Abstract
Phycobiliproteins, light-harvesting pigments found in cyanobacteria and in some eukaryotic algae, have numerous commercial applications in food, cosmetic, and pharmaceutical industries. Colorant production from cyanobacteria offers advantages over their production from higher plants, as cyanobacteria have fast growth rate and high photosynthetic efficiency and require less space. In this study, three cyanobacteria strains were studied for phycobiliprotein production and the influence of sodium nitrate, potassium nitrate and ammonium chloride on the growth and phycobiliprotein composition of the strains were evaluated. In the batch culture period of 12 days, Phormidium sp. and Pseudoscillatoria sp. were able to utilize all tested nitrogen sources; however, ammonium chloride was the best nitrogen source for both strains to achieve maximum growth rate μ = 0.284 ± 0.03 and μ = 0.274 ± 0.13 day−1, chlorophyll a 16.2 ± 0.5 and 12.2 ± 0.2 mg L−1, and phycobiliprotein contents 19.38 ± 0.09 and 19.99 ± 0.14% of dry weight, whereas, for Arthrospira platensis, the highest growth rate of μ = 0.304 ± 0.0 day−1, chlorophyll a 19.1 ± 0.5 mg L−1, and phycobiliprotein content of 22.27 ± 0.21% of dry weight were achieved with sodium nitrate. The phycocyanin from the lyophilized cyanobacterial biomass was extracted using calcium chloride and food grade purity (A620/A280 ratio > 0.7) was achieved. Furthermore, phycocyanin was purified using two-step chromatographic method and the analytical grade purity (A620/A280 ratio > 4) was attained. SDS-PAGE demonstrated the purity and presence of two bands corresponding to α- and β-subunits of the C-phycocyanin. The results showed that Phormidium sp. and Pseudoscillatoria sp. could be good candidates for phycocyanin production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria (blue-green algae) have emerged as an important source of high-value products, counting important bioactive and biotechnologically significant chemicals like phycobiliprotein (PBP), carotenoids, and polyunsaturated fatty acids with potential biotechnological application in food, cosmetic, pharmaceutical, and nutraceutical industries (Begum et al. 2016). PBP are water-soluble light-harvesting antenna complexes found in cyanobacteria, rhodophytes (red algae), and cryptophytes (Glazer and Apell 1977), which absorb energy in portions of visible spectrum that poorly utilized by chlorophyll and transfer electrons to the photosynthetic reaction centers to drive photosynthetic electron transport (Kirilovsky 2015). Based on their spectral properties, PBP are categorized into three main types, namely phycoerythrin (PE) (bright pink: λmax 490–570 nm), phycocyanin (PC) (dark cobalt blue: λmax 610–625 nm), and allophycocyanin (APC) (brighter aqua blue: λmax 650–660 nm) (Kuddus et al. 2013; Singh et al. 2015; Manirafasha et al. 2016). Cyanobacteria species are an intense and cost-effective source of the PBP, especially PC. Phycobilin pigments have gained importance as natural colorants in food and biotechnological industries by virtue of toxicity and carcinogenicity of synthetic colorants (Amchova et al. 2015; Dasgupta 2015). In addition, PC also exhibits several biological activities like anti-oxidant, anti-carcinogenic, anti-inflammatory, anti-angiogenic, and neuroprotective effects (Romay et al. 2003).
Cyanobacteria are considered as a potential candidate for PC production because of their rapid growth rate, CO2 fixation ability, high production capacity, growth on non-arable lands, and a wide variety of water sources, and at the same time, some also can survive under extreme environmental conditions (Encarnação et al. 2015). There are reports on many cyanobacterial species that are known to produce PC (Fatma 2009; Kuddus et al. 2013). Recently, cyanobacteria of the genus Scytonema isolated from various terrestrial habitats have also been studied for the composition and distribution of PBP (Asencio and Hoffmann 2013). Despite the huge variety of cyanobacteria containing PC, Arthrospira (Spirulina) platensis (Lee et al. 2016), Synechococcus (Gupta and Sainis 2010), Anabaena (Chakdar et al. 2014), Nostoc (Lee et al. 2017), and Oscillatoria (Soni et al. 2006) are currently used for commercial production of PC. The rhodophyte Galdieria sulphuraria has also been proposed for commercial production of PC; however, this species contains the cellulose-rich cell wall, causing difficulties in cell disruption and pigment extraction. Therefore, red algal strain has not yet been used for commercial production of PC (Buchweitz 2016).
The growth, biochemical composition, and pigment content of cyanobacteria vary from species to species and are affected by various physicochemical parameters (Manirafasha et al. 2016). The effect of physicochemical parameters, i.e., temperature, pH, light quality and intensity, nutrient limitation, and their synergistic interaction effect on PBP synthesis was extensively studied and reported by many researchers for cyanobacteria and rhodophyta (Fatma 2009; Chen et al. 2010; Johnson et al. 2014; Rizzo et al. 2015; Baer et al. 2016). Nitrogen is considered as one of the most important macronutrients for cyanobacterial growth and PBP synthesis since it is a fundamental element for the proteins, chlorophyll, and nucleic acid formation. Studies have shown that cyanobacteria are able to assimilate a variety of nitrogen sources including ammonium (NH4+), nitrate (NO3−), nitrite (NO2−), urea, and amino acids with several species also capable of fixing N2 (Herrero et al. 2001). However, comparatively few studies have investigated the effect of different nitrogen sources on growth and PBP content of cyanobacteria (Choi et al. 2003; Rückert and Giani 2004; Flores et al. 2005; Simeunović et al. 2013; Deschoenmaeker et al. 2017). To the best of our knowledge, the influence of different nitrogen sources on marine Phormidium and Pseudoscillatoria spp. growth and PBP composition (PC, APC, and PE) has not been studied. Therefore, this study was undertaken to test marine Phormidium sp., Pseudoscillatoria sp., and a brackish, alkaliphilic A. platensis strain for their abilities to produce PBP and to evaluate the influence of three different nitrogen sources (NaNO3, KNO3, NH4Cl) on growth and PBP content of these strains.
The specific objectives of this study were (i) to characterize marine cyanobacteria from Aegean Sea, Urla, Izmir, Turkey; (ii) to investigate the influence of different nitrogen sources on growth, and phycobiliprotein (PBP: PC, APC, and PE) composition of three cyanobacterial strains; and (iii) to develop a simple and efficient method for the extraction and purification of PC.
Material and methods
Strain, media, cultivation, and identification
The cyanobacterial strains were obtained from Ege University Microalgae Culture Collection (http://www.egemacc.com/cultures.php), Turkey (Arthrospira platensis EGEMACC 30, Phormidium sp. EGEMACC 72, and Pseudoscillatoria sp. EGEMACC 74). Phormidium sp. and Pseudoscillatoria sp. originated from Aegean Sea (Izmir/Turkey). Collection, isolation, and purification of strains were described in our previous study (Montalvão et al. 2016). The marine isolates were maintained and cultivated in BG-11 plus 25 g L−1 sea salt (Marinium reef sea salt) (indicated as sea salt BG-11) (Costa et al. 2016; Rippka et al. 1979) and A. platensis in Zarrouk’s medium (Zarrouk 1966) at 22 ± 1 °C under continuous illumination of 80 μmol photons m−2 s−1. Morphological observations were made using bright field (Olympus BX53, Japan) with × 60 and scanning electron microscopy (SEM) (Philips XL-30S FEG). The species were classified morphologically according to Desikachary (1959), Bourrelly (1970), and Rasoulouniriana et al. (2009).
DNA extraction and amplification
Isolated samples were harvested by centrifugation at 3500×g for 10 min. The concentrated cells were transferred into 1.5-mL microtubes and stored at − 20 °C. Genomic DNA was extracted using Quick-DNA Fungal/Bacterial MiniPrep kit (Zymo Research) according to the manufacturer’s instruction. Amplification was performed in 25 μL reactions using primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 809R (5′-GCTTCGGCACGGCTCGGGTCGATA-3′). The PCRs were performed using HelixAmp HyperSense DNA polymerase kit (NanoHelix), and conditions included preheating at 95 °C for 2 min, 35 cycles of denaturation at 95 °C for 20 s, annealing at 55 °C for 30 s, and extension at 72 °C for 2 min, followed by another 5 min extension at 72 °C. The resulting PCR products were verified by gel electrophoresis (1.0% agarose gel). The nucleotide sequences of PCR products were determined by a genetic analyzer (Applied Biosystems 3130XL with16-capillary array). The obtained sequences were compared and aligned with GenBank nucleotide sequences of some known cyanobacteria using NCBI-BLAST program to find the closest related organisms (http://www.ncbi.nlm.nih.gov/BLAST).
Inoculum preparation
Cultures were grown in 500-mL Erlenmeyer flasks containing 200 mL of standard (Zarrouk’s medium for A. platensis and sea salt BG-11 for Phormidium and Pseudoscillatoria spp.) medium on a rotary shaker at 120 rpm, 25 ± 2 °C, and 80 μmol photons m−2 s−1. Cells were harvested during the exponential growth phase, centrifuged, and pellet washed with physiological solution (0.9% NaCl) to remove nitrate. The recovered cells were then resuspended in nitrogen-free Zarrouk’s and sea salt BG-11 medium and used as inoculum at 10% volume for all experiments.
Characterization of growth and biochemical composition of the strains under different nitrogen sources
The strains were characterized in presence of different nitrogen sources to study their impact on growth and biochemical composition (chlorophyll a (Chl-a), total protein content, PBP). In the present study, Zarrouk’s and BG-11 media were selected as cultivation media since they were reported to be very convenient and effective for A. platensis and other cyanobacteria (Sassano et al. 2007; Manirafasha et al. 2016). The influence of different nitrogen sources was studied by replacing the original nitrogen source (NaNO3) in the Zarrouk’s (29.4 mM) and BG-11 (17.6 mM) media with equimolar concentration of KNO3 and NH4Cl in the experimental flasks, respectively. A set of three 500-mL conical flasks which contain 250 mL respective medium and 10% v/v inoculum for each nitrogen source was prepared and cultivated under previously optimized culture condition temperature of 33 ± 2 °C, irradiance of 44 μmol photons m−2 s−1 (LED CATA, Cool white), and agitation speed of 120 rpm (Khazi et al. 2018) over the period of 12 days. The growth, Chl-a, and phycobiliprotein content were monitored at 2-day intervals and the cultures were harvested after 12 days. High phycocyanin yielded culture biomass was taken for extraction and purification of phycocyanin.
Biomass and specific growth rate determination
Five milliliter of culture suspension was filtered through pre-dried and pre-weighed Whatman GF/C glass microfiber filters (Whatman UK) and washed with distilled water. Dry cell weights (DCWs) were calculated in g L−1 after the membrane was dried at 65 °C to a constant weight.
The specific growth rate (μ) and doubling time (td) were determined at the exponential growth phase of cultures through Chl-a concentration and time (Li et al. 2014) using Eqs. 1 and 2 respectively.
where X2 represents the Chl-a concentration (mg L−1) at time t2 (days) and X1 represents the initial concentration (mg L−1) at time t1 (days).
Chl-a extraction and quantification
Five milliliter of culture was centrifuged for 5 min at 10,000×g. The pellet was suspended in 5 mL methanol (100%) for 20 min in a water bath at 65 °C, followed by centrifugation at 4500×g for 5 min. The optical density of the supernatant was determined at 665 and 750 nm. The Chl-a concentration was calculated with the following equation (Eq. 3) (Demirel et al. 2015).
Total protein quantification
Protein concentration was measured by the Lowry method (Lowry et al. 1951) using bovine serum albumin as a standard.
Total protein content (%) was determined as indicated in Eq. 4:
where P (%) is the percent protein, X is total protein content, and Y is total biomass.
Spectroscopic determination of phycobiliprotein
Phycobiliproteins (PBP) (PC, APC, and PE) were extracted from cultures by the following method. Five milliliter of culture was centrifuged for 5 min at 10,000×g. The pellet was suspended in 5 mL of 100 mM Na phosphate buffer (pH 7). The suspension was sonicated (Bandelin, Sonopuls HD2070) at a frequency of 20 kHz for 2 min then centrifuged at 4500×g for 5 min. The PC, APC, and PE concentration in mg mL−1 was calculated from the optical densities at 562 nm (A562), 652 nm (A652), and 615 nm (A615), using Eqs. 5, 6, and 7 (Bennett and Bogorad 1973). The total phycobiliprotein content was calculated as shown in Eq. 8.
CaCl2 extraction, purification, and SDS-PAGE analysis of C-PC
PC extraction using CaCl2 solution was performed by a modified method (Herrera et al. 1989), in which, 50 mg of lyophilized cell mass was suspended in 5 mL of 1.5% CaCl2 solution and kept overnight (12 h) for extraction and centrifuged at 6000×g for 15 min. Then, the crude extract of PC was fractionated by precipitation with ammonium sulfate first at 30% and then at 70% saturation with continuous stirring for 2 h followed by centrifugation at 6000×g for 15 min and the residue was dissolved in 50 mM Na phosphate buffer (pH 7.0). Afterwards, ammonium sulfate precipitates were subjected to dialysis for overnight against 1 L Na phosphate buffer using dialysis tubing cellulose membrane (Sigma-Aldrich). Dialyzed sample was further purified by passing through Sephadex G-25 media packed in column (3 × 30 cm) pre-equilibrated and eluted with 50 mM Na phosphate buffer (pH 7.0). Collected fractions of size exclusion chromatography were further subjected to DEAE-Sephadex column (3 × 30 cm) and absorbed PC was eluted with a linearly increasing ionic concentration gradient of NaCl from 0.1 to 0.25 M. The purity of the fractions collected after each step was calculated according to the purity ratio (A620/A280) and the fraction showing maximum purity was selected for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
The purity and estimation of molecular weight of PC subunits were determined by SDS-PAGE in a vertical slab gel apparatus (Kuru Gel verti 1010YK, VWR) with 12.5% polyacrylamide slab gel (5% stacking gel) 1 mm thickness, according to the method described by Laemmli (1970). Electrophoresis was performed at 120 V and 20 mA for about 2–3 h at room temperature. After electrophoresis, bands were stained with Coomassie brilliant blue R-250. The sizes of the C-PC subunits bands were determined by comparison with standard PC (Sigma-Aldrich) and protein markers with a range of molecular weight from 7 to 175 kDa (New England BioLabs, USA).
Statistical analysis
All the experiments were conducted in triplicate and the data calculated were expressed as mean ± standard deviation. Statistical significance (p < 0.05) for the data obtained on the 12th day was analyzed using one-way analysis of variance (ANOVA) (biomass, Chl-a, total protein, and phycobiliprotein content as the response variables for the factors NaNO3, KNO3, and NH4Cl). When significant differences among groups were identified, multiple comparisons among means were made using the Tukey post hoc test with IBM SPSS Statistics version 23.0 software.
Results
Identification of cyanobacterial strains
Preliminary morphological observation of cyanobacterial cultures by bright field and scanning electron microscopy (SEM) analysis showed that the isolates (EGEMACC 72 and EGEMACC 74) belonged to the genus Phormidium and Pseudoscillatoria. The cells of isolate EGEMACC 72 were filamentous, filaments unbranched, more or less straight or curved, blue-green, 1.4–2 μm broad, thin layer of sheath, non-lamellated; trichomes 3–3.5 μm broad, trichomes not constricted at cross-walls, not granulate, end cells not attenuated, end cell rounded, and cross-wall not visible (Fig. 1a, c). The cells of isolate EGEMACC 74 were cylindrical, unbranched, blue-green, blunt cells at the ends, and trichomes 2.5–3.5 μm broad and were found to leave a thin mucilaginous trail as they glide, mucilaginous sheath absent (Fig. 1b, d). Furthermore, the isolates were identified based on partial 16 rRNA gene sequences, reviewed with BLASTn. The partial 16S rDNA gene sequence coding for the ribosomal RNA of the strains was sequenced and submitted to GenBank (Accession Numbers: HQ916865, HQ916861). The BLAST analysis of the corresponding sequences showed that the isolate EGEMACC 72 was closely related to Phormidium sp. (HQ916865) with sequence similarity of 92% as well as it was the representative of the genus Phormidium. The comparison of rDNA gene sequence (781 bp) revealed that the isolate EGEMACC 74 was closely related to Pseudoscillatoria sp. (HQ916861) with maximum sequence similarity of 97%.
Variation in growth rate, biomass, and Chl-a concentration with different nitrogen sources
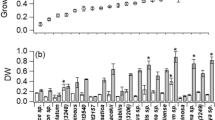
The impact of different nitrogen sources on growth, biomass production, and Chl-a content of cyanobacteria strains, i.e., A. platensis, Phormidium sp., and Pseudoscillatoria sp., were investigated. The time course profiles of the Chl-a concentration obtained in different nitrogen sources are shown in Fig. 2. The brackish and alkaliphilic A. platensis exhibited the fastest growth rate of 0.304 ± 0.0 day−1 in Zarrouk medium containing NaNO3 and reached the highest biomass of 1.56 ± 0.1 g L−1 and Chl-a content of 19.1 ± 0.5 mg L−1. This was followed by biomass of 1.08 ± 0.06 g L−1 and Chl-a of 16.27 ± 0.7 mg L−1 with the growth rate of 0.261 ± 0.0 day−1 in the medium with KNO3. The differences between the growth rate (ANOVA: d.f. = 1, F = 44.431, p = 0.003) and Chl-a (ANOVA: d.f. = 1, F = 31.756, p = 0.005) using NaNO3 and KNO3 were statistically significant. Conversely, the given NH4Cl concentration of 29.42 mM exhibited toxic effect on the growth of A. platensis. The marine isolates Phormidium sp. and Pseudoscillatoria sp. cultured in sea salt BG-11 medium with NH4Cl (17.6 mM) grew better and provided significantly higher biomass (1.225 ± 0.0 and 1.12 ± 0.01 g L−1), Chl-a (16.2 ± 0.5 and 12.2 ± 0.1 mg L−1), and the growth rates (0.284 ± 0.03 and 0.274 ± 0.13 day−1) than that with NaNO3 and KNO3 (Tukey’s post hoc: p = 0.000) (Table 1).
Variation in total protein and PBP content
Total protein profiles of examined cyanobacteria strains cultivated with three nitrogen sources are shown in Table 1. Protein content % (based on DCW) showed a gradual enhancement with the period of incubation for all investigated nitrogen source. The three examined strains had protein contents in the range of 35–45% under investigated culture conditions and nitrogen sources. The alkaliphilic A. platensis exhibited the highest protein content (45.5 ± 0.2% of dry weight) in Zarrouk’s medium with NaNO3 as a nitrogen source, followed by KNO3 (43.4 ± 0.1% of dry weight). Since A. platensis growth inhibition occurred with NH4Cl, the total protein and PBP content measurements were not conducted for these flasks, whereas marine isolates Pseudoscillatoria sp. and Phormidium sp. showed the maximum protein contents of 43.2 ± 0.2 and 38.3 ± 0.1% of dry weight in sea salt BG-11 medium with NH4Cl as nitrogen source. The total protein content of Pseudoscillatoria sp. (38.4 ± 0.1% of dry weight) with NaNO3 as nitrogen source was similar with NH4Cl (38.3 ± 0.1% of dry weight) as nitrogen source for Phormidium sp.
According to Table 1 and Fig. 3, A. platensis cultured in Zarrouk medium with NaNO3 as nitrogen source provided a significantly higher total PBP (22.27 ± 0.2% of dry weight) with the high PC content of 14.53 ± 0.16%, APC of 5.89 ± 0.05%, and PE of 1.84 ± 0.0% of dry weight under provided culture conditions (temperature of 33 ± 2 °C, irradiance of 44 μmol photons m−2 s−1, and agitation speed of 120 rpm) (ANOVA: d.f. = 1, F = 10.515, p = 0.032). This was followed by KNO3 (PBP 21.73 ± 0.19% of dry weight). The total PBP content of two marine strains grown with three different nitrogen sources was significantly different. Pseudoscillatoria sp. cultured in sea salt BG-11 medium with NH4Cl exhibited higher PBP content of 19.99 ± 0.14% with the PC (14.22 ± 0.15), APC (4.4 ± 0.01), and PE (1.36 ± 0.02) % of dry weight than those with other nitrogen sources (Tukey’s post hoc: p = 0.000), whereas Phormidium sp. cultured in sea salt BG-11 with NH4Cl produced 19.38 ± 0.09% PBP with PC (13.36 ± 0.10), APC (4.34 ± 0.06), and PE (1.67 ± 0.06) % of dry weight.
Extraction and purification of phycocyanin
After 12 h incubation of biomass with CaCl2 solution on rotary shaker, the supernatant was collected by centrifugation the purity ratio (A620/A280) of PC in crude extracts was observed as greater than 1 for all the tested samples (Table 2). In order to achieve reactive grade purity, the C-PC from crude extract was further purified by a three-step sequential method consisting of fractional ammonium sulfate precipitation, Sephadex G-25 gel filtration, and DEAE-Sephadex ion-exchange column chromatography. The results of the purification profile of C-PC are summarized in Table 2. The crude extract of PC was fractionated by precipitation with ammonium sulfate (first at 30% and then at 70% saturation). The resulting ammonium sulfate precipitates were dialyzed and the purity (A620/A280) after this step was calculated as greater than 2. The dialyzed samples were further purified by passing through Sephadex G-25 media, which resulted in increased purity ratio greater than 3, and after purification by DEAE-Sephadex, the attained purities of C-PC for A. platensis, Phormidium sp., and Pseudoscillatoria sp. were 4.33, 4.14, and 4.4, respectively.
Purity and molecular weight confirmation
The purity of fractions obtained after DEAE-Sephadex ion-exchange chromatography was confirmed using SDS-PAGE (Fig. 4). The α-PC subunits of A. platensis and Phormidium sp. have the same molecular weight of 18 kDa, whereas the molecular weights of β-subunits were 22 and 21.5 kDa (lanes B, C, and D), respectively. The molecular weights of α- and β-PC subunits of Pseudoscillatoria sp. were slightly different and calculated to be 17.5 and 21 kDa, respectively (lane E).
Discussion
Among the nutrients required for the growth of cyanobacteria, nitrogen is considered as an important element which is required for the growth and synthesis of proteins and other cellular components. Changes in nitrogen source and amount of cultivated media limit the growth and alter their pigment and biochemical composition. Growth on different nitrogen sources had different effects on the growth rate of studied cyanobacteria strains, and there was a significant difference among cultures containing NaNO3 and the other nitrogen sources (KNO3 and NH4Cl) in terms of the final cell density and Chl-a concentration. In the present study, it was found that nitrate salts (NaNO3 and KNO3) were more favorable for the growth of A. platensis than that of ammonium salts. The ammonium chloride (29.42 mM) in the medium changed the A. platensis biomass color from green to yellow and the cells died. Ferreira et al. (2010) also reported the similar problem during A. platensis growth with ammonium (15–16 mM) as nitrogen source. Regarding the mechanism of ammonia toxicity, it has been reported that ammonia affects the oxygen evolving complex (OEC) by displacing a water ligand to the outer manganese cluster of the OEC, leading to loss of PSII function (Drath et al. 2008; Gutierrez et al. 2016; Markou and Muylaert 2016). The growth inhibition/cell lysis of A. platensis with NH4Cl can be attributed to the fact that the ammonium concentration above 10 mM in the medium results in the decrease of pH by releasing H+ ions during ammonium utilization and uncontrolled ammonium influx (Converti et al. 2006; Kim et al. 2016). Therefore, when using ammonium salts as a nitrogen source for A. platensis and for other cyanobacteria, the concentration must be kept low (Ferreira et al. 2010), and the acidification of the medium during growth on NH4Cl should be resolved by increasing the buffering capacity of the growth medium by addition of HEPES-NaOH to the medium (Liotenberg et al. 1996; Sakamoto et al. 1998). In a review article, Collos and Harrison (2014) reported that the optimal ammonium concentration for cyanophyceae was 3000 μM, whereas 13,000 μM concentration was toxic.
On the other hand, the ammonium concentration of 17.6 mM in sea salt BG-11 medium did not show growth inhibitory effect and it was the most favorable nitrogen source for the cell growth and biomass production of Phormidium sp. and Pseudoscillatoria sp. than NaNO3 and KNO3. The higher growth rates and Chl-a contents of these strains with NH4Cl may be due to higher uptake and assimilation rates on ammonia than nitrate. Since ammonium is the reduced form of nitrogen, cell incorporates ammonia directly into the nitrogen metabolism whereas nitrate or nitrite must first be reduced to ammonium prior to its utilization.
According to Madkour et al. (2012), the protein biosynthesis in A. platensis increases with an increase of nitrogen concentration in the cultivation medium, whereas low nitrogen concentrations lead to a decrease in the level of these compounds. In the present study, 29.42 mM concentration of nitrogen source in Zarrouk’s and 17.6 mM in sea salt BG-11 medium yielded the total protein contents of 45.5 ± 0.2 and 43.5 ± 0.2% of dry weight in A. platensis and Pseudoscillatoria sp., respectively. The total percentage of protein was less when compared to other reported cyanobacterial strains. Rizzo et al. (2015) found a protein content value of 59% in A. platensis using Zarrouk’s medium after 18 days of growth under green light at an irradiance of 50 μmol photons m−2 s−1, whereas the present experiments were carried out at 44 μmol photons m−2 s−1 over the period of 12 days, so the obtained protein contents were lower than the results previously reported. The PBP contents of three cyanobacterial strains obtained in the present study was ranging from 16.92 ± 0.2–22.27 ± 0.2% of dry weight, which are remarkable in comparison with previously reported PBP contents of several cyanobacterial strains (8.4 ± 0.4–38.3 ± 1.9% of dry weight) (Khattar et al. 2015). The effect of using different nitrogen sources and their concentrations on PBP of A. platensis and other cyanobacterial strains has also been evaluated by other researchers (Vieira Costa et al. 2001; Madkour et al. 2012; Ajayan et al. 2012). With an increase in nitrogen concentration in the culture medium, PBP content in cyanobacteria increases to a certain extent and further increase does not affect the PBP production. It has been reported that the maximum C-PC content of A. platensis increased from 10% to nearly 12% as the initial nitrogen source concentration rose from 0.03 to 0.045 M, while further increasing the NaNO3 concentration did not enhance C-PC content or productivity (Chen et al. 2013). In contrary, nitrogen limitation in the medium triggers the degradation of PBP, since PBP serves as a nitrogen storage compound (Boussiba and Richmond 1980). Therefore, the availability of sufficient nitrogen is known to be an important factor for PBP production in cyanobacteria. In the present study, 29.42 mM concentration of NaNO3 in Zarrouk’s medium and 17.6 mM concentration of NH4Cl in sea salt BG-11 medium exhibited better results on PBP production of A. platensis (22.27 ± 0.21% of dry weight) and marine isolates Phormidium and Pseudoscillatoria (19.38 ± 0.09 and 19.99 ± 0.14% of dry weight).
PC is the main PBP in A. platensis and comprises up to 20% of its dry weight (Cuellar-Bermudez et al. 2015). The PC values found in the present study ranged from 12.1 ± 0.08 to 14.5 ± 0.16% of dry weight under different nitrogen sources evaluated. Similar to our findings, Ajayan et al. (2012) reported the PC values of A. platensis in the range of 9.92 ± 0.13–14.81 ± 0.2% which were obtained with KNO3 and urea as a nitrogen source, respectively. The three strains of Arthrospira were analyzed for their PC content by Bhattacharya and Shivaprakash (2005) and values obtained were within the range of 9.4–14.8%. The results of the present study demonstrated that PC content changed markedly depending on the nitrogen source. The PC content of Phormidium and Pseudoscillatoria sp. cells grown with ammonium was 5–15% higher than those cultured with nitrate salts. Similar to our results, phycobilisomes isolated from ammonium-grown Synechococcus sp. PCC 7002 cultures contained 10–20% more PC than those grown with nitrate (de Lorimier et al. 1992). The nitrogen source in culture medium markedly influenced the PE and PC content of the Calothrix sp. and observed that PE was 35% lower in ammonium compared to nitrate-grown cells, whereas PC was 46% higher (Liotenberg et al. 1996). In the present study, although the PC content was significantly higher in A. platensis compared with two marine strains, it is also important to consider the cost of nitrogen and other nutrients for developing cost-effective algal pigment production. To overcome high production costs, cheap resources should be found. Our study evaluated the possibility of using ammonium salts by marine strains, which were cheaper than nitrate salt. Therefore, Phormidium sp. and Pseudoscillatoria sp. could be a good candidate for large-scale PC production.
Since the maximum PBP yield was found in the cultures of Phormidium sp. and Pseudoscillatoria sp. with NH4Cl and A. platensis grown with NaNO3 and KNO3, these culture biomasses were selected to carry out extraction and purification steps. Many methods for PC extraction and purification have been described in the literature (de Jesús et al. 2016; Eriksen 2008). The efficiency of PC extraction may vary depending on the properties of the buffer, the duration of the extraction, biomass concentration, mode of cell disruption (physical/chemical cell disruption), and species of cyanobacteria. Therefore, no standard procedure was available for maximal extraction of PC from cyanobacterial cells (Sobiechowska-Sasim et al. 2014; Horváth et al. 2013). Among the various extraction methods, freezing and thawing was reported to be the most efficient method for PC extraction (Soni et al. 2006), whereas using ultrasonic bath in the presence of glass pearls in the A. platensis biomass has been reported to have 57% higher extraction efficiency than freezing and thawing method (Moraes et al. 2011). However, all these methods involved cell disruption which needs to supply some extra energy to promote cell breakage. In addition, mechanical methods of disintegration release a large number of contaminants from the cells which resulted in high viscosity and low purity in the crude extract. To circumvent these problems, we tested the extraction method using calcium chloride 1.5% salt solution without applying any cell disruption methods. The commercial use of PC in the food does not require high purity. Therefore, CaCl2 utilized in this study was found to be an effective salt solution to attain food grade purity (A620/A280 ratio greater than 0.7) in crude extracts. Various methods have been reported for the purification of PC from cyanobacteria but all these methods involve the combination of chromatographic and non-chromatographic methods such as aqueous two-phase extraction (Patil et al. 2008), ultrafiltration (Singh et al. 2009), expanded bed chromatography (Moraes et al. 2015; Jian-Feng et al. 2007), hydroxyapatite chromatography, ammonium sulfate precipitation, ion-exchange, and gel permeation chromatography (Zhang and Chen 1999; Kumar et al. 2014). Patil et al. (2008) employed a non-chromatographic (aqueous two-phase extraction) method to purify PC from A. platensis and achieved the final purity of 3.23, whereas with the combination of aqueous two-phase extraction and ion-exchange chromatography, the purity of 6.69 was achieved from A. platensis (Patil et al. 2006). Similarly, Singh et al. (2009) combined ultrafiltration and ion-exchange chromatography to obtain C-PC with A620/A280 of 4.1 from Phormidium ceylanicum. Chakdar et al. (2014) reported the purity of 2.75 from Anabaena variabilis CCC421 after precipitation with ammonium sulfate and DEAE-cellulose ion-exchange chromatography; however, this method does not satisfy the standard of reactive or analytical grade purity (A620/A280 ratio greater than 3.9 and 4). Depending on the application of PC, the method should be selected based on purification need. In the present study, our procedures having shown efficient separation and purification of C-PC from A. platensis, Phormidium sp., and Pseudoscillatoria sp. with purity ratio greater than 4 satisfy the standard for analytical grade. Therefore, this purification procedure was found to be suitable for small scale and an alternative for industrial scale.
In conclusion, our results showed that three different nitrogen sources had different effects on the growth and PBP accumulation in A. platensis, Phormidium sp., and Pseudoscillatoria sp. Among the three nitrogen sources, NH4Cl was found as a favorable source for growth and PC production of Phormidium and Pseudoscillatoria sp., whereas NaNO3 was found as an optimal nitrogen source for A. platensis. Calcium chloride utilized in the present study was found to be an effective salt solution to extract C-PC and achieve food grade purity (A620/A280 ratio greater than 0.7) in crude extracts, while reactive/analytic grade purity (A620/A280 ratio greater than 3.9) was achieved after Sephadex and DEAE-Sephadex ion-exchange chromatography. The strains Phormidium sp. and Pseudoscillatoria sp. isolated in the present study may prove to be promising cyanobacteria for C-PC production.
References
Ajayan KV, Selvaraju M, Thirugnanamoorthy K (2012) Enrichment of chlorophyll and phycobiliproteins in Spirulina platensis by the use of reflector light and nitrogen sources: an in-vitro study. Biomass Bioenergy 47(Supplement C):436–441
Amchova P, Kotolova H, Ruda-Kucerova J (2015) Health safety issues of synthetic food colorants. Regul Toxicol Pharmacol 73:914–922
Asencio AD, Hoffmann L (2013) Chemosystematic evaluation of the genus Scytonema (Cyanobacteria) based on occurrence of phycobiliproteins, scytonemin, carotenoids and mycosporine-like amino acid compounds. Eur J Phycol 48:331–344
Baer S, Heining M, Schwerna P, Buchholz R, Hübner H (2016) Optimization of spectral light quality for growth and product formation in different microalgae using a continuous photobioreactor. J Algal Res 14:109–115
Begum H, Yusoff FM, Banerjee S, Khatoon H, Shariff M (2016) Availability and utilization of pigments from microalgae. Crit Rev Food Sci Nutr 56:2209–2222
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Bhattacharya S, Shivaprakash M (2005) Evaluation of three Spirulina species grown under similar conditions for their growth and biochemicals. J Sci Food Agric 85:333–336
Bourrelly P (1970) Les Algues d'eau douce: Tome III: Les algues bleues et rouges, les Eugléniens, Peridiniens et Cryptomonadines Société nouvelle des Éditions Boubée, Paris
Boussiba S, Richmond AE (1980) C-phycocyanin as a storage protein in the blue-green alga Spirulina platensis. Arch Microbiol 125:143–147
Buchweitz M (2016) Natural solutions for blue colors in food. In: Handbook on natural pigments in food and beverages. Woodhead Publishing, pp 355–384
Chakdar H, Saha S, Pabbi S (2014) Chromatographic and spectroscopic characterization of phycocyanin and its subunits purified from Anabaena variabilis CCC421. Appl Biochem Microbiol 50:62–68
Chen H-B, Wu J-Y, Wang C-F, Fu C-C, Shieh C-J, Chen C-I, Wang C-Y, Liu Y-C (2010) Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem Eng J 53:52–56
Chen C-Y, Kao P-C, Tsai C-J, Lee D-J, Chang J-S (2013) Engineering strategies for simultaneous enhancement of C-phycocyanin production and CO2 fixation with Spirulina platensis. Bioresour Technol 145:307–312
Choi A, Kim S-G, Yoon B-D, Oh H-M (2003) Growth and amino acid contents of Spirulina platensis with different nitrogen sources. Biotechnol Bioprocess Eng 8:368–372
Collos Y, Harrison PJ (2014) Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar Pollut Bull 80:8–23
Converti A, Scapazzoni S, Lodi A, Carvalho J (2006) Ammonium and urea removal by Spirulina platensis. J Ind Microbiol Biotechnol 33:8–16
Costa MS, Rego A, Ramos V, Afonso TB, Freitas S, Preto M, Lopes V, Vasconcelos V, Magalhães C, Leão PN (2016) The conifer biomarkers dehydroabietic and abietic acids are widespread in Cyanobacteria. Sci Rep 6(23436). https://doi.org/10.1038/srep23436
Cuellar-Bermudez SP, Aguilar-Hernandez I, Cardenas-Chavez DL, Ornelas-Soto N, Romero-Ogawa MA, Parra-Saldivar R (2015) Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb Biotechnol 8:190–209
Dasgupta CN (2015) Algae as a source of phycocyanin and other industrially important pigments. In: Algal biorefinery: an integrated approach. Springer, Cham pp 253–276
de Jesús VC, Gutiérrez-Rebolledo GA, Hernández-Ortega M, Valadez-Carmona L, Mojica-Villegas A, Gutiérrez-Salmeán G, Chamorro-Cevallos G (2016) Methods for extraction, isolation and purification of C-phycocyanin: 50 years of research in review. Int J Food Nutr Sci 3(3):1
de Lorimier RM, Smith RL, Stevens SE (1992) Regulation of phycobilisome structure and gene expression by light intensity. Plant Physiol 98(3):1003–1010
Demirel Z, Imamoglu E, Conk Dalay M (2015) Fatty acid profile and lipid content of Cylindrotheca closterium cultivated in air-lift photobioreactor. J Chem Technol Biotechnol 90:2290–2296
Deschoenmaeker F, Bayon-Vicente G, Sachdeva N, Depraetere O, Cabrera Pino JC, Leroy B, Muylaert K, Wattiez R (2017) Impact of different nitrogen sources on the growth of Arthrospira sp. PCC 8005 under batch and continuous cultivation—a biochemical, transcriptomic and proteomic profile. Bioresour Technol 237(Suppl C):78–88
Desikachary TV (1959) Cyanophyta. Indian Council of Agriculture Research, New Delhi
Drath M, Kloft N, Batschauer A, Marin K, Novak J, Forchhammer K (2008) Ammonia triggers photodamage of photosystem II in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol 147:206–215
Encarnação T, Pais AA, Campos MG, Burrows HD (2015) Cyanobacteria and microalgae: a renewable source of bioactive compounds and other chemicals. Sci Prog 98:145–168
Eriksen NT (2008) Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14
Fatma T (2009) Screening of cyanobacteria for phycobiliproteins and effect of different environmental stress on its yield. Bull Environ Contam Toxicol 83:509–515
Ferreira LS, Rodrigues MS, Converti A, Sato S, Carvalho JCM (2010) A new approach to ammonium sulphate feeding for fed-batch Arthrospira (Spirulina) platensis cultivation in tubular photobioreactor. Biotechnol Prog 26:1271–1277
Flores E, Frías JE, Rubio LM, Herrero A (2005) Photosynthetic nitrate assimilation in cyanobacteria. Photosynth Res 83:117–133
Glazer AN, Apell GS (1977) A common evolutionary origin for the biliproteins of cyanobacteria rhodophyta and cryptophyta. FEMS Microbiol Lett 1:113–116
Gupta A, Sainis JK (2010) Isolation of C-phycocyanin from Synechococcus sp., (Anacystis nidulans BD1). J Appl Phycol 22:231–233
Gutierrez J, Kwan TA, Zimmerman JB, Peccia J (2016) Ammonia inhibition in oleaginous microalgae. J Algal Res 19:123–127
Herrera A, Boussiba S, Napoleone V, Hohlberg A (1989) Recovery of c-phycocyanin from the cyanobacterium Spirulina maxima. J Appl Phycol 1:325–331
Herrero A, Muro-Pastor AM, Flores E (2001) Nitrogen control in cyanobacteria. J Bacteriol 183:411–425
Horváth H, Kovács AW, Riddick C, Présing M (2013) Extraction methods for phycocyanin determination in freshwater filamentous cyanobacteria and their application in a shallow lake. Eur J Phycol 48:278–286
Jian-Feng N, Guang-Ce W, Lin X-Z, Zhou B-C (2007) Large-scale recovery of C-phycocyanin from Spirulina platensis using expanded bed adsorption chromatography. J Chromatogr B 850:267–276
Johnson EM, Kumar K, Das D (2014) Physicochemical parameters optimization, and purification of phycobiliproteins from the isolated Nostoc sp. Bioresour Technol 166:541–547
Khattar JIS, Kaur S, Kaushal S, Singh Y, Singh DP, Rana S, Gulati A (2015) Hyperproduction of phycobiliproteins by the cyanobacterium Anabaena fertilissima PUPCCC 410.5 under optimized culture conditions. J Algal Res 12:463–469
Khazi M, Demirel Z, Conk DM (2018) Enhancement of biomass and phycocyanin content of Spirulina platensis. Front Biosci 10:276–286
Kim G, Mujtaba G, Lee K (2016) Effects of nitrogen sources on cell growth and biochemical composition of marine chlorophyte Tetraselmis sp. for lipid production. Algae 31:257–266
Kirilovsky D (2015) Modulating energy arriving at photochemical reaction centers: orange carotenoid protein-related photoprotection and state transitions. Photosynth Res 126:3–17
Kuddus M, Singh P, Thomas G, Al-Hazimi A (2013) Recent developments in production and biotechnological applications of C-phycocyanin. Biomed Res Int 1(9). doi:https://doi.org/10.1155/2013/742859
Kumar D, Dhar DW, Pabbi S, Kumar N, Walia S (2014) Extraction and purification of C-phycocyanin from Spirulina platensis (CCC540). Indian J Plant Physiol 19:184–188
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee S-H, Lee JE, Kim Y, Lee S-Y (2016) The production of high purity phycocyanin by Spirulina platensis using light-emitting diodes based two-stage cultivation. Appl Biochem Biotechnol 178:382–395
Lee NK, Oh H-M, Kim H-S, Ahn C-Y (2017) Higher production of C-phycocyanin by nitrogen-free (diazotrophic) cultivation of Nostoc sp. NK and simplified extraction by dark-cold shock. Bioresour Technol 227:164–170
Li Y, Lin Y, Loughlin PC, Chen M (2014) Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris—a filamentous cyanobacterium containing chlorophyll f. Front Plant Sci 5:67
Liotenberg S, Campbell D, Rippka R, Houmard J, de Marsac NT (1996) Effect of the nitrogen source on phycobiliprotein synthesis and cell reserves in a chromatically adapting filamentous cyanobacterium. Microbiology 142:611–622
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Madkour FF, Kamil AE-W, Nasr HS (2012) Production and nutritive value of Spirulina platensis in reduced cost media. Egypt J Aquat Res 38:51–57
Manirafasha E, Ndikubwimana T, Zeng X, Lu Y, Jing K (2016) Phycobiliprotein: potential microalgae derived pharmaceutical and biological reagent. Biochem Eng J 109:282–296
Markou G, Muylaert K (2016) Effect of light intensity on the degree of ammonia toxicity on PSII activity of Arthrospira platensis and Chlorella vulgaris. Bioresour Technol 216:453–461
Montalvão S, Demirel Z, Devi P, Lombardi V, Hongisto V, Perälä M, Hattara J, Imamoglu E, Tilvi SS, Turan G (2016) Large-scale bioprospecting of cyanobacteria, micro-and macroalgae from the Aegean Sea. New Biotechnol 33:399–406
Moraes CC, Sala L, Cerveira GP, Kalil SJ (2011) C-phycocyanin extraction from Spirulina platensis wet biomass. Braz J Chem Eng 28:45–49
Moraes CC, Sala L, Ores JC, Braga ARC, Costa JAV, Kalil SJ (2015) Expanded and fixed bed ion exchange chromatography for the recovery of C-phycocyanin in a single step by using lysed cells. Can J Chem Eng 93:111–115
Patil G, Chethana S, Sridevi AS, Raghavarao KSMS (2006) Method to obtain C-phycocyanin of high purity. J Chromatogr 1127:76–81
Patil G, Chethana S, Madhusudhan MC, Raghavarao KSMS (2008) Fractionation and purification of the phycobiliproteins from Spirulina platensis. Bioresour Technol 99:7393–7396
Rasoulouniriana D, Siboni N, Ben-Dov E, Kramarsky-Winter E, Loya Y, Kushmaro A (2009) Pseudoscillatoria coralii gen. nov., sp. nov., a cyanobacterium associated with coral black band disease (BBD). Dis Aquat Org 87:91–96
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Rizzo RF, BdNCd S, GFPdSd C, Passos TS, Nascimento MA, Guerra HD, CGd S, Dias DS, Domingues JR, KGd L-A (2015) Production of phycobiliproteins by Arthrospira platensis under different lightconditions for application in food products. Food Sci Technol (Campinas) 35(2):247–252
Romay C, Gonzalez R, Ledon N, Remirez D, Rimbau V (2003) C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci 4:207–216
Rückert GV, Giani A (2004) Effect of nitrate and ammonium on the growth and protein concentration of Microcystis viridis Lemmermann (Cyanobacteria). Braz J Bot 27:325–331
Sakamoto T, Delgaizo VB, Bryant DA (1998) Growth on urea can trigger death and peroxidation of the cyanobacterium Synechococcus sp. strain PCC 7002. Appl Environ Microbiol 64:2361–2366
Sassano C, Gioielli L, Almeida K, Sato S, Perego P, Converti A, Carvalho J (2007) Cultivation of Spirulina platensis by continuous process using ammonium chloride as nitrogen source. Biomass Bioenergy 31:593–598
Simeunović J, Bešlin K, Svirčev Z, Kovač D, Babić O (2013) Impact of nitrogen and drought on phycobiliprotein content in terrestrial cyanobacterial strains. J Appl Phycol 25:597–607
Singh NK, Parmar A, Madamwar D (2009) Optimization of medium components for increased production of C-phycocyanin from Phormidium ceylanicum and its purification by single step process. Bioresour Technol 100:1663–1669
Singh NK, Sonani RR, Rastogi RP, Madamwar D (2015) The phycobilisomes: an early requisite for efficient photosynthesis in cyanobacteria. EXCLI J 14:268
Sobiechowska-Sasim M, Stoń-Egiert J, Kosakowska A (2014) Quantitative analysis of extracted phycobilin pigments in cyanobacteria—an assessment of spectrophotometric and spectrofluorometric methods. J Appl Phycol 26:2065–2074
Soni B, Kalavadia B, Trivedi U, Madamwar D (2006) Extraction, purification and characterization of phycocyanin from Oscillatoria quadripunctulata—isolated from the rocky shores of Bet-Dwarka, Gujarat, India. Process Biochem 41:2017–2023
Vieira Costa JA, Cozza KL, Oliveira L, Magagnin G (2001) Different nitrogen sources and growth responses of Spirulina platensis in microenvironments. World J Microbiol Biotechnol 17:439–442
Zarrouk C (1966) Contribution l'étude d'une cyanophycée. Influence de divers facteurs physiques et chimiques sur la croissance et la photosynthése de Spirulina maxima. Thesis, Universite de Paris, Paris
Zhang Y-M, Chen F (1999) A simple method for efficient separation and purification of c-phycocyanin and allophycocyanin from Spirulina platensis. Biotechnol Tech 13:601–603
Acknowledgments
The authors wish to thank Dr. Esra Imamoglu and Fakhra Liaqat who assisted in the proofreading of the manuscript. Authors would also like to thank the reviewers for their insightful comments that helped improve our paper.
Funding
This work was financially supported by Ege University Scientific Research Projects (BAP) Project No. 16-FBE-005. Also thanks to the Turkish Government for providing scholarship to pursue this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khazi, M.I., Demirel, Z. & Dalay, M.C. Evaluation of growth and phycobiliprotein composition of cyanobacteria isolates cultivated in different nitrogen sources. J Appl Phycol 30, 1513–1523 (2018). https://doi.org/10.1007/s10811-018-1398-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1398-1