROS inhibition in HepG2 cells
ROS, including free radicals such as the hydroxyl radical and the superoxide anion, not only play an important role in the cell, but can also induce damage in cells and can lead to the generation of various diseases (Birben et al. 2012). The non-fluorescent probe 5-(and 6) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) diffuses into the cell, where its acetate groups are cleaved by intracellular esterases to non-fluorescent H2DCF, and this is oxidized in the presence of ROS in dichlorofluorescein (DCF); thus, quantification of DCF can be used as an indication of ROS levels. The effect of fucoidans on ROS inhibition was evaluated in HepG2 cells. The cells were seeded onto 96-well plates (5 × 103 cells well−1) at 37 °C for 48 h. When the cells reached 80–90% confluence, the medium was replaced and the cells were preincubated with CM-H2DCFDA 1 mM for 30 min at 37 °C in the absence of the light; thereafter, the cells were treated with the fucoidans (0.125, 0.25, 0.5, and 1 mg mL−1) in the presence of 100 mM H2O2 for 30 min. Fluorescence was measured at 492/495-nm excitation and 517/527-nm emission in a multi-detection reader (GloMax-Multi + Detection System with Instinct Software). Non-treated cells, 100 mM H2O2-, and 1 mM NACT-treated cells were employed as negative, damage and protection controls, respectively.
Determination of GSH levels and CAT activity in HepG2 cells
Glutathione (GSH) is a tripeptide consisting of glutamine, cysteine, and glycine, and is a key component of the non-enzymatic antioxidant cell defense against OS. Catalase (CAT) is a homotetramer belonging to the group of monofunctional CATs. GSH functions directly by scavenging ROS or acting as a substrate for glutathione peroxidase (GPx) and glutathione S-transferase (GST) (Zhang and Forman 2012), whereas CAT removes H2O2, when at high concentrations, forming H2O and O2, and at low H2O2 concentration, CAT acts periodically by oxidizing its substrate (Popovic et al. 2008). The HepG2 cell line was seeded in a 25-cm2 flask for 72 h at 37 °C; when cells reached 80–90% confluence, the medium was replaced, and thereafter, the cells were treated with the fucoidans (0.5 and 1 mg mL-1) for 24 h at 37 °C. Cell damage was induced with 400 mM of EtOH for 24 h at 37 °C. After that, the cells were suspended in PBS, washed twice, sonicated, and centrifuged at 10,000×g for 15 min. The supernatant obtained was divided in three aliquots: one used for the determination of the protein content (Bradford assay), the second one for the quantification of GSH levels (glutathione assay kit, Sigma-Aldrich CS0260); and the third aliquot to measure CAT activity (CAT assay kit, Sigma-Aldrich CAT100). Cells without EtOH treatment were negative control, and cells treated with EtOH and EtOH + silymarin were damage and standard control of protection, respectively.
Statistical analysis
All the fucoidan concentrations were evaluated in duplicate at each experiment, and the experiments were performed in triplicate; the results are expressed as mean ± standard deviations (SD). One-way analysis of variance (ANOVA) was used to assess significant differences among treated groups followed by the Dunnett test. Statistical analyses were performed using the GraphPad Prism ver. 4.0 software (USA) to establish statistical significance; values of p < 0.05 were considered significant in all cases.
Results
Chemical analysis and fucoidan composition
The chemical composition and fucoidan yield of the tropical brown seaweeds are shown in Table 1. Dictyota ciliolata and P. sanctae-crucis fucoidans showed a higher content of neutral carbohydrates (65 and 56.8%, respectively), sulfate ester groups (5.4 and 5.1%, respectively) and uronic acids (13.9 and 11.8%, respectively), when compared to S. fluitans fucoidan (50.5, 3.7, and 6.5%), whereas protein content was higher in S. fluitans 7.3% (D. ciliolata 5.3% and P. sanctae-crucis 5.1%). Total phenolic content was higher in D. ciliolata fucoidan (2%) and S. fluitans fucoidan (1.8%) when compared to that of P. sanctae-crucis fucoidan (1.2%).
The FTIR spectra of the fucoidan extracted from D. ciliolata, S. fluitans and P. sanctae-crucis were determined and compared to fucoidan standard from Fucus vesiculosus (Fig. 1). Two bands in the 3600–200-cm−1 region, characteristic of all polysaccharides, are evident in all samples. A broad band of around 3346 cm−1 assigned to OH, and the H2O stretching sugar ring vibration, and another located at 2941 cm−1 corresponding to the C-6 of the pyranoid ring of fucose unit are evident (García-Rios et al. 2012; Manoj et al. 2013). Other signals that were recorded at 1036 cm−1 were assigned to the guluronic and mannuronic acid residue (an acidic polysaccharide). An intense band at 1628 cm−1 was noted and could be related with the uronic acid content (García-Ríos et al. 2012; Manoj et al. 2013), which is in agreement with the values obtained by chemical analysis.
All three fucoidans exhibited a broad band at 1255 cm−1, which is assigned to the sulfate ester groups (S=O) that are characteristic of fucoidans (García-Ríos et al. 2012; Manoj et al. 2013). Two additional bands were also observed: one at 845 cm−1 indicating the presence of S=O groups in the axial position of the C-4 fucopyranose residue and the other at 825 cm−1, assigned to S=O groups located at C-2 and C-3 in the equatorial position (García-Ríos et al. 2012; Manoj et al. 2013), confirming that the majority of sulfate groups are localized in the C-4 of fucose.
Radical scavenging activities and cytotoxicity
DPPH radical scavenging activity of the three fucoidans is illustrated in Fig. 2. All extracted fucoidans showed a concentration-dependent increase pattern of DPPH scavenging activity, with its maximal activity (27, 22 and 14%, respectively) at a concentration of 2 mg mL−1.
Effect of Dictyota ciliolata (FDc), Padina sanctae-crucis (FPs), and Sargassum fluitans fucoidans (FSf) on the DPPH scavenging of free radicals in a cell-free system. The amount of the DPPH radical was determined spectrophotometrically at 517 nm. Results are expressed as means ± standard deviations (SD) (n = 3). Ascorbic acid (AA) was used as reference control
Our results also demonstrated that all three fucoidans did not induce cell damage or compromise viability of HepG2 and Hek-293 cells at 2 mg mL−1 (data not shown from the cell viability study employing the MTT assay). Based on our results, 1 mg mL−1 was chosen as the maximumz dose to study the protective effect of fucoidans against OS in a HepG2 cell model of H2O2-induced toxicity.
Protective effect of fucoidans on HepG2 cells
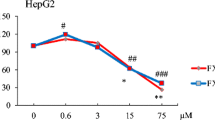
Incubation of HepG2 cells with 1 mM H2O2 for 3 h (damage control) decreases cell viability by 70% with respect to the untreated cells (negative control), while pretreatment of HepG2 cells for 24 h with NACT reduced the damage induced by H2O2, increasing cell viability by 40% more than the damage control (Fig. 3). However, pretreatment of HepG2 cells for 24 h with D. ciliolata fucoidan demonstrated a 12% increase on cell viability at 1 mg mL−1, significantly different from that of the damage control. The same was also observed for S. fluitans fucoidan at the same concentration, whereas pretreatment of HepG2 cells with P. sanctae-crucis fucoidan protected the cells against OS induced by H2O2 by increasing 17 and 19% cell viability at 0.5 and 1 mg mL−1, respectively, and significantly different from the damage control.
Protective effect of Dictyota ciliolata (FDc), Padina sanctae-crucis (FPs), and Sargassum fluitans fucoidans (FSf) on H2O2-induced damage in HepG2 cells. HepG2 cells were pretreated with four concentrations of FDc, FPs, and FSf, for 24 h; then, damage was induced with 1 mM H2O2 for 3 h, and cell viability was determined by ATP assay. Results are expressed as means ± standard deviations (SD) (n = 3) normalized to the negative control. Cells treated only with 1 mM H2O2 were the damage control, and cells treated with 1 mM NACT + 1 mM H2O2 were employed as the protection control. One-way analysis of variance (ANOVA) followed by the Dunnett posttest: *p < 0.05; **p < 0.01 vs. H2O2
Effect of fucoidans on intracellular ROS levels in HepG2 cells
The fluorescence spectrometric data (DCF) of ROS inhibition in H2O2-treated HepG2 cells are presented in Fig. 4. HepG2 cells treated with 100 mM H2O2 significantly increased intracellular ROS levels by 57% in comparison to non-treated cells (baseline); however, pretreatment with 1 mM NACT reduced ROS levels induced by H2O2 by 57% and almost 50% below baseline. Pretreatment with D. ciliolata fucoidan at 0.125, 0.25, 0.5, and 1 mg mL−1 significantly decreased ROS generation in relation to the damage control by 17, 29, 57, and 57%, respectively. P. sanctae-crucis fucoidan was only active at 0.5 and 1 mg mL−1, with ROS inhibition values of 36 and 46%, respectively. Pretreatment of HepG2 cells with S. fluitans fucoidan at 0.25, 0.5 and 1 mg mL−1 completely decreased the damage induced by H2O2 and also the baseline, by 21, 29 and 45% respectively. The 1 mg mL−1 dose was similar to the effect shown by the standard NACT. For both P. sanctae-crucis and S. fluitans fucoidans, the ROS levels were significantly different from the damage control.
Effect of Dictyota ciliolata (FDc), Padina sanctae-crucis (FPs), and Sargassum fluitans fucoidans (FSf) on free radical scavenging in a cell system. HepG2 cells were simultaneously treated with four concentrations of FDc, FPs, FSf, and 100 mM H2O2 during 30 min. Reactive oxygen species (ROS) generation was detected by fluorescent probe CM-H2DCFDA. Results are expressed as means ± standard deviations (SD) (n = 3), normalized with the negative control. Cells treated only with 100 mM H2O2 were the damage control, and cells treated with 1 mM NACT + 100 mM H2O2 were the protection control. One-way analysis of variance (ANOVA) followed by the Dunnett posttest: *p < 0.05; **p < 0.01; vs. H2O2
Intracellular concentration of reduced glutathione and CAT activity
Treatment of HepG2 cells with 400 mM of EtOH decreased GSH levels by 70% in relation to baseline levels (100%), while pretreatment for 24 h with 0.05 mg mL−1 of silymarin restored the GSH level to that of the baseline in comparison with the damage control (Fig. 5). Pretreatment for 24 h with D. ciliolata fucoidan at 0.5 and 1 mg mL−1 restored GSH levels by 15 and 18%, respectively, in comparison with the damage control, whereas pretreatment with P. sanctae-crucis fucoidan restored the level of GSH by 30 and 57%, significantly different from the damage control. S. fluitans fucoidan was effective only at 1 mg mL−1, with a GSH restoration by 19% in relation to the damage control. The effect observed can be due to the ability of S. fluitans fucoidan to induce GSH synthesis or to their ability to increase or enhance the enzymatic defense system.
Effect of Dictyota ciliolata (FDc), Padina sanctae-crucis (FPs), and Sargassum fluitans fucoidans (FSf) on the intracellular level of glutathione (GSH). HepG2 cells were pretreated with two concentrations of FDc, FPs, and FSf for 24 h, and then, damage was induced with 400 mM ethanol (EtOH) for 24 h and the GSH level was determined. Results are expressed as means ± standard deviations (SD) (n = 3) normalized to the negative control. Cells treated only with 400 mM EtOH were the damage control, and cells treated with silymarin 0.05 mg mL−1 (Sm) + 400 mM EtOH were the protection control. One-way analysis of variance (ANOVA) followed by the Dunnett posttest: *p < 0.05; **p < 0.01; vs. EtOH
In Fig. 6, we present the CAT activity of treated HepG2 cells. The CAT activity was reduced by 35% in relation to baseline (100%) when treated with 400 mM EtOH. As expected, pretreatment with 0.05 mg mL−1 of silymarin avoided the CAT depletion induced by EtOH, reaching a value similar to baseline. Pretreatments with 0.5 and 1 mg mL−1 of D. ciliolata fucoidan restored CAT activity by 23 and 35%, respectively, with similar effects exhibited by the silymarin. Pretreatment with P. sanctae-crucis fucoidan significantly increased CAT activity by 23 and 29% in comparison with the damage control, while in S. fluitans fucoidan, the CAT activity was restored to 23 and 29%, respectively, in relation to the damage control.
Effect of Dictyota ciliolata (FDc), Padina sanctae-crucis (FPs), and Sargassum fluitans fucoidans (FSf) on catalase activity (CAT). HepG2 cells were pretreated with two concentrations of FDc, FPs, and FSf for 24 h; then, damage was induced with 400 mM ethanol (EtOH) for 24 h and the GSH level was determined. Results are expressed as means ± standard deviations (SD) (n = 3) normalized to the negative control. Cells treated only with 400 mM EtOH were the damage control, and cells treated with silymarin 0.05 mg mL−1 (Sm) + 400 mM EtOH were the protection control. One-way analysis of variance (ANOVA) followed by the Dunnett posttest: *p < 0.01; **p < 0.05; vs. EtOH
Discussion
The antioxidant potential of fucoidans is one of the most studied subjects to be used in the prevention or treatment of OS disorders or associated diseases (Chale-Dzul et al., 2015; Suleira et al. 2016). This activity has been correlated with fucoidan chemical composition. In the present study, we described the chemical composition and the protective effect of fucoidans extracted from the tropical brown seaweeds D. ciliolata, P. sanctae-crucis, and S. fluitans. The chemical composition may influence fucoidan activity and could be related to the ecology of the species studied. In this regard, we found a high content of neutral carbohydrates, sulfate ester groups, and uronic acids, in the fucoidans extracted from intertidal species belonging to the order Dictyotales (D. ciliolata and P. sanctae-crucis), and lower levels in S. fluitans (Fucales), which thrives in the sublittoral zone. It has been proposed that seaweeds growing at the intertidal are exposed to higher desiccation and light intensity, environmental stresses that may contribute to higher fucoidan synthesis (Black 1954). Similar chemical compositions from those found in the species studied have been described for fucoidan extract from Dictyota dichotoma (Eluvakkal et al. 2010), Padina boergesenii (Kordjazi et al. 2013), Padina gymnospora (Praveen and Chakaborty 2013), Sargassum siliquosum (Vasquez et al. 2012), Sargassum graminifolium (Zhang et al. 2012) and Sargassum wightii (Marudhupandi and Ajith 2013). FTIR spectra were used to characterize the fucoidans extracted and corroborated the presence of a intense band related with the uronic acid content, a broad band assigned to the sulfate ester groups (S=O) and more interesting two additional bands: one indicating the presence of S=O groups in the axial position of the C-4 fucopyranose residue and a second one assigned to S=O groups located at C-2 and C-3 in the equatorial position (García-Ríos et al. 2012; Manoj et al. 2013). This confirms that the majority of sulfate groups of the fucoidans are localized in the C-4 of fucose; sulfate content and position have been correlated with their antioxidant potential (Marudhupandi et al. 2014).
The major fucoidan impurities described so far which exert low or poor antioxidant activities are proteins (Ananthi et al. 2010; Audibert et al. 2010) and phenolic compounds (Audibert et al. 2010). In this regard, our results showed similar protein contents in D. ciliolata fucoidan to that reported for Dictyota caribaea (García-Ríos et al. 2012), another tropical species. For P. sanctae-crucis, a 1.5-fold more protein was found than those reported in Padina perindusiata and Padina tetrastromatica (García-Ríos et al. 2012; Manoj et al. 2013), while in Sargassum fluitans, a 2.5-fold more protein was found when compared to Sargassum tenerrimun and Sargassum filipendula (García-Ríos et al. 2012; Manoj et al. 2013). These differences in protein content have been directly correlated to different factors, including species, collection site and seasonality (Balboa et al. 2013). Phenolic compounds are frequently extracted with fucoidans due their great affinity for carbohydrates and they usually cannot be separated during the extraction procedure. Therefore, its measurement in the fucoidan extract is important since the presence of phenolic compounds may mask or influence fucoidan biological activity. High content of phenolic content in D. ciliolata, S. fluitans and P. sanctae-crucis fucoidans is reported in this study; however, previous studies evaluating fucoidan extracts from Dictyota or Padina species do not report phenolic contents, while those from Sargassum muticum reported values similar to our results for S. fluitans (Balboa et al. 2013).
The antioxidant potential of the fucoidans from tropical seaweeds was evaluated using a cell-free system. D. ciliolata fucoidan showed a higher DPPH scavenging effect, when compared to P. sanctae-crucis and S. fluitans fucoidans; this may be attributed to its higher phenolic and sulfate content (Table 1). Costa et al. (2010) described the antioxidant activity of the sulfated polysaccharides isolated from Dictyota menstrualis and Dyctiota mertensii with maximal hydroxyl radical scavenging of 8.7 and 7.5%, respectively, whereas that from Dictyota cervicornis and Dictyota deliculata with superoxide radical scavenging of 29 and 32%, respectively, all of them at a concentration of 0.5 mg mL−1. Praveen and Chakaborty (2013) described the antioxidant effect of the fractionated sulfated polysaccharide from P. tetrastromatica and P. gymnospora with DPPH scavenging of 77 and 44%, respectively, at a concentration of 1 mg mL−1, while Zhang et al. (2012) reported the antioxidant effect of fucoidans from S. graminifolium with a DPPH scavenging of 65% at 1 mg mL−1. A similar pattern (76%) was found for S. siliquosum extracts at a concentration of 2.5 mg mL−1 (Vasquez et al. 2012) and for S. tenerrimun (64%) at 1 mg mL−1 (Vijayavaskar and Vaseela 2012). On the contrary, Costa et al. (2010) have shown that fucoidans obtained from S. filipendula does not possess antioxidant activity. This may be related to the sulfate content, since most of the authors have concluded that the antioxidant effect of fucoidans is due to their amount and position of sulfate ester groups in the molecule.
Although many fucoidans show antioxidant potential, they may also exert cytotoxic effects undesirable to their use as functional foods (Vo and Kim 2013). None of fucoidans extracted from the tropical seaweeds studied showed cytotoxicity on HepG2 and Hek-92 cells. This is important since fucoidans from D. cervicornis, D. deliculata, D. menstrualis and D. mertensii have shown cytotoxic effects on cervical carcinoma (HeLa) cell line at 1 mg mL−1 (Costa et al. 2010). Marques et al. (2012) reported the cytotoxic effect of fucoidan from P. gymnospora on mouse peritoneal macrophage cell line at 0.5 mg mL−1, whereas S. schroderi and S. filipendula showed cytotoxicity on cervical carcinoma (HeLa) cell line at 1 mg mL−1 (Costa et al. 2010). Previous studies reported no cytotoxicity of fucoidans isolated from D. ciliolata, S. fluitans and P. sanctae-crucis on hepatocellular carcinoma (HepG2), breast adenocarcinoma (MCF-7), prostate carcinoma (LNCaP), and Hek-293 cell lines at the evaluated maximal concentration of 0.5 mg mL−1, similar to our results (Caamal-Fuentes et al. 2014).
The three fucoidans evaluated in this study also showed a dose-dependent intracellular ROS-scavenging effect. It is noteworthy that S. fluitans fucoidan was more effective by diminishing the induced experimental damage, a similar pattern also observed for the D. ciliolata fucoidan. Fucoidan fractions from Sargassum horneri have shown protective effects in a cell line model at the maximal concentration of 0.5 mg mL−1 (Wen et al. 2014), while no reports were found for fucoidans isolated from Dictyota or Padina species using similar models. The protection assay against OS in HepG2 cells is widely utilized for screening extracts or drugs with a hepatoprotective potential; thus, our results indicated the enormous potential of fucoidan extracts as potential candidates for hepatoprotective agents (Thabrew et al. 1997), although more experiments may be required to corroborate this hypothesis. Some authors have attributed the protective effect against OS induced by fucoidans to their capacity to directly scavenge free radicals, their ability to chelate ferrous and copper ions, and their capacity to regulate the enzymatic defense system that controls intracellular ROS generation (Hong et al. 2011). Moreover, this has also been associated with the content and position of sulfate groups in the fucoidans (Li et al. 2008; Chattopadhyay et al. 2010; Marudhupandi et al. 2014).
The levels of reduced GSH and CAT activity used as markers in non-enzymatic and enzymatic defense systems help us to determine whether fucoidans are capable of regulating these systems. All the fucoidan extracts evaluated restored the GSH levels, with the most effective effects for the P. sanctae-crucis fucoidan. For the CAT activity, a similar pattern was observed, and also, D. ciliolata fucoidan reached a restoration similar to that observed for the positive control of protection (silymarin). Popovic et al. (2008) have explained the relation between CAT activity and GSH levels; these authors claim that a reduction of GSH leads to an accumulation of H2O2 and consequently, an increase in CAT activity, while Hong et al. (2011) postulated that fucoidans could interact directly at the genic level of transcription and induce an increase in the enzymes, GPX, sodium dismutase (SOD), and CAT, involved in the antioxidant defense system. However, in order to elucidate the mechanism by which fucoidans evaluated in this study induce an increase in CAT activity, more research is needed.
To our knowledge, this is the first study evaluating the protective potential against the OS of fucoidans from the tropical brown seaweeds D. ciliolata, S. fluitans and P sanctae-crucis on cell lines. From our results, we can conclude that fucoidans obtained from these three species protect HepG2 cells from OS through regulation of the glutathione level and CAT activity. However, further in vivo and clinical studies are necessary to demonstrate their protective effect and to be considered as candidates for the prevention of OS-related disorders.
References
Ananthi S, Raghavendran HR, Sunil AG, Gayathri V, Ramakrishnan G, Vasanthi HR (2010) In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (marine brown alga). Food ChemToxicol 48:187–192
Attard E (2013) A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Cent Eur J Biol 8:48–53
Audibert L, Fauchon M, Blanc N, Hauchard D, Ar Gal E (2010) Phenolic compounds in the brown seaweed Ascophyllum nodosum: distribution and radical-scavenging activities. Phytochem Anal 21:399–405
Balboa EM, Conde E, Moure A, Falqué E, Domínguez H (2013) In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem 138:1764–1785
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Black WAP (1954) The seasonal variation in the combined L-fucose content of the common British Laminariaceae and Fucaceae. J Sci Food Agr 5:445–448
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Bradford MM (1976) A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brieger K, Schiavone S, Miller F, Krause KH (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:1–14
Caamal-Fuentes E, Chale-Dzul J, Moo-Puc R, Freile-Pelegrín Y, Robledo D (2014) Bioprospecting of brown seaweed (Ochrophyta) from the Yucatan Peninsula: cytotoxic, antiproliferative and antiprotozoal activities. J Appl Phycol 26:1009–1017
Castro LSEPW, de Sousa PT, Castro AJG, da Silva Nascimento Santos M, Soriano EM, Leite EL (2015) Potential anti-angiogenic, antiproliferative, antioxidant, and anticoagulant activity of anionic polysaccharides, fucans, extracted from brown algae Lobophora variegata. J Appl Phycol 27:1315–1325
Chale-Dzul J, Moo-Puc R, Robledo D, Freile-Pelegrín Y (2015) Hepatoprotective effect of the fucoidan from the brown seaweed Turbinaria tricostata. J Appl Phycol 27:2123–2135
Chattopadhyay N, Ghosh T, Sinha S, Chattopadhyay K, Karmakar P, Ray B (2010) Polysaccharides from Turbinaria conoides: structural features and antioxidant capacity. Food Chem 118:823–829
Costa LS, Fidelis GP, Cordeiro SL, Oliveira RM, Sabry DA, RB NRT, Costa MS, Almeida-Lima J, Farias EH, Leite EL, Rocha HA (2010) Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother 64:21–28
Dickinson BC, Chang CJ (2012) Chemistry and biology of reactive oxygen species in signaling or stress response. Nat Chem Biol 7:504–511
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Eluvakkal T, Sivakumar SR, Arunkumar K (2010) Fucoidan in some Indian brown seaweeds found along the Coast Gulf of Mannar. Int J Bot 6:176–181
Filisetti-Cozzi TM, Carpita NC (1991) Measurement of uronic acids without interference from neutral sugars. Anal Biochem 197:157–162
Foley SA, Mulloy B, Tuohy MG (2011) An unfractionated fucoidan from Ascophyllum nodosum: extraction characterization, and apoptotic effects in vitro. J Nat Prod 74:1851–1861
Freile-Pelegrín Y, Robledo D (2013) Bioactive phenolic compounds from algae. In: Hernández-Ledesma B, Herrero M (eds) Bioactive compound form marine foods: plant and animal sources. Wiley-Blackwell, Madrid, pp 113–129
García-Ríos V, Ríos-Leal E, Robledo D, Freile-Pelegrín Y (2012) Polysaccharides composition from tropical brown seaweeds. Phycol Res 60:305–315
Hong SW, Jung KH, Lee HS, Zheng HM, Choi MJ, Lee C, Hong SS (2011) Suppression by fucoidan of liver fibrogenesis via the TGF-β/Smad pathway in protecting against oxidative stress. Biosci Biotechnol Biochem 75:833–840
Ikeguchi M, Saito H, Miki Y, Kimura T (2015) Effect of fucoidan dietary supplement on the chemotherapy treatment of patients with unresectable advanced gastric cancer. J Cancer Ther 6:1020–1026
Irhimeh MR, Fitton JH, Lowenthal RM (2009) Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagul Fibrinolysis 20:607–610
Jackson SG, McCandless EL (1978) Simple, rapid, turbidometric determination of inorganic sulfate and/or protein. Anal Biochem 90:802–808
Kordjazi M, Shabbanpour B, Zabihi E, Faramarzi MA, Feizi F, Ahmadi GH, Feghhi MA, Hosseini SA (2013) Sulfated polysaccharides purified from two species of Padina improve collagen and epidermis formation in the rat. Int J Mol Cell Med 2:156–163
Li B, Lu F, Wei X, Zhao R (2008) Fucoidan: structure and bioactivity. Molecules 13:1671–1695
Lowenthal RM, Fitton JH (2015) Are seaweed-derived fucoidans possible future anti-cancer agents? J Appl Phycol 27:2075–2077
Manoj SG, Mahesh Kumar PS, Vasanthi M, Achary A (2013) Anticoagulant property of sulphated polysaccharides extracted from marine brown algae collected from Mandapam Island, India. Afr J Biotechnol 12:1937–1945
Marques CT, Azevedo CT, Nascimiento MS, Medeiros VP, Alves LG, BenevidesNM RHA, Leite EL (2012) Sulfated fucans extracted from algae Padina gymnospora have anti-inflammatory effect. Rev Bras Pharmacogn 22:115–122
Marudhupandi T, Ajith TT (2013) Antibacterial effect of fucoidan from Sargassum wrightii against the chosen human bacterial pathogens. Int Curr Pharm J 2:156–158
Marudhupandi T, Kumar T, Senthil SL, Devi K (2014) In vitro antioxidant properties of fucoidan fractions from Sargassum tenerrimum. Pak J Biol Sci 17:402–407
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 65:55–63
Negishi H, Mori M, Mori H, Yamori Y (2013) Supplementation of elderly Japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J Nutr 143:1794–1798
Ngo DH, Wijesekara I, Vo TS, Ta QV, Kim SK (2011) Marine food-derived functional ingredients as potential antioxidants in the food industry: an overview. Food Res Int 44:523–529
Popovic M, Janicijevic-Hudomal S, Kaurinovic B, Rasic J, Trivic S (2008) Effects of various drugs on alcohol-induced oxidative stress in the liver. Molecules 13:2249–2259
Praveen NK, Chakaborty K (2013) Antioxidant and anti-inflammatory potential of the aqueous extract and polysaccharide fraction from brown marine macroalgae Padina sp. from Gulf of Mannar of Peninsular India. J Coast Life Med 1:19–20
Robledo D (1998) Seaweed resource of Mexico. In: Critchley A, Ohno M (eds) Seaweed resources of the world. Kanagawa International Fisheries Training Center, Japan International Cooperative Agency (JICA), Japan, pp 331–342
Ross V, Joven A, Donnie RJ, Marianne M, Katherine P, Carla P (2012) Hepatoprotective effects of aqueous sulfated polysaccharide extract from Sargassum siliquosum JG Agardh on paracetamol-induced oxidative liver toxicity and antioxidant properties. Int J Pharm Front Res 2:15–27
Sharma OP, Bhat TK (2009) DPPH antioxidant assay revisited. Food Chem 113:1202–1205
Suleria HAR, Gobe G, Masci P, Osborne SA (2016) Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci Technol 50:44–55
Thabrew M, Hughes RD, McFarlane IG (1997) Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J Pharm Pharmacol 49:1132–1135
Vasquez R, Apostol J, Ramos JD, Morales M, Padiernos K, Pangilinan C, Payuran C, Princesa J (2012) Hepatoprotective effects of aqueous extract form Sargassum siliquosum J.G. Agardh on paracetamol-induced oxidative liver toxicity and antioxidant properties. Int J Pharm Front Res 2:15–27
Vijayavaskar P, Vaseela N (2012) In vitro antioxidant properties of sulfated polysaccharide from brown marine algae Sargassum tenerrimum. Asian Pac J Trop Dis 2:S890–S896
Vo TS, Kim SK (2013) Fucoidans as a natural bioactive ingredient for functional foods. J Funct Foods 5:16–27
Wen ZS, Liu LJ, OuYang XK, Qu YL, Chen Y, Ding GF (2014) Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264.7 cells. Int J Biol Macromol 68:98–106
Xu Y, Zhang Q, Luo D, Wang J, Duan D (2017) Low molecular weight fucoidan ameliorates the inflammation and glomerular filtration function of diabetic nephropathy. J Appl Phycol 29:531–542
Zhang CY, Wu WH, Wang J, Lan MB (2012) Antioxidant properties of polysaccharide from the brown seaweed Sargassum graminifolium (Turn.), and its effects on calcium oxalate crystallization. Mar Drugs 10:119–130
Zhang H, Forman HJ (2012) Glutathione synthesis and its role in redox signaling. SeminCell Dev Biol 23:722–728
Acknowledgments
We wish to thank CONACyT PDC PN 2014 (248004) for funding this project. The authors want to express their acknowledgment to Dr. José Luis Godínez (IB-UNAM) for seaweed species identification and C. Chávez Quintal (CINVESTAV) and R. Pérez Cabeza de Vaca (IF305 UNAM) for their technical assistance during analysis. We also thank Foundation IMSS for the grant received by Rosa Esther Moo-Puc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chale-Dzul, J., Freile-Pelegrín, Y., Robledo, D. et al. Protective effect of fucoidans from tropical seaweeds against oxidative stress in HepG2 cells. J Appl Phycol 29, 2229–2238 (2017). https://doi.org/10.1007/s10811-017-1194-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1194-3