Abstract
Brown seaweeds are rich in polysaccharides, such as fucoidan (FUC) which has shown beneficial effects in several medical conditions. The aim of the present study was to assess the antioxidant, anti-inflammatory, and hepatoprotective properties of Colpomenia sinuosa– and Sargassum prismaticum–isolated FUC in vitro and in vivo. The hot acid extraction method was used to isolate FUC from C. sinuosa (FCS) and S. prismaticum (FSP) species. The antioxidant, anticancer, as well as the effect on neurotransmitter-degrading enzyme and disaccharidase activities were measured using standard protocols. Moreover, the hepatoprotective effect of two FCS doses (100 and 200 mg/kg) on paracetamol-administered rats (one dose of 1 g/kg) were evaluated by measuring blood liver function markers, hepatic pro-oxidants as malondialdehyde (MDA) and nitric oxide (NO), antioxidants as glutathione (GSH) and glutathione peroxidase (GPx), proinflammatory markers as inducible nitric oxide synthase (iNOS), interleukin 1β (IL-1β), tumor necrosis factor-α (TNF-α), and liver histology. The crude fucoidan yield was 15.6% and 14.8% of C. sinuosa and S. prismaticum dry weights, respectively. The antioxidant effects and cytotoxic activity on hepatic cancer cell were higher for FCS than FSP. Moreover, in vivo data showed that FCS administration at both doses significantly improved liver functions and alleviated histological alterations, hepatic inflammation, and oxidative stress following paracetamol intake. In conclusion, fucoidan exerts anti-inflammatory, antidigestive enzyme activity, antioxidant, anticancer, and hepatoprotective effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seaweeds are rich in biologically active compounds (Chew et al. 2008) that possess antioxidant, antimicrobial, antifungal, antiviral, anticancer, anticoagulant, and anti-inflammatory effects. These activities are attributed to different secondary metabolites, formed due to oxidative effects that face the immobile algae (Souza et al. 2012). Among seaweeds, Sargassum is a brown to olive green colored macro-algae of the brown algae family and can reach impressive lengths of 3–4 or even 16 m; numerous species are distributed throughout the temperate and tropical oceans of the world, especially in the coast of the Mediterranean (Elabbar et al. 2014). Sargassum has been used traditionally for treating scrofula, goiter, tumor, edema, testicular pain, and swelling (Chakraborty et al. 2009). Algin is a carbohydrate present in Sargassum and is used in textile, paper, and pharmaceutical industries (Chia et al. 2015). Sargassum also had been studied extensively for its high antioxidant activity and anticancer effect against lung and melanoma cells (Ale et al. 2011a). However, little information about Sargassum prismaticum species’ biological activity and polysaccharides is available compared with those of other Sargassum species.
Furthermore, Colpomenia sinuosa (C. sinuosa) belongs to the order of Scytosiphonales, and the family Scytosiphonaceae, is an edible brown algae (Tannoury et al. 2016; Tannoury et al. 2017). Morphology of C. sinuosa bears a sessile thallus which is attached by a broad base; it is leathery and globular, hollow when young, and becoming expanded and folded as it grows older (Parsons 1982). Historically, it was found that this species occurs mostly in the Mediterranean (Cotton 1908). C. sinuosa is used as human food, due to their antioxidant properties and being a good source of phenols, folic, vitamins, and amino acids (Gyi and Htun 2012). C. sinuosa is rich in ash content and unsaturated fatty acids, fucosterols, stigmasterols, the amino acid lysine, and palmitic acid (Cotton 1908; Parsons 1982; Shaikh et al. 1991; Khin Khin Gyi and Htun 2012; Pereira et al. 2012; Tabarsa et al. 2012). The presence of the free monosaccharides galactose, glucuronic acid, and xylose in C. sinuosa did not differ significantly among seasons of collection (Al Monla et al. 2020). Likewise, fucoxanthin isolated from this seaweed significantly increased percentage of death in breast cancer cells (Karkhane Yousefi et al. 2018). C. sinuosa expressed the highest fucoidanase activity and the highest fucoidan content which can be used as an antioxidant, antibacterial, and antidiabetic medication (El Shora et al. 2018).
Moreover, fucoidans (FUCS) are a family of fucose-containing sulfated polysaccharides (FCSPs) with variable chemical structures (Ale et al. 2011b). In 1913, Kylin isolated brown seaweed FUCs, which are characterized by the presence of l-fucose residues, along with low amounts of other monosaccharides and proteins. Fucoidans have attracted the interest of many research groups because it exhibits promising biological activities such as anticancer, antiangiogenic, anti-inflammatory, antiviral, and antioxidant effects (Koyanagi et al. 2003; Chotigeat et al. 2004; Lin et al. 2016). In addition, FUC can alleviate the symptoms of liver disease, osteoarthritis, and kidney disease and reduce the risk of radiation damage (Fitton 2011).
Several drugs are metabolized mainly in the liver, and their overdose can induce hepatitis or hepatotoxicity. To alleviate this toxicity, researchers have examined the hepatoprotective effects of several naturally isolated compounds. One of these compounds was FUC which was found to increase hepatic growth factor (HGF), hepatic antioxidant capacity, and stellate cell apoptosis; therefore, it prevents liver cell death and fibrosis induced by chemical injuries (Bilan et al. 2006; Abdel-Daim et al. 2020a, b; Hong et al. 2012).
Thus, the aims of this work were to isolate FUCs from Colpomenia sinuosa and Sargassum prismaticum and to evaluate the biological activities of isolated FUCs (in vitro) and test the hepatoprotective ability of the most potent FUC (in vivo).
Materials and methods
Fucoidan extraction and characterization
Seaweeds collection
Fresh marine seaweeds were collected in April 2015 from Abu-Qir shore (Alexandria, Mediterranean Sea) and the shore behind National Institute of Oceanography and Fishers (NIOF, Hurghada, Red Sea) and were authenticated by Dr. Mona Ismail (Taxonomy and Biodiversity of Aquatic Biota Lab, NIOF, Alexandria, Egypt) as C. sinuosa and S. prismaticum. The collections were made from low tidal and subtidal regions (up to 1–1.5 m depth) by hand picking. Sediment and epiphytes removal from the collected materials was done by washing with marine water in the field then the samples were in wet conditions. In the laboratory, several washing steps were done by tap and distilled water to remove all undesired materials (mud, salt, sand, and foreign substances). Finally, seaweed was dried and ground to fine powder form.
Fucoidan extraction
The dried algal material (250 g) was soaked with a mixture of ethanol:formaldehyde:distilled H2O (80:5:15) overnight to flush out chlorophylls and other pigments. This mixture was decanted, and the remaining algal material was defatted with acetone. The extraction method was done twice in Soxhlet apparatus by using a 0.1-M HCl containing 4% CaCl2 solution at 70 °C. After extraction, alginates were precipitated by cooling, then the supernatant was collected after centrifugation for 10 min (4000 rpm, 10, 4 °C). The supernatant was neutralized with ammonium carbonate powder. Residual polysaccharides were then precipitated overnight by adding 2.5 times volume of ethanol. Then, ethanol was decanted and the moist polysaccharides were centrifuged at 10,000 rpm for 15 min. The obtained precipitate was dissolved into 10 ml distilled water and was then freeze-dried (von Andrea Désirée Holtkamp 2009).
Chemical analysis
The carbohydrate content was estimated by Molish test as mentioned in Elzagheid (2018), while fucose was measured by Dische’s method (Dische and Shettles 1948) in which the boiled acidified extract interacts with cysteine hydrochloride and the concentration of the colored product was estimated at 420 nm.
Fourier transform infrared analysis
The pellet of fucoidan (1 mg) was mixed with potassium bromide (100 mg), and infrared (IR) spectral (400 to 4000 cm−1) analysis was performed using Shimadzu Fourier transform infrared (FTIR) 8300 instrument, Japan.
Assessment of in vitro biological activities
Preparation of enzyme sources
The liver and brain tissues from normal rats were immediately removed after animals’ scarification and were washed with cold saline solution (0.9% NaCl). Then, 1 g of liver tissue was homogenized in 9 ml of 0.1 M phosphate buffer (pH 7.4) and centrifuged at 6000 rpm for 10 min; the collected supernatant (enzyme source) was used to estimate DPPH reductase activity (Shaban et al. 2003). The brain tissue homogenate (4%) was prepared in the same buffer containing 0.001 M Na2 EDTA, 0.2 M NaCl, and 0.5% Triton X-100, then centrifuged and the collected supernatant was used as a source for determination of neurotransmitter-degrading enzymes as acetylcholine esterase (AChE) and monoamine oxidase (MAO). The protein content in both supernatants was determined according to Lowry’s method (Lowry et al. 1951).
Anticancer activity
The cytotoxic effects of FCS and FSP on HCT-116 (colon adenocarcinoma), MCF-7 (breast adenocarcinoma), and HepG-2 (liver adenocarcinoma) cell lines were examined using standard protocols in which doxorubicin was used as a positive control. Monolayer cells (10,000 counts) were grown in growth medium (RPMI-1640 containing 10% inactivated fetal calf serum and 50 μg/ml gentamycin) for 24 h at 37 °C and 5% CO2. After that, cells were washed with phosphate buffer saline (0.01 M, pH 7.2) and incubated with 100 μl from different dilutions of FCS, FSP, or doxorubicin at 37 °C for another 24 h. The cell viability was determined using the crystal violet staining method, followed by cell lysis using 33% glacial acetic acid, and the absorbance (ODt) was read at 590 nm using an ELISA reader (sunrise, TECAN Inc, USA). The 50% inhibitory concentration (IC50) was estimated according to Gangadevi and Muthumary (2007).
Antioxidant properties
The DPPH scavenging activities of different concentrations of FCS and FSP were estimated according to Liyana-Pathirana and Shahidi (2005). The DPPH reductase activity was measured according to Yim et al. (2004), while the ferric reducing antioxidant power assay (FRAP) was done according to Yen and Der Duh (1993).
Effects on digestive enzymes
Lactase, maltase, and α-amylase activities in the presence of different concentrations of FCS and FSP were estimated according to general enzymatic methods. The end-product, glucose, was measured by using glucose oxidase reagent. The liberated glucose from the enzymatic action (mmol) was calculated by using calibration curve and then the enzymatic activity was measured by using this equation: IU (mmol/min) = number of mmol of glucose / time of incubation (min).
Anti-inflammatory activity
The NO radical scavenging activity was measured according to Nabavi et al. (2008). The human red blood corpuscles (HRBC) membrane-stabilizing method was used to detect the in vitro anti-inflammatory activity of FCS and FSP as per Vadivu and Lakshmi (2008).
Effects on neurotransmitter-degrading enzymes
The activity of AChE was assessed using the methods of Ellman (1959), and the MAO activity was measured according to Sandler et al. (1981).
Assessment of in vivo FCS hepatoprotective effect
Animals
Thirty adult male rats (aged 3 months; weight: 150–200 g) were provided from the animal house, Faculty of Agriculture, Alexandria University and were housed in polypropylene cages at 50–60% humidity, 23 ± 2 °C temperature, and 12 h light/dark cycle. All animal experiments were conducted according to the Animal Ethics Committee guidelines of the National Health and Medical Research Council (NHMRC) and recommended guidelines of the Egyptian Ministry of Health and Population, High Committee of Medical Specialties, Arab Republic of Egypt. This study was approved by the Committee application for the Institutional Animal Care and Use Committees (IACUCs), Pharmaceutical and Fermentation Industries Development Centre (PFIDC), SRTA-City, Alexandria, Egypt; the approval number was IACUC # 15-1P-9020.
Animal design
Five groups (each group of 6 rats) were used in this study as follows: group I (normal controls) rats were orally administrated 5 ml/100 g of 1% polyethylene glycol (PEG) for 8 days. Group II rats were orally administrated 5 ml/100 g of 1% PEG for 7 days then paracetamol (PCM, 3 g/kg b.wt.) was orally administered at the 8th day to induce hepatotoxicity. Groups III, IV, and V rats were orally administered 100 mg/kg FCS, 200 mg/kg FCS, or 100 mg/kg silymarin for 7 days, respectively, then paracetamol was orally administrated at a dose of 3 g/kg b. wt. on the eighth day (Ross et al. 2012; Alam et al. 2017).
After 24 h of the last dose, the blood of anesthetized rats (ketamine (70 mg/kg) plus xylazine (7 mg/kg), IP) was collected in plain tubes. The serum was isolated by centrifugation at 3000 rpm (4 °C for 20 min). After scarification by cervical dislocation (Ross et al. 2012), the liver was removed and washed with ice-cold saline, dried, and then weighed. The relative liver weight was calculated using the following rule: liver weight/body weight × 100. The liver tissue was divided into two parts: The first one was fixed in 10% neutral formalin solution for histological study, while the other part was used to prepare liver homogenate as described in “Preparation of enzyme sources.”
Assessment of liver serum function tests
Commercial kits were used to determine the serum levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin, cholesterol, albumin (ALB), total protein (TP), and serum lactate dehydrogenase (LDH) according to manufacturer’s instructions.
Assessment of hepatic oxidative stress parameters
The hepatic MDA concentration was measured using the reaction described by Tappel and Zalkin (1959). Then, the concentration of MDA (nmol/mg) was calculated using the equation: At / 0.156 where At is the absorbance of the tested sample and 0.156 is the extension coefficient. Tissue NO concentrations were assessed according to Qebesy et al. (2015). Hepatic GSH levels were measured according to the methods of Beutler et al. (1963), while glutathione peroxidase (GPx) activity was evaluated as per Paglia and Valentine (1967).
Assessment of hepatic inflammatory parameters
The levels of inducible nitric oxide synthase (iNOS), interleukin 1β (IL-1β), and tumor necrosis factor-α (TNF-α) were quantified in the hepatic homogenate supernatant using ELISA kits (CUSABIO, China), according to the manufacturer’s protocol.
Histopathological examination
The tissues were embedded in paraffin wax, then 6-mm-thick sections were cut. The eosin and hematoxylin dye was used to stain the sections. Light microscopic photomicrographs were used to evaluate the histological changes as fibrosis, fatty infiltration, centrilobular necrosis, and lymphocyte infiltration.
Statistical analysis
Data were analyzed by SPSS software package version 20.0 (Armonk, NY: IBM Corp). Normally distributed quantitative variables were described using mean and standard deviation and the comparison between more than two groups was conducted by F test (ANOVA) and post hoc LSD test. The significance was assessed at p < 0.05.
Results
Fucoidan characterization
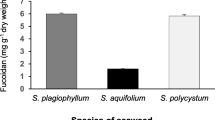
In this study, the obtained crude powder was about 39 g and 37 g from C. sinuosa and S. prismaticum, respectively. Colorimetric identification showed that the isolated powder was carbohydrates, containing fucose residues (as FUC). An IR spectrum within the range of 400–4000 cm−1 (Fig. 1a, b) was used to confirm the presence of FUC. A broad IR band was observed at ≈ 3430 cm−1, which corresponds to OH and H2O stretching vibration. The IR bands at ≈ 1420–2520 cm−1 were due to the presence of aliphatic (CH) stretching vibrations in the pyranoid ring and C–O–C stretching of the glycosidic bonds. Intense absorption in these regions is the common characteristic for all polysaccharides. A strong absorption band was observed at 1083 cm−1, which is attributed to the O=S=O stretching of sulfates, which is a good marker of sulfated polysaccharides. The IR band observed at ≈ 875 cm−1 is attributed to the C–O–S bending vibration of sulfate substituents.
In vitro biological activities
Anticancer effect
Both FCS and FSP showed cytotoxic effects on HCT-116, MCF-7, and HepG-2 cell lines; however, this effect was less potent than doxorubicin (Fig. 2); the estimated IC50 of both extracts against each cell line was higher than that of the reference drug (Table 1). FSP showed a more potent cytotoxic effect on MCF-7 and HepG-2 than FCS, while both showed similar cytotoxic effects on HCT-116.
Antioxidant properties
The first assay used to determine the antioxidant properties of FCS and FSP was DPPH scavenging assay. Figure 3a shows that both FCS and FSP had free radical scavenging activity, which was directly proportional to the concentration gradient. The DPPH-scavenging activity of FCS (with IC50 of 16.3 μg/ml) was higher than that of FSP (with IC50 of 43.2 μg/ml) (Table 1). The second assay was DPPH reductase inhibition, which reflects the activity of NADPH-cytochrome P450 reductase (CPR) system. Both FCS and FSP slightly inhibited the CPR enzyme to levels lower than those of vitamin C (Fig. 3b); the IC50 of FCS and FSP were 520 and 886.4 μg/ml, respectively, while for vitamin C was 128 μg/ml. The third assay was ferric-reducing activity (FRA) in which the ability of FUCs to reduce ferric to ferrous. Figure 3c shows that the FRA was directly proportional to different FUC concentrations. Moreover, the activities of both FCS and FSP were higher than that of vitamin C. Table 1 shows that FCP has the highest FRA activity followed by FSP and finally vitamin C where EC50 were 4.9, 10.3, and 19.6 μg/ml, respectively.
Antidigestive enzyme effect
Figure 4 shows that FCS and FSP significantly inhibited the activities of all digestive enzymes tested in the present study and that this inhibitory activity was directly proportional to FUC concentration gradient. FCS was a more potent inhibitor for maltase and amylase than FCS; the IC50 of FCS for maltase and amylase were 51.2 ± 3.5 and 269.4 ± 15.2 μg/ml, respectively, while the IC50 for FSP were 348.6 ± 10.5 and 929.8 ± 23.4 μg/ml, respectively. Both FCS and FSP showed similar inhibitory activities on lactase enzyme (Table 1).
Anti-inflammatory activity
Figure 5 shows that the NO scavenging activity was directly proportional to fucoidan concentration gradient. FCS (with IC50 of 2.9 ± 0.005 μg/ml) was a more potent anti-inflammatory than FPS (with IC50 of 4.2 ± 0.02 μg/ml) (Table 1). Further, FCS (with EC50 of 88.3 μg/ml) was more efficient in preventing RBC hemolysis than FSP (with EC50 of 364.8 μg/ml).
Effects on neurotransmitter-degrading enzymes
Figure 6 shows that both FCS and FSP had inhibitory effects on AChE and MAO, and these inhibitory effects were directly proportional to FUC concentrations. In the case of AChE, FCS (IC50 38.4 ± 3.1) was a more potent inhibitor than FSP (IC50: 50.6 ± 2.5 μg/ml; p < 0.05), while both of them exhibited similar MAO inhibitory effects (similar IC50 values) (Table 1).
In vivo hepatoprotective agent
Table 2 shows that all groups had the same body and absolute liver weights, as well as relative liver weight, at p < 0.05. Table 3 shows that PCM administration was associated with significantly elevated serum levels of AST, ALP, ALT, LDH, bilirubin and cholesterol, as well as significantly reduced serum ALB and TP levels. FCS at both doses significantly improved all these blood parameters (p < 0.05).
Table 3 indicates that PMC induced marked oxidative stress in the hepatic tissue. The administration of PMC was associated with significantly increased (p < 0.05) MDA, NO, iNOS, IL-1β, and TNF-α when compared to control rats. Treatment with FCS at both doses significantly improved these parameters in comparison to PCM-intoxicated rats, but it failed to restore them to control levels (Table 4).
Histopathological study showed that liver tissue sections from PCM-intoxicated rats exhibited centrilobular hepatic necrosis, infiltrating lymphocytes, cell degeneration, hepatocytes inflammation, and fat droplet. Treatment with FCS 100 mg/kg and silymarin alleviated PCM-induced histopathological changes, while treatment with 200 mg/kg FCS restored the hepatic architecture to normal (Fig. 7).
Discussion
Fucoidans extracted from the same species growing at different locations have various bioactivities (Kim et al. 2010). This could be attributed to the difference vicinity, seaweed growing conditions, extraction, and analytical methods (Bilan et al. 2006). Therefore, it was important to isolate FUCs from Egyptian seaweeds and investigate their action in vitro and in vivo. The isolated FUCs showed cytotoxic effect against breast, colon, and hepatic cancer cell lines. Several previous studies proved that FUCs inhibit the proliferation of cancer cells through activation of caspase-8-dependent pathway and inhibition of cytosolic Bax which consequently increase cytosolic cytochrome c (Kim et al. 2010), enhancing immune surveillance and response, suppression of angiogenesis, and reduction of cancer oxidative stress environment (Zorofchian Moghadamtousi et al. 2014).
Due to a lack of benchmark for antioxidant assay, several antioxidant assays must be conducted in order to establish the overall antioxidant capacity of a sample (Fedorov et al. 2013). Therefore, three methods were used in the current study to assess the antioxidant properties of both FCS and FSP. Our findings proved that both FCS and FSP had the capacity to prevent DPPH auto-oxidation and reactive oxygen species (ROS) formation through NADPH oxidation and electron donation to ferric chloride to produce ferrous chloride (Skriptsova 2015). Former findings revealed that sulfated polysaccharides like fucoidan present significant antioxidant system in vitro (O’Sullivan 2013) due the presence of –OSO3H groups which contain a highly activate hydrogen atom. Moreover, FUC’s chelating properties and antioxidant activities are affected by the presence of other monosaccharide and polysaccharide chains (Souza et al. 2012).
Cancer cells consume more glucose during metabolism to fuel malignant proliferation; therefore, a glucose depletion strategy could offer therapeutic benefits in cancer (Jang et al. 2013). There are several methods to control the blood glucose level, one of them is the control of carbohydrate breakdown and glucose intestinal absorption by inhibiting pancreatic α-amylase and intestinal disaccharidases (α-glycosidase/maltase and lactase) (Costa et al. 2010). The inhibition of amylase, maltase, and sucrase is an effective strategy to control postprandial hyperglycemia (Da Silva et al. 2009). In agreement with our results that confirmed the inhibitory effects of FCS and FSP on these digestive enzymes, a previous studies demonstrated that fucoxanthin-rich extract from the brown seaweed Sargassum hemiphyllum can inhibit α-glucosidase, α-amylase, sucrase, and maltase activities and increase insulin secretion in vitro (Fred-Jaiyesimi et al. 2009; Skriptsova et al. 2010).
Nitric oxide has an antitumor activity and is generated by various NADPH-dependent enzymes called NO synthases (NOS) (El Shora et al. 2018). During inflammation, the formation of NO is increased, which may cause undesirable deleterious effects. Yang et al. (2006) concluded that FUC inhibits NO production in macrophages activated by LPS or TNF-α through the selective inhibition of AP-1 activation. Moreover, liberation of lysosomal enzymes is involved in both acute and chronic inflammation. The antihemolytic effect of FCS and FSP confirmed its ability to stabilize the lysosomal membrane which in turn mitigates the inflammatory response. In agreement with our results, FUC from Sargassum vulgare prevented erythrocyte membrane damage (Dore et al. 2013).
Acetylcholinesterase and MAO-B inhibitors are used to increase acetylcholine concentration and dopamine, respectively, which in turn improves or stabilizes the symptoms of Alzheimer’s disease (Dorosti and Jamshidi 2016). Published data highlighted a correlation between antioxidant power and the anticholinesterase activity (Muhammad et al. 2016). In agreement with this finding, both FSC and FSP acted as potent AChE and MAO inhibitors. It is proved that several polysaccharides could be considered memory and learning enhancers (Asker et al. 2015). In agreement with our results, FUC extracted from Ecklonia cava showed significant in vitro antioxidant activity, inhibitory effect against AChE, and neuronal cell-protective effect (Park et al. 2018).
Altogether, FUC possesses antioxidant, anti-inflammatory, hypoglycemic, and cell cycle regulatory effects; therefore, it could be used as a hepatoprotective agent against PCM-induced liver injury.
Although paracetamol is a safe drug at its therapeutic dose, it is converted into a highly toxic substance (in overdose) that damages kidney and liver cells by inducing necrosis and severe inflammation. Hepatic cell death occurs due to the formation of highly reactive PCM metabolite by cytochrome P450 that conjugated progressively with GSH leading to GSH depletion and mitochondrial dysfunction (Simeonova et al. 2013; Park et al. 2018). Yan et al.’s (2018) study proved that PCM increased TBARS level and decreased GSH level. Oxidative stress alters the cell membrane permeability, triggers leukocytes infiltration, and stimulates Kupffer cell. These abnormalities stimulate the production of NO through expression of iNOS and proinflammatory cytokines like IL-1β, IL-6, and TNF-α (McGill and Jaeschke 2013). Due to oxidative stress and inflammation, hepatic necrosis and cellular damage occur leading to liberation of liver enzymes into the blood (Ross et al. 2012; McGill and Jaeschke 2013; Simeonova et al. 2013; Yan et al. 2018). These alterations prove that PCM induces hepatic abnormalities that are similar to acute hepatitis, which is characterized by changes in liver function tests (elevation of AST, ALT, ALP, LDH, bilirubin, and reduction of TP and ALB) (Park et al. 2018). Our data are in agreement with (Alam et al. 2017) who reported that PCM increased ALT, AST, and bilirubin levels in mice. Histopathological results confirmed the alterations that took place in PCM-intoxicated group as centrilobular necrosis, infiltrating lymphocytes, cell degeneration, and fat droplet accumulation (Simeonova et al. 2013).
Because hepatic oxidative stress and inflammation are the possible mechanisms of PCM-induced hepatotoxicity, the administration of antioxidants could protect against hepatotoxicity. It has been demonstrated that the marine brown algae extracts containing antioxidant have hepatoprotective effects (Ross et al. 2012). According to our in vitro results, FCS is a potent antioxidant and anti-inflammatory agent; therefore, it could be interesting to examine it as a hepatoprotective agent against PCM-induced liver injury. Our results proved that FCS prevented PCM-induced hepatic oxidative stress and inflammation as it decreased TBARS, NO, TNF-α, IL-1β, IL-6, and iNOS, while it increased GSH and GPX activities and restored blood routine parameters nearly to normal which consequently prevented leukocyte inflammation, steatosis, and hepatic necrosis.
In agreement with our results, several findings reported that sulfated polysaccharides preserve the integrity of hepatocytes membrane and prevent cell membrane damage by reactive metabolites; therefore, they restore the activities of hepatic marker enzymes to normal levels (Tittarelli et al. 2017). Moreover, sulfated polysaccharides activate cellular antioxidants status and consequently prevent oxidative cell injury (Costa et al. 2010). Moreover, FUC protected liver from injury by increasing HGF expression (Bilan et al. 2006). Moreover, it was found that FUC inhibited liver fibrogenesis and induced hepatic stellate cell apoptosis, and these actions could be attributed to its sulfate content, which is important for its bioactivity.
Conclusion
In the present study, FUCs were isolated from the Egyptian alga species, C. sinuosa and S. prismaticum, and their structure was confirmed by FTIR and colorimetric methods. Experimental studies of FCS and FSP exhibited considerable hypoglycemic, hypocholesteremic, antioxidant, antidementia, and anticancer activities. The present study provides the first investigation of the in vivo hepatoprotective effect of FUC from Colpomenia sinuosa against PCM-induced hepatotoxicity. Fucoidan exhibited antioxidant and anti-inflammatory properties that protected the hepatocytes from inflammation and membrane damage.
Data availability statement
All required data will be available with the corresponding author upon request.
Abbreviations
- (AChE):
-
Acetylcholinesterase
- (ALT):
-
alanine transaminase
- (ALB):
-
albumin
- (ALP):
-
alkaline phosphatase
- (AST):
-
aspartate aminotransferase
- (FRAP):
-
ferric-reducing antioxidant power assay
- (F):
-
fucoidan
- (FCSPs):
-
fucose-containing sulfated polysaccharides
- (GSH):
-
glutathione
- (GPx):
-
glutathione peroxidase
- (HCC):
-
hepatic cancer cell
- (HRBC):
-
human red blood corpuscles
- inducible nitric oxide synthase:
-
(iNOS)
- (IC50):
-
inhibitory concentration 50%
- (IL-1β):
-
interleukin 1β
- (LDH):
-
lactate dehydrogenase
- (MDA):
-
malondialdehyde
- (MAO):
-
monoamine oxidase
- (NO):
-
nitric oxide
- (PCM):
-
paracetamol
- (PEG):
-
polyethylene glycol
- (ROS):
-
reactive oxygen species
- (TP):
-
total protein
- (TNF-α):
-
tumor necrosis factor-α
References
Abdel-Daim MM, Dawood MAO, Aleya L, Alkahtani S (2020a) Effects of fucoidan on the hematic indicators and antioxidative responses of Nile tilapia (Oreochromis niloticus) fed diets contaminated with aflatoxin B1. Environ Sci Pollut Res 27(11):12579–12586
Abdel-Daim MM, Abushouk AI, Bahbah EI, Bungău SG, Alyousif MS, Aleya L, Alkahtani S (2020b) Fucoidan protects against subacute diazinon-induced oxidative damage in cardiac, hepatic, and renal tissues. Environ Sci Pollut Res 27(11):11554–11564
Alam J, Mujahid M, Badruddeen et al (2017) Hepatoprotective potential of ethanolic extract of Aquilaria agallocha leaves against paracetamol induced hepatotoxicity in SD rats. J Tradit Complement Med 7:9–13. https://doi.org/10.1016/j.jtcme.2015.12.006
Ale MT, Maruyama H, Tamauchi H et al (2011a) Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int J Biol Macromol 49:331–336. https://doi.org/10.1016/j.ijbiomac.2011.05.009
Ale MT, Mikkelsen JD, Meyer AS (2011b) Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar Drugs 9:2106–2130
Asker MMS, Ibrahim AY, Mahmoud MG, Mohamed SS (2015) Production and characterization of exopolysaccharide from novel Bacillus sp. M3 and evaluation on development sub-chronic aluminum toxicity induced Alzheimer’s disease in male rats. Am J Biochem Biotechnol 11:92–103. https://doi.org/10.3844/ajbbsp.2015.92.103
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882
Bilan MI, Grachev AA, Shashkov AS et al (2006) Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr Res 341:238–245. https://doi.org/10.1016/j.carres.2005.11.009
Chakraborty C, Hsu C-H, Wen Z-H, Lin C-S (2009) Anticancer drugs discovery and development from marine organisms. Curr Top Med Chem 9:1536–1545. https://doi.org/10.2174/156802609789909803
Chew YL, Lim YY, Omar M, Khoo KS (2008) Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT Food Sci Technol 41:1067–1072. https://doi.org/10.1016/j.lwt.2007.06.013
Chia YY, Kanthimathi MS, Khoo KS et al (2015) Antioxidant and cytotoxic activities of three species of tropical seaweeds. BMC Complement Altern Med 15. https://doi.org/10.1186/s12906-015-0867-1
Chotigeat W, Tongsupa S, Supamataya K, Phongdara A (2004) Effect of Fucoidan on disease resistance of black tiger shrimp. Aquaculture 233:23–30. https://doi.org/10.1016/j.aquaculture.2003.09.025
Costa LS, Fidelis GP, Cordeiro SL et al (2010) Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother 64:21–28. https://doi.org/10.1016/j.biopha.2009.03.005
Cotton AD (1908) The appearance of Colpomenia sinuosa in Britain. Bull Misc Inf (Royal Gard Kew) 2:73–77. https://doi.org/10.2307/4111835
Da Silva PM, Kwon YI, Apostolidis E et al (2009) Potential of Ginkgo biloba L. leaves in the management of hyperglycemia and hypertension using in vitro models. Bioresour Technol 100:6599–6609. https://doi.org/10.1016/j.biortech.2009.07.021
Dische Z, Shettles LB (1948) A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J Biol Chem 175:595–603
Dore CMPG, Faustino Alves MGDC, Pofírio Will LSE et al (2013) A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr Polym 91:467–475. https://doi.org/10.1016/j.carbpol.2012.07.075
Dorosti N, Jamshidi F (2016) Plant-mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: synthesis, characterization, and evaluation of anticancer and antibacterial activity. J Appl Biomed 14:235–245. https://doi.org/10.1016/j.jab.2016.03.001
El Shora HM, Abou El Wafa GS, Abu Eftouh NM (2018) Fucoidan and Fucoidanase from brown seaweeds and applications. Int J Curr Microbiol App Sci 7:3707–3715. https://doi.org/10.20546/ijcmas.2018.702.440
Elabbar FA, Alziny FA, Elgabaeli MG (2014) Chemical screening, biological activity and antioxidant activity screening of some algae from Libyan coast Pelagia Research. Library. 4:31–33
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Fedorov SN, Ermakova SP, Zvyagintseva TN, Stonik VA (2013) Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar Drugs 11(12):4876–4901
Fitton JH (2011) Therapies from fucoidan; multifunctional marine polymers. Mar Drugs 9:1731–1760
Fred-Jaiyesimi A, Kio A, Richard W (2009) α-Amylase inhibitory effect of 3β-olean-12-en-3-yl (9Z)-hexadec-9-enoate isolated from Spondias mombin leaf. Food Chem 116:285–288. https://doi.org/10.1016/j.foodchem.2009.02.047
Gangadevi V, Muthumary J (2007) Preliminary studies on cytotoxic effect of fungal taxol on cancer cell lines. Afr J Biotechnol 6:1382–1386. https://doi.org/10.5897/AJB2007.000-2194
Hong SW, Lee HS, Jung KH et al (2012) Protective effect of fucoidan against acetaminophen-induced liver injury. Arch Pharm Res 35:1099–1105. https://doi.org/10.1007/s12272-012-0618-5
Elzagheid MI (2018) Laboratory activities to introduce carbohydrates qualitative analysis to college students. World J Chem Educ 6:82–86. https://doi.org/10.12691/wjce-6-2-1
Jang M, Kim SS, Lee J (2013) Cancer cell metabolism: Implications for therapeutic targets. Exp Mol Med 45:e45
Karkhane Yousefi M, Seyed Hashtroudi M, Mashinchian Moradi A, Ghasempour AR (2018) In vitro investigating of anticancer activity of focuxanthin from marine brown seaweed species. Glob J Environ Sci Manag 4:81–90. https://doi.org/10.22034/gjesm.2018.04.01.008
Gyi KK, Htun US (2012) The morphology and distribution of Colpomenia sinuosa (Mertens ex Roth) Derbes & Solier (Scyosiphonales, Phaeophyta) from Myanmar. Dep Mar Sci 5:1–21
Kim JA, Kong CS, Kim SK (2010) Effect of Sargassum thunbergii on ROS mediated oxidative damage and identification of polyunsaturated fatty acid components. Food Chem Toxicol 48:1243–1249. https://doi.org/10.1016/j.fct.2010.02.017
Koyanagi S, Tanigawa N, Nakagawa H et al (2003) Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem Pharmacol 65:173–179. https://doi.org/10.1016/S0006-2952(02)01478-8
Lin HTV, Lu WJ, Tsai GJ et al (2016) Enhanced anti-inflammatory activity of brown seaweed Laminaria japonica by fermentation using Bacillus subtilis. Process Biochem 51:1945–1953. https://doi.org/10.1016/j.procbio.2016.08.024
Liyana-Pathirana CM, Shahidi F (2005) Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chem 53:2433–2440. https://doi.org/10.1021/jf049320i
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/0922-338X(96)89160-4
McGill MR, Jaeschke H (2013) Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 2174–2187
Al Monla R, Dassouki Z, Kouzayha A et al (2020) The cytotoxic and apoptotic effects of the brown algae colpomenia sinuosa are mediated by the generation of reactive oxygen species. Molecules 25:1993. https://doi.org/10.3390/molecules25081993
Muhammad A, Tel-Çayan G, Öztürk M et al (2016) Phytochemicals from Dodonaea viscosa and their antioxidant and anticholinesterase activities with structure–activity relationships. Pharm Biol 54:1649–1655. https://doi.org/10.3109/13880209.2015.1113992
Nabavi SM, Ebrahimzadeh MA, Nabavi SF et al (2008) Determination of antioxidant activity, phenol and flavonoid content of Parrotia persica Mey. Pharmacologyonline 2:560–567
O’Sullivan AMN (2013) Cellular and in-vitro models to assess antioxidant activities of seaweed extracts and the potential use of the extracts as ingredients thesis presented by. PhD thesis, Universtiy Coll Cork
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169. https://doi.org/10.5555/uri:pii:0022214367900765
Park SK, Kang JY, Kim JM et al (2018) Protective effect of fucoidan extract from Ecklonia cava on hydrogen peroxide-induced neurotoxicity. J Microbiol Biotechnol 28:40–49. https://doi.org/10.4014/jmb.1710.10043
Parsons MJ (1982) Colpomenia (endlicher) derbès et solier (phaecphyta) in New Zealand. New Zeal J Bot 20:289–301. https://doi.org/10.1080/0028825X.1982.10428496
Pereira H, Barreira L, Figueiredo F et al (2012) Polyunsaturated fatty acids of marine macroalgae: Potential for nutritional and pharmaceutical applications. Mar Drugs 10:1920–1935. https://doi.org/10.3390/md10091920
Qebesy HS, Zakhary MM, Abd-alaziz MA et al (2015) Tissue levels of oxidative stress markers and antixidants in breast cancer patients in relation to tumor grade. Al-ahar assiut Med J 13:10–17
Ross V, Joven A, Donnie RJ et al (2012) Hepatoprotective effects of aqueous sulfated polysaccharide extract from Sargassum siliquosum J.G. Agardh on paracetamol-induced oxidative liver toxicity and antioxidant properties. Int J Pharm Front Res 2:15–27
Sandler M, Reveley MA, Glover V (1981) Human platelet monoamine oxidase activity in health and disease: a review. J Clin Pathol 292–302
Shaban NZ, Helmy MH, El-Kersh MAR, Mahmoud BF (2003) Effects of Bacillus thuringiensis toxin on hepatic lipid peroxidation and free-radical scavengers in rats given alpha-tocopherol or acetylsalicylate. Comp Biochem Physiol - C Toxicol Pharmacol 135:405–414. https://doi.org/10.1016/S1532-0456(03)00142-X
Shaikh W, Shameel M, Ahmad VU, Usmanghani K (1991) Phycochernical studies on Colpomenia sinuosa (Scytosiphonales, Phaeophyta). Bot Mar 34:77–79. https://doi.org/10.1515/botm.1991.34.2.77
Simeonova R, Vitcheva V, Kondeva-Burdina M et al (2013) Hepatoprotective and antioxidant effects of saponarin, isolated from gypsophila trichotoma wend. on paracetamol-induced liver damage in rats. Biomed Res Int 2013:757126. https://doi.org/10.1155/2013/757126
Skriptsova AV (2015) Fucoidans of brown algae: Biosynthesis, localization, and physiological role in Thallus. Russ J Mar Biol 41:145–156
Skriptsova AV, Shevchenko NM, Zvyagintseva TN, Imbs TI (2010) Monthly changes in the content and monosaccharide composition of fucoidan from Undaria pinnatifida (Laminariales, Phaeophyta). J Appl Phycol 22:79–86. https://doi.org/10.1007/s10811-009-9438-5
Souza BWS, Cerqueira MA, Bourbon AI et al (2012) Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll 27:287–292. https://doi.org/10.1016/j.foodhyd.2011.10.005
Tabarsa M, Rezaei M, Ramezanpour Z et al (2012) Fatty acids, amino acids, mineral contents, and proximate composition of some brown seaweeds. J Phycol 48:285–292. https://doi.org/10.1111/j.1529-8817.2012.01122.x
Tannoury MY, Elia JM, Saab AM et al (2016) Evaluation of cytotoxic activity of Sargassum vulgare from the Lebanese coast against Jurkat cancer cell line. J Appl Pharm Sci 6:108–112. https://doi.org/10.7324/JAPS.2016.60619
Tannoury MY, Saab AM, Elia JM et al (2017) In vitro cytotoxic activity of Laurencia papillosa, marine red algae from the Lebanese coast. J Appl Pharm Sci 7:175–179. https://doi.org/10.7324/JAPS.2017.70328
Tappel AL, Zalkin H (1959) Inhibition of lipide peroxidation in mitochondria by vitamin E. Arch Biochem Biophys 80:333–336. https://doi.org/10.1016/0003-9861(59)90259-0
Tittarelli R, Pellegrini M, Scarpellini MG et al (2017) Hepatotoxicity of paracetamol and related fatalities. Eur Rev Med Pharmacol Sci 21:95–101
Vadivu R, Lakshmi KS (2008) In vitro and In vivo anti-inflammatory activity of leaves of Symplocos cochinchnensis (Lour) Moore ssp laurina. Bangladesh J Pharmacol 3:121–124. https://doi.org/10.3329/bjp.v3i2.956
von Andrea Désirée Holtkamp M (2009) Isolation, characterisation, modification and application of Fucoidan from Fucus vesiculosus. Analysis
Yan M, Huo Y, Yin S, Hu H (2018) Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol 17:274–283
Yang JW, Yoon SY, Oh SJ et al (2006) Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem Biophys Res Commun 346:345–350. https://doi.org/10.1016/j.bbrc.2006.05.135
Yen GC, Der Duh P (1993) Antioxidative properties of methanolic extracts from peanut hulls. J Am Oil Chem Soc 70:383–386. https://doi.org/10.1007/BF02552711
Yim SK, Yun SJ, Yun CH (2004) A continuous spectrophotometric assay for NADPH-cytochrome P450 reductase activity using 1,1-diphenyl-2-picrylhydrazyl. J Biochem Mol Biol 37:629–633. https://doi.org/10.5483/bmbrep.2004.37.5.629
Zorofchian Moghadamtousi S, Karimian H, Khanabdali R et al (2014) Anticancer and antitumor potential of fucoidan and fucoxanthin, two main metabolites isolated from brown algae. Sci World J 2014:768323
Acknowledgements
This work was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Funding
This work was supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Author information
Authors and Affiliations
Contributions
Idea and protocol design: M.E.A., A.E, M. A. A., D.A.G., and M.M.E. Methodology and experimentation: M.E.A., A.E, M. A. A., D.A.G., and M.M.E. Data analysis: M.E.A., A.E, M. A. A., D.A.G., M.M.E., and M. M. A-D. Funding: I.S.A., S. A., and M. M. A-D. All authors shared draft writing. All authors approved the submission.
Corresponding authors
Ethics declarations
Conflict of interest statement
The authors declare that they have no conflict of interest.
Consent for publication
All authors approve this submission.
Consent to participate
Not applicable as the study did not include human subject.
Ethics approval
All animal experiments were conducted according to the Animal Ethics Committee guidelines of the National Health and Medical Research Council (NHMRC) and recommended guidelines of the Egyptian Ministry of Health and Population, High Committee of Medical Specialties, Arab Republic of Egypt. This study was approved by a Committee application for the Institutional Animal Care and Use Committees (IACUCs), Pharmaceutical and Fermentation Industries Development Centre (PFIDC), SRTA-City, Alexandria, Egypt; the approval number was IACUC # 15-1P-9020.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Atya, M.E., El-Hawiet, A., Alyeldeen, M.A. et al. In vitro biological activities and in vivo hepatoprotective role of brown algae-isolated fucoidans. Environ Sci Pollut Res 28, 19664–19676 (2021). https://doi.org/10.1007/s11356-020-11892-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11892-9