Abstract
The present study focuses on finding a good source of phycobiliproteins (PBP). We report a new cyanobacterium Nodularia sphaerocarpa PUPCCC 420.1 as a good producer of PBP. The organism produced 445.6 μg PBP mg−1 dry biomass. The growth and PBP production of organism were optimized by varying pH of growth medium, nitrogen sources, light quality and sugars. The optimized conditions for PBP production were as follows: pH 8.0, 5 mmol L−1 KNO3, 10 mmol L−1 NaNO2, 0.5% sucrose and green light. The PBP production under these conditions ranged from 486 to 676.3 μg mg−1 dry biomass. The PBP were more stable when stored in alkaline pH at 4 °C under dark. As per survey of literature, except for Anabaena fertilissima, the amount of PBP is significantly higher than the amount of PBP produced by other cyanobacteria. Thus, this organism is a good candidate for the PBP production at commercial level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Development of healthy food, free from chemical additives is currently seen as very important. Pigments such as phycobiliproteins, carotenoids and chlorophyll as natural colourants in food are gaining importance over the synthetic ones as they are non-toxic and non-carcinogenic (Chaneva et al. 2007). Production of colourants from cyanobacteria can offer advantages over their extraction from higher plants as they require less space, have a short life cycle and a high rate of biomass production (Hancock 1997).
Phycobiliproteins (PBP) are naturally occurring water-soluble fluorescent pigments produced by cyanobacteria and some eukaryotic algae (Pandey et al. 2013). They serve as accessory or antenna pigments for the photosynthetic light-harvesting complex (Moreno et al. 1995; Sekar and Chandramohan 2008). They may account for up to 60% of cyanobacterial cellular protein and also serve as an additional source of nitrogen reserve in cyanobacteria (Soni et al. 2008). PBP are organized in supramolecular complex called phycobilisomes which are assembled in a regular array on the outer surface of thylakoid membrane and lie adjacent to the photosynthetic reaction centre of PSII in cyanobacteria and red algae (Sidler 1994; MacColl 1998). Based on the spectroscopic properties, these pigments are classified as phycoerythrin (PE, λmax 540–570 nm), phycocyanin (PC, λmax 590–630 nm) and allophycocyanin (APC, λmax 620–655 nm) depending upon their absorption maxima (Ducret et al. 1998; Viskari and Coyler 2002).
PBPs are widely being used as natural colourant in food, nutraceutical and cosmetics industry (Spolaore et al. 2006; Pandey et al. 2013; Sonani et al. 2015). Currently, nutraceutical segment in food industry is booming at 5% per annum and is estimated between 6 billion and 60 billion USD $ in present day global market (Rodríguez-Sánchez et al. 2012). PBP have also been utilized for photodynamic therapy by making use of its function as a photosensitizer (He et al. 1997; Zhang et al. 2000) and may have applications in medicine (Xia et al. 2016). Due to these properties, these pigments are source of attraction for research by scientific communities. Major organisms which are exploited for commercial production of phycobiliproteins are limited to cyanobacteria Arthrospira (Spirulina), Gleotrichia natans and the rhodophyte Porphyridium (Roman et al. 2002; Spolaore et al. 2006). Since the phycobiliproteins have an array of applications, there is a need to explore more cyanobacteria for large-scale PBP production. Thus, present research was focused on the production and condition optimization of PBP by cyanobacterium Nodularia sphaerocarpa PUPCCC 420.1.
Material and methods
The cyanobacterium Nodularia sphaerocarpa PUPCCC 420.1 was isolated by our laboratory from cold deserts lake near Koksar village (32°24′30″N; 77°15′5″E) of district Lahaul-Spiti, Himachal Pradesh, India, and raised to axenic cultures through plating technique (Stanier et al. 1971). The cyanobacterium was identified based on morphological characters such as trichome/filament shape, cell dimensions, shape, size and position of heterocyst, presence or absence of sheath using the following monographs (Desikachary 1959; Komárek 2013). The cell size of the cyanobacterium was determined by using an ocular micrometer. The identification was confirmed by 16S rRNA gene sequence (Singh et al. 2014). The pure cultures of the organism were grown in slightly modified Chu-10 medium (Safferman and Morris 1964), where calcium nitrate was replaced with an equimolar amount of calcium chloride. The nutrient medium contained (g L−1) CaCl2·2H2O, 0.232; K2HPO4, 0.01; MgSO4·7H2O, 0.025; Na2CO3, 0.02; Na2SiO3·5H2O, 0.044; ferric citrate, 0.0035; citric acid, 0.0035. The cultures were incubated in a culture room at 28 °C ± 2 °C and illuminated for 14 h daily with light intensity of 44.5 μmol photons m−2 s−1 at the surface of culture vessels.
Condition optimization for phycobiliprotein production
The growth of the organism was monitored at regular interval as an increase in dry weight biomass with time. The experiment was conducted in 250-mL culture flask containing 100-mL culture medium. Exponentially growing cultures, 8 days old, were washed twice with sterilized double distilled water and added in basal medium to give initial absorbance of 0.1 at 720 nm. Ten-microlitre culture was withdrawn, centrifuged at 5000×g for 10 min, and the obtained cell pellet washed twice with double distilled water was oven dried at 70 °C for 24 h. The weight of dry biomass was determined. The growth and PBP content were optimized by varying culture conditions such as pH (6.0, 7.0, 8.0 and 9.0) of growth medium, nitrogen sources KNO3 and NaNO2 (2–15 mmol L−1) and sugars (glucose, fructose, sucrose; 0.5 and 1%). The effect of red light (RL), blue light (BL), green light (GL) and yellow light (YL) on growth and total PBP production was studied by illuminating the culture vessels wrapped with the cellophane papers of respective colours. The irradiance received by the cells inside the flask measured by Digital Luxmeter (Model MS6610) was 14.8, 24.7, 27.1 and 32.1 μmol photons m−2 s−1 for RL, BL, GL and YL, respectively. The transmission spectra of coloured cellophane papers as measures with a UV-Visible spectrophotometer (Shimadzu, model UV-1280) are given in Fig. 1. At any one time, one parameter was varied keeping others constant.
Extraction and quantification of phycobiliproteins
For extraction of phycobiliproteins, a known volume of homogenous suspension of culture was centrifuged at 5000 rpm for 10 min, and the pellet obtained was resuspended in known volume of water. The contents were then subjected to freeze and thaw cycle till all the pigments were released from the cells. The contents were centrifuged at 5000 xg for 10 min, and the absorbance of supernatant was measured at 562, 615 and 652 nm. The total phycobiliprotein (total PBP), phycocyanin (PC), allophycocyanin (APC) and phycoerythrin (PE) were quantified following the equations given by Bennett and Bogorad (1973).
Total protein content was measured according to the method of Lowry et al. (1951). Total carbohydrate was estimated following the methods of Ashwell (1957). To 1 mL of the thick cell suspension of culture, 4 mL of 0.2% anthrone reagent (prepared in concentrated sulphuric acid) was added and thoroughly mixed. The tubes were kept in boiling water bath for 10 min. After this, tubes were allowed to cool at room temperature and absorbance was taken at 540 nm. Standard curve was prepared by using glucose. Total lipids were estimated according to the method of Bligh and Dyer (1959).
Stability of phycobiliproteins
Three parameters such as light and dark, temperature (28,18 and 4 °C) and pH (6–9) were chosen to study the stability of crude PBP. A known volume of crude PBP extract was kept under above-mentioned conditions separately keeping one parameter varied and others constant. The absorbance of extract was measured, and time in days for 50% decrease of PBP was determined.
Statistical analysis
All the data are the average of three independent experiments ± standard deviation (SD). Data were statistically analysed by one-way analysis of variance and Tukey’s post hoc significance difference test. All statistical analyses were tested against the probability value at 95% confidence level (p < 0.05) using GraphPad Prism 6.0 version (www.graphpad.com).
Results
Selection of the test organism
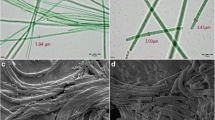
In a preliminary experiment, 20 cyanobacterial species isolated from cold desert area of Lahaul-Spiti, Himachal Pradesh, India, were screened for production of PBP. Among these, the cyanobacterium Nodularia sphaerocarpa PUPCCC 420.1 produced maximum amount of PBP equivalent to 44.4% of the dry weight (Table 1). The cyanobacterium is a diazotroph, unbranched and filamentous. The filaments are solitary, straight, bended or spirally coiled with a thick colourless sheath; trichome blue green, cells are short, discoid 4.0 ± 0.8 μm wide and 2.4 ± 0.8 μm long; heterocysts are sub-spherical, 5.0 ± 0.8 μm wide and 2.8 ± 0.4 μm long and are broader than the vegetative cells. The cyanobacterium belongs to order Nostocales of class Cyanophyceae (Fig. 2).
Growth of the microorganism
The pH values of growth medium, nitrogen sources, light quality and sugars were selected to optimize the conditions for growth and phycobiliprotein production. The results revealed that the growth was maximum (160 mg dry biomass L−1) when grown in medium having slightly alkaline pH 8. The supplementation of 5 mmol nitrate L−1 (210 mg dry biomass L−1) and 10 mmol nitrite L−1 (204 mg dry biomass L−1) in basal medium exhibited maximum growth. Among sugars, 0.5% sucrose in the culture medium supported maximum growth (247 mg dry biomass L−1) of N. sphaerocarpa. Among different colours of light, the incubation of the cultures in white light showed more growth, equivalent to 160 mg dry biomass L−1 (Fig. 3).
Growth of Nodularia sphaerocarpa on 8 days under varied culture conditions. n = 9, error bar: SD. Data in figures with same small letter are not significantly different from each other at 95% confidence level (p < 0.05). Light: green light (GL), yellow light (YL), blue light (BL), red light (RL). Sugars: glucose (Glc), sucrose (Suc), fructose (Fuc)
Phycobiliprotein production
The production of PBP under the above-mentioned conditions was also compared. The results revealed that a maximum amount of total PBP (445.6 μg mg−1 dry wt. biomass) was produced in the medium with pH 8. Nodularia sphaerocarpa produced 486 μg total PBP mg−1 dry wt. biomass in the cultures grown under green light. On the other hand, when the cultures were grown in medium with 5 mmol nitrate L−1 and 10 mmol nitrite L−1, total PBP production was increased by 47 and 52%, respectively, over the control cultures. The supplementation of 0.5% sucrose in basal medium increased the total PBP production by 42% compared to the control. The increase in total PBP, PC, APC and PE under studied conditions was almost similar, indicating that PC, APC and PE individually contributed to increase in the level of total PBP in this organism (Figs. 4, 5, 6 and 7). The increase in total PBP content of N. sphaerocarpa in green light was mainly due to enhancement of PC (17% increase) and APC (12% increase). The supplementation of either 5 mmol nitrate L−1, 10 mmol nitrite L−1 or 0.5% sucrose resulted in increase in PC (80–92%) and APC (42–65%) as compared to the control cultures (Figs. 5 and 7). The relative biochemical content of N. sphaerocarpa grown under control and optimized condition is presented in Fig. 8. The total proteins as well as PBP were enhanced significantly in optimized conditions compared to control conditions.
Comparison of PC, APC and PE of Nodularia sphaerocarpa on 8 days when grown in medium having different concentration of potassium nitrate and sodium nitrite. n = 9, error bar: SD. Data in figures with same small letter are not significantly different from each other at 95% confidence level (p < 0.05)
Comparison of PC, APC and PE of Nodularia sphaerocarpa on 8 days when illuminated with different light colours. n = 9, error bar: SD. Data in figures with same small letter are not significantly different from each other at 95% confidence level (p < 0.05). Light: white light (WL), green light (GL), yellow light (YL), blue light (BL), red light (RL)
Comparison of PC, APC and PE of Nodularia sphaerocarpa on 8 days when grown in medium having different concentration of sugars. n = 9, error bar: SD. Data in figures with same small letter are not significantly different from each other at 95% confidence level (p < 0.05). Sugars: glucose (Glc), sucrose (Suc), fructose (Fuc)
Comparison of total protein, total PBP, total carbohydrate and total lipids of Nodularia sphaerocarpa on 8 days grown in control and optimized conditions. n = 9, error bar: SD. Data in each parameter of figure at different time interval are significantly different from each other at 95% confidence level (p < 0.05)
Stability of phycobiliproteins
Three parameters such as light/dark, temperature and pH were chosen to study the stability of crude PBP extract of N. sphaerocarpa. The results revealed that the crude PBP extract was more stable in the dark compared to storage in the light at room temperature. A decrease of 50% in crude PBPs was observed on day 10 in the dark and day 6 in the light (Fig. 9a). The crude PBPs were more stable at 4 °C in dark with a 50% decrease on day 22. There was a 50% decrease in crude PBPs on days 17 and 10 at 18 and 28 °C, respectively (Fig. 9b). PBPs were more stable in acidic conditions in the dark with a 53.3% decrease on day 6. In alkaline conditions (pH 9.0), a decrease of 64% in PBP was observed in just 4 days (Fig. 9c).
Discussion
Phycobiliproteins have been reported to have great potential for use in food and pharmaceutical industries (Pandey et al. 2013; Johnson et al. 2014). However, their economically viable production on a commercial scale has been challenging. Inspite of the enormous diversity in algae, only few algal species, Arthrospira (Spirulina) and Porphyridium, are exploited for the production of PBPs (Roman et al. 2002). Thus, there is a need to identify and exploit hyperproducers of PBP. A number of earlier studies were focussed on the PBP production from mesophilic cyanobacteria (Moreno et al. 1995; Hemlata and Fatima 2009; Khattar et al. 2015). During the present study, 20 cyanobacterial species isolated from cold desert of Himachal Pradesh, India, were screened for phycobiliproteins production. Among these, Nodularia sphaerocarpa PUPCCC 420.1 produced a high amount of total PBP (44.4% of dry biomass).
It is well known that physical as well as nutrient parameters such as pH of the medium, light quality, nitrogen sources and sugars affected the growth of cyanobacteria (Hemlata and Fatima 2009; Khattar et al. 2015; Tiwari et al. 2015). During the present investigation, these conditions were optimized by taking one parameter at a time. The results revealed that pH of growth medium, supplementation of nitrate and nitrite as nitrogen source or sucrose as sugars significantly affected the growth of N. sphaerocarpa. Nodulatia sphaerocarpa PUPCCC 420.1 showed maximum growth (159.6 mg dry wt. biomass L−1) in basal medium having pH 8. Similar to our study, the optimum growth of a mesophilic cyanobacterium Anabaena fertilissima was reported in alkaline pH 9.5 (Khattar et al. 2015). The growth of Anabaena sp. NCCU-9 was maximum in pH range 6–10 (Hemlata and Fatima 2009). Incubation of cultures of N. sphaerocarpa PUPCCC 420.1 under different light colours did not support the growth of organism up to the level of growth in control culture under white light (Fig. 3). Our observations are in agreement with Madhyastha and Vatsala (2007) who reported high biomass production in Spirulina fusiformis cultures in white light followed by blue and green light. Oberhaus et al. (2007) observed more growth of Planktothrix agardhii and P. rubescens under white light at 25 °C as compared to other light colours. Blue light enhanced the biomass production in Pseudanabaena sp. and Nanochloris spp. as compared to white light (Mishra et al. 2012; Vadiveloo et al. 2015, 2016). The high biomass production by Calothrix elenkinii was observed in the cultures grown under red light followed by blue and green light (Velu et al. 2015). Better growth of N. sphaerocarpa in white light is due to the fact that these received higher irradiances compared to the other colours (Vadiveloo et al. 2015).
The incubation of the cultures in 5 mmol nitrate L−1 and 10 mmol nitrite L−1 supported maximum growth of N. sphaerocarpa. Moore et al. (2002) reported more growth of Prochlorococcus and Synechococcus sp. in NH4 + and urea supplemented medium as compared to NO3 − supplemented medium. The growth of Nostoc flagelliforme was enhanced by 19.8% as compared to the control cultures when grown in medium containing urea as nitrogen source in presence of blue light (Han et al. 2016). Another study found that sodium nitrate as nitrogen source did not support the growth of the cyanobacteria Nostoc and Anabaena (Simeunovic et al. 2013).
Among various sugars, sucrose (0.5%) proved to be a good source of organic carbon for the growth of N. sphaerocarpa compared to fructose and glucose exhibiting 55% more growth in presence of 0.5% sucrose than control cultures. Sugarcane molasses having sucrose has been reported to be the most promising substrate for the production of biomass of Nostoc (Borsari et al. 2007). The supplementation of glucose and sucrose in medium stimulated the growth of Calothrix and Anbaena azollae, respectively (Prasanna et al. 2004, 2006).
Incubation of cultures in green light enhanced the production of total PBP in N. sphaerocarpa (Fig. 6). The enhancement of total PBP production in green light is due to increase in PE which may be due to chromatic adaptation (Bezy et al. 2011; Velu et al. 2015). Green light also enhanced the production of PBP in Fremyella diplosiphon and Arthrospira (Spirulina) platensis (Oelmuller et al. 1988; Babu et al. 1991). Hemlata and Fatima (2009) observed more PBP in Anabaena NCCU-9 under white light. Blue light enhanced the synthesis of phycobiliprotein in Anabaena ambigua, Anabaena fertillissima Westiellopsis iyengarii, Spirulina fusiformis and Nostoc sphaeroides (Madhyastha and Vatsala 2007; Vijaya and Anand 2009; Ashok Kumar and Narayanaswamy 2010; Khattar et al. 2015; Ma et al. 2015). These reports suggest that response of cyanobacteria varied with the quality of light. As compared to nitrogen free or NH4 + containing medium, the supplementation of 5 mmol nitrate L−1 and 10 mmol nitrite L−1 resulted in increase of 47–52% in the amount of total PBP of N. sphaerocarpa. Our observations are in agreement with Soltani et al. (2007) who have observed high amount of PBP in Fischerella grown in nitrate containing medium. Phycobiliproteins serve as nitrogen source, and excess of nitrogen available to cyanobacterial cell may be stored in the form of PBP under nitrogen sufficient conditions. Total PBP in Nostoc strain S36 was very high when grown in N2-free medium, but in Anabaena S28, it was high when grown in nitrogen containing medium (Simeunovic et al. 2012).
The supplementation of 0.5% of sucrose increased total PBP by 41% in N. sphaerocarpa. Significant increase in total PBP in the sucrose containing cultures of Anabaena azollae and Anabaena fertilissima has been reported (Prasanna et al. 2006; Khattar et al. 2015). It has been reported that molasses of sugar act as a good substrate for the production of PBP by Nostoc sp. (Borsari et al. 2007). The addition of sucrose in culture medium increased PBP by 30–90% in A. azollae (Venugopal et al. 2006). The increase in production of PBS in presence of sucrose may be due to increased energy linked assimilation and ATP production as reported in other cyanobacteria (Prasanna et al. 2004).
Phycocyanin, allophycocyanin and phycoerythrin increased by 17, 12 and 6%, respectively, when cultures of N. sphaerocarpa were incubated in green light. The results indicate that cells growing under GL were in a state of high transfer of excitation energy from phycobilisomes/PS-II supercomplex to PS-I. This transfer allowed GL capture by PBS to ultimately drive both PS-I and PS-II photochemistry more efficiently (Campbell 1996; Mishra et al. 2012). The cyanobacterium Pseudoanabaena exhibited maximum amount of PE (39 mg L−1) in green light and PC (11 mg L−1) in red light (Mishra et al. 2012). The addition of 5 mmol nitrate L−1 or 10 mmol nitrite L−1 increased PC, APC and PE content of Nodularia sphaerocarpa PUPCCC 420.1 by 34–91%. It has been observed that deficiency of nitrogen resulted in loss of these pigments in cyanobacteria Oscillatoria splendida and Pseudanabaena sp. (Laura et al. 1987). Addition of 0.5% sucrose in the growth medium resulted in 12–70% increase in PC, APC and PE. Chen and Zhang (1997) observed high production of PC in Arthrospira plantensis when it was grown in glucose containing medium. Lebedeva et al. (2005) observed increase in PE and PC content of Calothrix sp. with the addition of glucose in growth medium. N. sphaerocarpa exhibited maximum production of 120 μg PC mg−1 dry biomass, 40 μg APC mg−1 dry biomass and 283 μg PE mg−1 dry biomass in cultures grown in medium having pH 8. Maurya et al. (2014) observed maximum PBP production by A. plantensis at pH 7.0. PBP production was maximum at pH 8 in Synechocystis sp., Gloecapsa sp., Anabaena sp. and Lyngbya sp. (Hemlata and Fatima 2009).
Since phycobiliproteins have wide applications, it is important that these proteins remain stable. Thus, stability of crude phycobiliproteins produced by N. sphaerocarpa was studied under different condition of light and temperature. The PBP were more stable when incubated in dark at room temperature. The stability of PBP further increased up to 20 days when stored at 4 °C under dark in acidic conditions (pH 6). PBP of Lyngbya arboricola were more stable at 4 °C than 25 °C (Tripathi et al. 2007). Antelo et al. (2008) reported that phycocyanin of A. platensis was more stable when incubated at temperature 50–55 °C at acidic pH 6. The addition of preservatives may further enhance PBP stability (Kannaujiya and Sinha 2016).
In conclusion, the N. sphaerocarpa produced 445.6 mg PBP L−1 dry biomass under the control conditions which was enhanced to 653, 676 and 629 mg L−1 with the addition of 5 mmol nitrate L−1, 10 mmol nitrite L−1 and 0.5% sucrose, respectively. As per available literature, except for Anabaena fertilissima, the amount of PBP is significantly higher than the amount of PBP produced by other strains of cyanobacteria (Table 2). Thus, this organism is a good candidate for the phycobiliprotein production at the commercial level. Furthermore, PBP of N. sphaerocarpa was more stable when stored in alkaline conditions at 4 °C in the dark.
References
Ajyan KV, Selvaraju M, Thirugnanamoorthy K (2015) Enrichment of chlorophyll and phycobiliproteins in Spirulina platensis by the use of reflector light and nitrogen source: an in-vitro study. J Biomass Bioenerg 47:436–441
Antelo FS, Costa JAV, Kalil SG (2008) Thermal degradation kinetics of the phycocyanin from Spirulina platensis. J Biochem Eng 41:43–47
Ashok kumar P, Narayanaswamy A (2010) Studies on growth and phycobilin pigments of the cyanobacterium Westiellopsis iyengarii. Int J Biotechnol Biochem 6:315–323
Ashwell G (1957) Colorimetric analysis of sugars. Method Enzymol 3:73–105
Babu TS, Kumar A, Varma AK (1991) Effect of light quality on phycobilisome components of the cyanobacterium Spirulina platensis. J Plant Physiol 95:492–497
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in filamentous blue green algae. J Cell Biol 58:419–433
Bezy RP, Wiltbanka L, Kehoea DM (2011) Light dependent attenuation of phycoerythrin gene expression reveals convergent evolution of green light sensing in cyanobacteria. Proc Natl Acad Sci U S A 108:18542–18547
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borsari RRJ, Morioka LRI, Ribeiro MLL, Buzato JB, Pinotti MHP (2007) Mixotrophic growth of Nostoc sp. on glucose, sucrose and sugarcane molasses for phycobiliprotein production. J Acta Sci Biol Sci 29:9–13
Campbell D (1996) Complementary chromatic adaptation alters photosynthetic strategies in the cyanobacterium Calothrix. J Microbiol 142:1255–1263
Chaneva G, Furnadzhieva S, Minkova K, Lukavsky J (2007) Effect of light and temperature on the cyanobacterium Arthronema africanum—a prospective phycobiliprotein-producing strain. J Appl Phycol 19:537–544
Chen F, Zhang Y (1997) High cell density mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzym Microb Technol 20:221–224
Desikachary TV (1959) Cyanophyta. Indian Council of Agriculture Research, New Delhi
Ducret A, Muller SA, Goldie KN, Hefti A, Sidler WA, Zuber H, Engel A (1998) Reconstitution, characterization and mass analysis of cyanobacterium Anabaena sp. PCCC 7120. J Mol Biol 278:369–388
Hemlata, Fatima T (2009) Screening of cyanobacteria for phycobiliproteins and effect of different environmental stress on its yield. Bull Env Contam Toxicol 83:509–515
Han PP, Shen SG, Yang HY, Yao SU, Tan ZL, Zhong C, Jia SR (2016) Applying the strategy of light environment control to improve the biomass and polysaccharide production of Nostoc flagelliforme. J Appl Phycol. doi:10.1007/s10811-016-0963-8:1-11
Hancock M (1997) Potential for colourants from plant source in England and Wales. ADAS Boxworth, Cambridge
He JA, Hu YJ, Jiang LJ (1997) Photodynamic action of phycobiliproteins: in situ generation of reactive oxygen species. J Biochem Biophys 1320:167–174
Johnson EM, Kumar K, Das D (2014) Physicochemical parameters optimization and purification of phycobiliproteins from isolated Nostoc sp. J Bioresour Technol 166:541–547
Kannaujiya VK, Sinha RP (2016) Thermokinetic stability of phycocyanin and phycoerythrin in food-grade preservatives. J Appl Phycol 28:1063–1070
Khattar JIS, Kaur S, Kaushal S, Singh Y, Singh DP, Rana S, Gulati A (2015) Hyperproduction of phycobiliproteins by the cyanobacteria Anabaena fertilissima PUPCCC 410.5 under optimized culture conditions. J Algal Res 12:463–469
Komárek J (2013) Cyanoprokaryota. 3. Heterocytous genera. In: B. Büdel, G. Gärtner, L. Krienitz, M. Schagerl (Eds.), Süswasserflora von Mitteleuropa. Springer Spektrum, Berlin, p. 1130
Laura CI, Dubaca IP, Thomas JP (1987) The effects of nitrogen deficiency on pigment and lipids of cyanobacteria. J Plant Physiol 83:838–843
Lebedeva NV, Biochenko VA, Semenova LR, Pronina NA, Stadnichuk IN (2005) Effect of glucose during photoheterotrophic growth of cyanobacterium Calothrix sp. PCC 7601 capable for chromatic adaptation. Russ J Plant Physol 52:235–241
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with Folin phenol reagent. J Biol Chem 193:265–275
Ma R, Lu F, Bi Y, Hu Z (2015) Effect of light intensity and quality on phycobiliprotein accumulation in the cyanobacterium Nostoc sphaeroides Kützing. Biotechnol Lett 37:1663–1669
MacColl R (1998) Cyanobacterial phycobilisome. J Struct Biol 124:311–334
Madhyastha HK, Vatsala TM (2007) Pigment production by Spirulina fussiformis in different photophysical conditions. J Biomol Eng 24:301–305
Maurya SS, Maurya JN, Pandey VD (2014) Factors regulating phycobiliprotein production in cyanobacteria. Int J Curr Microbiol App Sci 3:764–771
Mishra SK, Shrivastav A, Maurya RR, Patidar SK, Haldar S, Mishra S (2012) Effect of light quality on the C-phycoerythrin production in marine cyanobacteria Pseudanabaena sp. isolated from Gujarat coast, India. J Protein Express Purif 81:5–10
Moore LR, Post AF, Rocap G, Chisholm SW (2002) Utilization of different nitrogen sources by the marine cyanobacteria Prochlorococcus and Synechococcus. J Limnol Oceanogr 47:989–996
Moreno J, Rodriguez H, Vargas MA, Rivas J, Guerrero MG (1995) Nitrogen-fixing cyanobacteria as source of phycobiliprotein pigments. Composition and growth performance of ten filamentous heterocystous strains. J Appl Phycol 7:17–23
Oberhaus L, Briand JF, Leboulanger C, Jacquet S, Humbert JF (2007) Comparitive effect of quality and quantity of light and temperature on the growth of the Planktothrix agardhii and Planktothrix rubescens. J Phycol 43:1191–1199
Oelmuller R, Grossman AR, Briggs WR (1988) Photo reversibility of the effect of red and green light pulses on the accumulation in darkness of mRNAs coding for phycocyanin and phycoerythrin Fremyella diplosiphon. J Plant Physiol 88:1084–1090
Ojit SK, Indrama T, Gunapati O, Avijeet SO, Subhalaxami SA, Silvia C, Indira DW, Romi K, Minerva S, Thadoi DA, Tiwari ON, Sharma GD (2015) The response of phycobiliproteins to light qualities in Anabaena circinalis. J Appl Biol Biotechnol 3:1–6
Pandey VD, Pandey A, Sharma V (2013) Biotechnological applications of cyanobacterial phycobiliproteins. J Curr Microbiol Appl Sci 2:89–97
Prasanna R, Pabby A, Singh PK (2004) Effect of glucose and light/dark environment on pigmentation profiles in Calothrix elenkenii. Folia Microbiol 49:26–30
Prasanna R, Venugopal V, Sood A, Jaiswal P, Kaushik BD (2006) Stimulation and pigment accumulation in Anabaena azollae strains: effect of light intensity and sugars. Folia Microbiol 51:50–56
Ranjitha K, Kaushik BD (2005) Purification of phycobiliproteins from Nostoc muscorum. J Sci Ind Res 64:372–375
Rodríguez-Sánchez R, Ortiz-Butrón R, Blas-Valdivia B, Hernández-García A, Cano-Europa E (2012) Phycobiliproteins or C-phycocyanin of Arthrospira (Spirulina) maxima protect against HgCl2-caused oxidative stress and renal damage. J Food Chem 135:2359–2365
Roman RB, Alvarez-Pez JM, Fernandez FGA, Grima EM (2002) Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J Biotechnol 93:78–85
Safferman RS, Morris ME (1964) Growth characteristics of the blue green algal virus LPP-1. J Bacteriol 88:771–775
Sekar S, Chandramohan M (2008) Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol 20:113–136
Sidler WA (1994) Phycobilisome and phycobiliprotein structure. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer Academic, Dordrecht, pp 140–205
Simeunovic JB, Markovic SB, Kovac DJ, Misan AC, Mandic AI, Svircev ZB (2012) Filamentous cyanobacteria from Vojvodina region as the source of phycobiliproteins pigments as potential natural colourants. Food Feed Res 39:23–32
Simeunovic J, Beslin K, Svircev Z, Kovac D, Babic O (2013) Impact of nitrogen and drought on phycobiliprotein content in terrestrial cyanobacterial strains. J Appl Phycol 25:597–607
Singh Y, Khattar JIS, Singh DP, Rahi P, Gulati A (2014) Limnology and cyanobacterial diversity of high altitude lakes of Lahaul-Spiti in Himachal Pradesh, India. J Biosci 39:1–15
Soltani N, Khavari-Nejad RA, Yazdi MT, Shokravi S (2007) Growth and some metabolic features of cyanobacterium Fischerella sp. FS18 in different combined nitrogen sources. J Sci Repub Iran 18:123–128
Sonani RR, Rastogi RP, Madamwar D (2015) Antioxidant potential of phycobiliproteins: role in anti-aging research. Biochem Anal Biochem 4:1–8
Soni B, Trivedi U, Madamwar D (2008) A novel method of single step hydrophobic interaction chromatography for the purification of phycocyanin from Phormidium fragile and its characterization for antioxidant property. Bioresour Technol 99:188–194
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Stanier RY, Kunisawa R, Mandel MG, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bact Rev 35:171–205
Tiwari ON, Indrama T, Singh KO, Singh OA, Oinam G, Koijam L, Subhalaxmi A, Thadoi A, Indira W, Silvia C, Khangembam R, Shamjetshabam M, Premi P, Bidyababy T, Sarabati K, Sharma GD (2015) Enumeration, pigment analysis and nitrogenase activity of cyanobacteria isolated from unexplored rice fields of Manipur, India falling under Indo-Burma biodiversity hotspots. Int J Curr Microbiol App Sci 4:666–680
Tripathi SN, Kapoor S, Shrivastava A (2007) Extraction and purification of an unusual phycoerythrin in a terrestrial desiccation tolerant cyanobacterium Lyngbya arboricola. J Appl Phycol 19:441–449
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res 8:121–127
Vadiveloo A, Moheimani NR, Kosterrink NR, Cosgrove JJ, Parlevliet D, Gonzalez-Garcia C, Lubian LM (2016) Photosynthetic performance of two Nannochloropsis spp under different filtered light spectra. Algal Res 19:168–177
Velu V, Narayanaswamy M, Balan KS (2015) Enhancing the phycobilin pigment synthesis in Calothrix elenkinii through optimization of light conditions. Int J Sci Nature 6:88–91
Venugopal V, Prasanna R, Sood A, Jaiswal P, Kaushik BD (2006) Stimulation of pigment accumulation in Anabaena azollae strain: effect of light intensity and sugars. Folia Microbiol 51:50–56
Vijaya V, Anand N (2009) Blue light enhance the pigment synthesis in cyanobacterium Anabaena ambigua Rao (Nostocales). J Agri Biol Sci 4:36–43
Viskari JP, Coyler CL (2002) Separation and quantification of phycobiliproteins using phytic acid in capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr 972:269–276
Xia D, Liu B, Xin W, Liu T, Sun J, Liu N, Qin S, Du Z (2016) Protective effects of C-phycocyanin on alcohol-induced subacute liver injury in mice. J Appl Phycol 28:765–772
Zhang SP, Zhao ZQ, Jiang LJ (2000) Photosensitized formation of singlet oxygen by phycobiliproteins in natural aqueous solutions. J Free Radical Res 33:489–496
Acknowledgements
The authors thank Head, Department of Botany, Punjabi University, Patiala and Coordinator, DRS SAP-II of UGC and FIST of DST, New Delhi for infrastructure and laboratory facilities. Financial assistance received from Department of Science and Technology, New Delhi under WOS-A scheme, Project no. SR/WOS-A/LS-17/2013 to Shveta Kaushal is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaushal, S., Singh, Y., Khattar, J.I.S. et al. Phycobiliprotein production by a novel cold desert cyanobacterium Nodularia sphaerocarpa PUPCCC 420.1. J Appl Phycol 29, 1819–1827 (2017). https://doi.org/10.1007/s10811-017-1093-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1093-7