Abstract
A green microalga, Acutodesmus sp., a close relative of Acutodesmus deserticola, was isolated from the wastewater discharges of an oil refinery in India. This study examined the effects of light intensity, temperature, pH, and high-CO2 treatments (up to 20 %) on the growth of the alga and investigated the effects of different CO2 treatments on its macromolecular composition (protein, carbohydrate, and lipids). Under controlled laboratory conditions, the alga showed high growth rates over a wide range of light (up to 700 μmol photons m−2 s−1), temperature (up to 40 °C), and pH (5–10) conditions. In the stationary phase, the highest protein and carbohydrate content was found to be 71.52 and 40.72 % of dry weight at 5 and 15 % CO2, respectively. After 5 days of cultivation, the maximum dry weight biomass attained in these cultures was 1.149, 1.99, 1.75, and 1.65 g L−1 at 5, 10, 15, and 20 % CO2, respectively, indicating that this strain has significant tolerance to CO2. These results indicate that this strain is a promising candidate for use in biofixation of CO2 from the flue gases emitted by industries, and it also has a strong potential as a feedstock for value-added substances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing industrialization is driving an increase in CO2 concentrations in the atmosphere. Around half of the total anthropogenic CO2 emitted each year accumulates in the atmosphere, and the rest is deposited in terrestrial and oceanic sinks (Ritschard 1992; Ramaraj et al. 2014). This increasing accumulation of atmospheric CO2 is a major cause of global warming, which causes significant problems in the functioning of ecosystems and has a potential impact on global economics and on society. Therefore, the amelioration of CO2 outputs is of high priority.
Numerous technologies are available for CO2 capture and fixation, which include absorption, adsorption, mineral sequestration, oceanic sequestration, and geological sequestration (Bachu 2008; Centi and Perathoner 2011; Mondal et al. 2012). However, these approaches are expensive, consume energy, and present other significant challenges. Some of these challenges are non-renewability of the adsorbent materials (e.g., LiOH), a high space requirement for storage, potential leakage of CO2 over time (Kumar et al. 2010; Seth and Wangikar 2015) and, in the case of oceanic nutrient enrichment and CO2 sequestration, possible negative impacts on the functioning of the ecosystem (Glibert et al. 2008). For CO2 amelioration, we need systems that are less complex, require small capital investment, and have low operating costs; the systems also need to be affordable by small-scale CO2 generators. Hence, biological processes that involve algal photosynthesis for CO2 utilization have attracted great attention as strategic alternatives that are both environmentally sustainable and economically feasible. Algal assimilation of CO2 will not lead to sequestration of C but will potentially ameliorate rising CO2 levels if the use of the products of fixation allows by-passing of the use of fossil fuels.
Microalgae and cyanobacteria are oxygenic phototrophs present in many habitats on earth. For more than 75 years, they have played a role in wastewater treatment and have been used in the production of bioactive compounds through the mass cultivation of some genera such as Dunaliella, Spirulina, Chlorella, and Haematococcus (Vonshak and Richmond 1988; Ben-Amotz and Avron 1990; Spolaore et al. 2006; Gouveia et al. 2007). The mass culture of algae now holds an important position as a potential candidate in the development of third-generation biofuels and as a major player in CO2 sequestration. Such cultures have the potential for higher photosynthetic rates (photon conversion efficiency) compared to even the fastest-growing terrestrial plants or crops, which leads to 10–50 times more efficiency in biofixation of CO2 (Cheah et al. 2015; Lara-Gil et al. 2014). Though there are contradictory reports which suggests that algal mass cultures are less, not more, productive than terrestrial plants (Walker 2009), algae still have an edge over higher plants, as they do not require high-value arable land, can be harvested more frequently, and can efficiently accumulate large quantities of lipids (for biodiesel), carbohydrates (for bioethanol), proteins, and number of other bioactive compounds such as antiviral and antibacterial agents (Ho et al. 2012; Miranda et al. 2012).
Our research focuses on isolating and characterizing microalgae that can efficiently convert CO2 emissions, which are present in industrial flue gases, into organic matter. Numerous research studies that discuss either CO2-mitigation ability or thermotolerance or high-light tolerance of microalgae have been reported to date. However, these have largely ignored the combined effects of all these extreme conditions, which can be the actual situation in the commercial application of microalgae; for example, power-plant flue gases not only contain high concentrations of CO2 but also have high temperatures, up to 40–50 °C (after desulfurization) (Basu et al. 2013). As part of our research, we are focusing on petroleum industry emissions. The petroleum refining process typically produces and disposes of large volumes of gases, of which CO2 is a major component. Not all algae can tolerate many of these gases; even CO2 can inhibit the growth of many species at the high concentrations found in flue gases (Cheng et al. 2006; Rahaman et al. 2011). Assuming that microalgae growing in ponds situated in the locality of these oil refineries are tolerant to the environmental conditions prevalent in these areas, any process that is developed using these local strains will be independent of outside strain suppliers and such species or strains would be well adapted to the particular cultivation conditions present in the vicinity of the refinery plant. Hence, we screened and isolated an alga from a refinery wastewater pond, which showed a combination of high-temperature, high-light, and high-CO2 tolerance. We then examined the macromolecular composition of this alga under different CO2 treatments and compared our results with previously reported literature.
Materials and methods
Isolation of algal species
Water samples were collected from the guard pond present in the effluent treatment unit of an oil refinery located in the northern part of India (27° 28′ N, 77° 41′ E). This pond stores water from the different units of the petroleum refining process, including domestic sewage water from the refinery township. Samples were collected, in the month of February, during the peak of the day when the atmospheric temperature was close to 21 °C, stored in plastic bottles, transferred to the laboratory, and processed within 24 h of their collection. Since the pond was open to the air, CO2 concentrations were assumed to be in equilibrium with that in air (404 ppm) and the pH was 6.2.
Five mL of each water sample was transferred to a 250-mL conical flask that contained 50 mL of autoclaved Bold’s Basal (BB) medium that was prepared according to the medium recipe reported by CSIRO Marine and Atmospheric Research, Australia (http://www.marine.csiro.au/microalgae/methods/Media%20CMARC%20recipes.htm#BB). The flask was incubated in a shaker with an agitation speed of 125 rpm at 37 °C and 1 % CO2, under continuous illumination of 250 μmol photons m−2 s−1. After 5 days of incubation, an aliquot of 2 mL (pre-cultured sample) was transferred to another 250 mL conical flask that contained a fresh 50-mL BB medium, supplemented with 25 μL of 40 mg mL−1 of the antibiotic agent neomycin sulfate and 5 μL of 0.5 g mL−1 (ethanol suspension) of the antifungal agent, nystatin. After 1 week of incubation, from this enrichment culture, serial dilutions were made in BB medium, and 100 μL from 10−3, 10−4, and 10−5 dilution tubes was spread onto Petri plates that contained BB medium solidified with 2 % Bacto-Agar. The plates were incubated under the same light, temperature, and CO2 conditions for 8 days. Single colonies were picked from each agar plate and streaked on new plates; this process was repeated until a pure unialgal culture was obtained. Purity was checked through microscopic observation. Also, the presence of any bacterial contamination was tested by regularly spreading 100 μL of cultures on Luria agar (LA) plates and incubating them at 37 °C for 48 h. Cultures showing bacterial growth were discarded.
Two green algae were isolated. They were sent to GeneOmbio Technologies Pvt. Ltd., Pune, India, for species identification through 18s rRNA gene sequencing. Homology study of the 18 S rRNA gene sequence of the strains was carried out using National Center for Biotechnology Information (NCBI) nucleotide BLAST searches, and the neighbor-joining method (based on distance matrix data) was used to construct the phylogenetic tree (data not shown) using the MEGA 6.0 software package. One of these strains was identified as Acutodesmus sp. (see “Results”) and was used in the experiments described here.
Studies under different experimental conditions
To determine the effects of varying culture conditions on the growth kinetic parameters of Acutodesmus sp., experiments were performed under various conditions of light, temperature, pH, and CO2 concentrations, and the optimal conditions necessary for proliferation and CO2 fixation by this alga were obtained.
Acclimatization of the inoculum under the same conditions as were used in experiments is important in order to reduce the adaptation (lag) phase during the experiments (Yun et al. 1997; de Morais and Costa 2007). Hence, we always provided our alga two cycles of acclimatization under the same conditions (3 days each) before starting each experiment. All experiments were run with three independent replicate cultures, until the stationary phase of growth was reached.
Batch cultures for light, temperature, and pH tolerance studies were grown in a Photon System Instruments (PSI, Czech Republic) Multi-Cultivator MC 1000-OD, which contains eight 100 mL cultivation vessels, all immersed in a temperature-controlled water bath under controlled light, and aeration conditions. Each vessel can have an independent and different light intensity and the growth of cultivated organisms can be measured in the culture vessels by optical density at wavelengths of 680 and 720 nm. For our experiments, the initial OD was set at 0.1 at 720 nm and the cultures were aerated with filtered ambient air at constant and equal airflow rates in all the vessels. To examine the effect of light, we cultivated our Acutodesmus strain under different light intensities, that is, 50, 100, 200, 300, 400, 500, and 700 μmol photons m−2 s−1. For temperature variation studies, the alga was cultivated at five different temperatures, that is, 23.5, 30, 37, 40, and 43 °C under an optimal light intensity obtained from the previous experiment. The effect of pH was studied under optimal light and temperature conditions in the range of pH 4–11. We used three different biological buffers—MES (pH range 5.5–6.7), HEPES (pH range 6.8–8.2), and CHES (pH range 8.6–10.0)—in order to maintain a constant pH during this experiment and to see the actual effect of pH on the growth of the microalga. The buffer concentration was maintained at 20 mM in each medium.
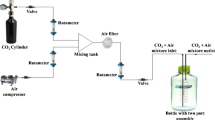
Industrial flue gases typically contain 12–20 % CO2. Therefore, we tested the effect of different CO2 concentrations between 0.04 % (ambient) and 20 % on our alga, by conducting CO2 tolerance experiments as batch cultures in 500 mL Erlenmeyer flasks that contained 200 mL of working media. The initial cell concentration was 5 × 104 cells mL−1. Flasks were incubated in a Kuhner LT XC (Lab Therm) incubator shaker at 37 °C under continuous illumination of 250 μmol photons m−2 s−1 with cool white fluorescent light and at an agitation speed of 125 rpm. The pH of the cultures was monitored at the end of each experiment, that is, at 120 h. Different CO2 levels were applied by replacing the atmosphere in the incubator with the requisite CO2 concentration and maintained with the help of in-built NDIR (nondispersive infrared) CO2 sensors.
Determination of cell concentration, dry cell weight, and growth kinetic parameters
The biomass of the cultures grown in the PSI Multi-Cultivator was monitored by measuring the built-in capacity to measure optical density at 720 nm. For the CO2 experiments, the optical density of sub-samples of cultures was measured at 730 nm.
The dry cell weight (DCW) was measured at the end of each experiment by filtering 50 mL algal suspension through a pre-weighed dried filter paper (Whatman CF/C, diameter 47 mm). The loaded filter paper was washed twice with distilled water to remove any dissolved solutes from residual medium, dried in an oven at 100 °C for 70 min, and reweighed. The dry weight of the blank filter was then subtracted from the filters with biomass to obtain the final algal biomass in g L−1.
The time course profiles of optical density were used to determine the maximum specific growth rate (μ max, day−1) from exponential regression of the logarithmic portion of the growth curve versus time.
Determination of biochemical composition
Microalgal composition in terms of protein, carbohydrate, and lipids was measured for each CO2 treatment. In each experiment, samples were collected at two points in time during the growth curve: once at 72 h (late exponential phase) and another at 120 h (late stationary phase), and all the measurements were done on triplicate cultures.
Carbohydrate analysis
The total sugar content was determined using the phenol sulfuric acid method of Dubois et al. (1956) which was modified to suit our experiment. Culture of 1.0 OD at 730 nm was collected and centrifuged at 6800×g for 10 min at 4 °C. The supernatant was discarded and the pellet was washed twice with deionized water to remove any medium constituents. The pellet was resuspended in deionized water, and 0.2 mL of this sample was used for analysis. Different sugar concentrations (dextrose; 0–0.1 mg mL−1) were used to prepare standards. Phenol (0.2 mL of a 5 % w/v solution, in water) was then added to the samples and to the standards. Without mixing, 1 mL of concentrated sulfuric acid was added, and the tubes were allowed to stand for 10 min at room temperature and were then mixed gently. The samples were incubated for 30 min in a water bath maintained at 35 °C and the absorbance was measured at 490 nm.
Protein analysis
The white pellet obtained after ethanol extraction of cells was used for protein determination by the Folin–Lowry method (Lowry et al. 1951). The pellet was resuspended in 500 μL of 1 % SDS/0.1 M NaOH. Reagent A (500 μL of 1:1:1:1 ratio of CTC (CTC: 0.1 % CuSO4·5H2O + 0.2 % NaK tartrate +10 % Na2CO3), 10 % SDS, 0.8 M NaOH and dH2O) was added and mixed well with the resuspended pellet, and the tubes were allowed to stand for 10 minutes at room temperature. Reagent B (250 μL of a solution of one volume of Folin-Ciocalteu reagent and five volumes of dH2O) was added to the samples; the tubes were immediately vortexed and then allowed to stand for 30 minutes at room temperature. The absorbance was measured at 750 nm. The amount of total protein in the sample was calculated from a standard curve made with different concentrations of bovine serum albumin (BSA) dissolved in 1 % SDS/0.1 M NaOH (0–1.0 mg mL−1); 0.5 mL of 1 % SDS/0.1 M NaOH, treated as per the sample, was used as a blank.
Neutral lipid analysis using an improved Nile Red method
Nile Red (9-(diethylamino)-5H-benzo[a]phenoxazin-5-one) is a lipophilic fluorescent dye used to quantify intracellular neutral lipids present in prokaryotic and eukaryotic cells. However, most of the green algae, including Scenedesmus, have a thick and rigid cell wall, which may inhibit the penetration of Nile Red to the cell and its binding with intracellular lipids to give the required fluorescence (Ren et al. 2013). Several studies have used dimethyl sulfoxide (DMSO) as a stain carrier, which probably affects the integrity of cell wall and plasmalemma and improves the diffusion of Nile Red (Chen et al. 2009; Doan and Obbard 2011). Therefore, for our measurements, we re-examined the original technique with regards to DMSO concentration, Nile Red concentration, staining time, and algal cell concentration using our species as a model organism in order to improve the lipid-staining efficiency.
We tested a range of cell concentrations from 5 × 104 to 1 × 106 cells mL−1 and obtained a linear relationship between cell concentration and fluorescence intensity for all concentrations up to 5 × 105 cells mL−1. Therefore, in order to maintain consistency, we used a cell density of 5 × 105 cells mL−1 for all our measurements; 200 μL of cell suspension was added to the wells of a 96-well micro-titre plate. Nile Red dye (0.8 μL of a solution 0.25 mg mL−1 in 100 % acetone) was added to each well followed by 1.5 μL of pure DMSO. The plate was placed in the fluorescence plate reader (Tecan plate reader, Infinite M200 Pro) and shaken for 30 minutes. The excitation and emission wavelengths were set at 480 and 570 nm, respectively. An unstained algal suspension was used as a blank and the relative fluorescence intensity of Nile Red was attained after subtraction of both the autofluorescence of microalgae and the self-fluorescence of Nile Red.
Statistical analysis
Statistical significance of the differences among means in relation to CO2 treatments was analyzed using repeated-measures (RM) one-way analysis of variance (ANOVA) with a Greenhouse–Geisser correction, followed by a Tukey multiple-comparison test. Values of P ≤ 0.05 were considered statistically significant.
Results
Isolation and identification of the microalgae
One of the two strains isolated was identified as Acutodesmus sp., belonging to the subgenus of Scenedesmus, a member of the Scenedesmaceae. Based on electron microscopy studies, the genus Acutodesmus is characterized by longitudinal or net-like ridges produced by inner cellulosic cell walls. Further, NCBI BLAST searches confirmed that the obtained sequence was homologous to Acutodesmus deserticola (Hegewald et al. 2013; Lewis and Flechtner 2004), and the strain was then used in the experiments reported here.
Effect of light on growth of Acutodesmus
The highest light intensity used here (700 μmol photons m−2 s−1) was close to one third of the maximal sunlight intensity observed under natural conditions (Kirk 2010; Park et al. 2011). This strain was not sensitive to these high light levels and achieved a specific growth rate of 0.0366 h−1 at this maximum intensity. However, the optimal light intensity was 500 μmol photons m−2 s−1, sustaining a maximum specific growth rate of 0.043 h−1 under ambient CO2 and 37 °C temperature conditions (Fig. 1a).
Effect of temperature on growth of Acutodesmus
The growth rate increased approximately linearly from 23.5 to 37 °C, and the highest maximum specific growth rate was found at 37 °C. The growth of the alga was abruptly affected upon further increase up to 40 °C in temperature, and was completely inhibited at 43 °C, at which the cells died (Fig. 1b).
Effect of pH on growth of Acutodesmus
The growth rate was almost constant over the pH range 7.0–9.0, and the maximum growth rate of 0.0364 h−1 was obtained at pH 8.0. The growth rate decreased both above and below this optimal value and was completely inhibited at the end points, that is, at pH 4.0 and 11.0. Under these pH conditions, the color of Acutodesmus sp. changed completely from green to white within 48 h and the cells died. As shown in Fig. 1c, the alga thus has a broad-range response against pH values.
Effect of CO2 concentrations on growth of Acutodesmus
Under ambient conditions, the growth curve of Acutodesmus sp. showed a negligible lag phase, while lag phase durations of 12 h for 5 %, 24 h for 10 %, and 15 and 36 h for 20 % CO2 were observed (data not shown). As indicated in Fig. 2, the lowest growth rate of 0.0283 h−1 occurred when the alga was grown in ambient CO2 conditions, while the maximum growth rate of 0.102 h−1 was obtained at 10 % CO2 and the growth rate was 0.085 h−1 at 20 % CO2 (F 1.432, 2.864 = 121.3, P = 0.0018).
At high CO2, the pH of the culture remained in the range of 6–7 (pH 6.96–6.44), indicating that high CO2 concentrations were able to nullify the pH increase that would be a normal consequence of high rates of photosynthesis. In trials at different CO2 concentrations, the highest final cellular biomass (1.991 g L−1) was produced at 10 % CO2. Although the lag phase was slightly longer for 20 % CO2, the exponential phase growth rate was high, and cultures still produced a cellular biomass of 1.646 g L−1 (Fig. 2). No inhibitory effects, relative to ambient levels, of high CO2 concentrations at the levels used here were detected.
Effect of CO2 concentrations on biochemical composition of Acutodesmus
Protein
Overall, different growth stages as well as varying CO2 treatments affected the protein content of cells. The total protein content (mg mL−1) increased as the culture moved from the late exponential (72 h) to the late stationary phase (120 h), due to higher biomass in the stationary phase. However, the protein content per cell decreased as cultures moved into their late stationary phase, most likely as a result of N-limitation. At 72 h, the highest total protein content of 0.716 mg mL−1 was found in the fastest growing cultures under the 10 % CO2 treatment, which increased up to 0.952 mg mL−1 at 120 h. However, when normalized to the OD at 730 nm, 5 % CO2-treated cells had the maximum protein content per biomass, and there was no significant difference between the protein contents of 10, 15, and 20 % CO2-treated cells (F 1.940, 3.880 = 0.09694, P = 0.9051) (Fig. 3). Protein levels ranging from 28.8 to 71.5 % of the dry biomass (at 120 h), depending upon the CO2 treatment, were observed in this study (Table 1).
Carbohydrate
There was a significant effect of the treatment by CO2 and the growth stages on the CHO content of cells (Fig. 3). Among all treatments, the maximum CHO content was observed in cells grown with 15 % CO2, and increased from 0.45 mg mL−1 at 72 h to 0.70 mg mL−1 at 120 h. The CHO levels declined on further increasing the CO2 concentration up to 20 % (F 1.167, 2.334 = 275.6, P = 0.0018). When normalized to OD at 730 nm, the CHO content per cell dropped on moving from the late exponential to the late stationary phase. The CHO content ranged from 18.9 to 40.72 % of the dry biomass (at 120 h), depending on the CO2 treatments (Table 1).
Neutral lipids
As is evident from Figs. 3d and 4, the protein content (% dry wt) dropped on moving from 72 to 120 h, while a gradual increase in the neutral lipid content was observed on moving from the late exponential to the late stationary phase for all CO2 treatments. The fluorescence intensity changes (reflecting changes in neutral lipid content) were as follows: 0.04 % CO2 (15.28 %), 5 % CO2 (17.95 %), 10 % CO2 (17.563 %), 15 % CO2 (15.319 %), and 20 % CO2 (11.67 %). The neutral lipid content of the cultures also differed among different CO2 treatments within the same growth stage. There was not much variation between the fluorescence intensity of cells in the ambient and 5 % CO2 treatments but, as shown in Fig. 4, the neutral lipid content increased dramatically in the samples treated with 10 % CO2, where the maximum lipid accumulation occurred. The lipid content decreased at higher CO2 treatments (F 1.000, 1.000 = 3293, P = 0.0111).
Changes in the content of total carbohydrate (a, b) and total protein (c, d) of Acutodesmus sp. at two different growth stages (72 h = late exponential phase; 120 h = late stationary phase) under different CO2 treatments [Ambient (0.04 %)  , 5 %
, 5 %  , 10 %
, 10 %  , 15 %
, 15 %  , 20 % v/v
, 20 % v/v
 ]. Data represent mean ± SEM of three replicates
]. Data represent mean ± SEM of three replicates
Neutral lipid content indicated by fluorescence emission of Nile Red stained Acutodesmus sp. at two different growth stages (72 h = late exponential phase; 120 h = late stationary phase) under different CO2 treatments. [Ambient (0.04 %)  , 5 %
, 5 %  , 10 %
, 10 %  , 15 %
, 15 %  , 20 % v/v
, 20 % v/v
 ]. Data represent mean ± SEM of three replicates
]. Data represent mean ± SEM of three replicates
Discussion
To make the commercial application of microalgae successful, careful selection of fast-growing, extremes-tolerant, and high-energy-content microalgal strains is a primary requirement. A large number of strains of Scenedesmus/Acutodesmus (of which S01801 is a recognized sub-genus) have been used for photosynthetic CO2 mitigation, wastewater treatment, and biofuel production (Doria et al. 2012; Basu et al. 2013; Zhang et al. 2014). Here, we add to the information on the uses of this genus by our isolation of an Acutodesmus sp. from industrial wastewater; the examination of its biomass productivity, light, temperature, pH, and CO2-tolerance range or ability; and the macromolecular composition under varying CO2 concentrations.
Light intensity, its duration and wavelength, directly affect the growth and photosynthesis of microalgae. Insufficient light may lead to growth limiting, and too much light to photo-oxidation and photoinhibitory conditions (Al-qasmi et al. 2012). Park et al. (2011) reported that photosynthesis in most algae saturates at a solar radiation intensity of ̴ 200 μmol photons m−2 s−1, which is around 10–17 % of summer/winter maximum outdoor sunlight intensity, though some species have been reported to saturate at higher light levels (Ho et al. 2012; Liu et al. 2012; Treves et al. 2013). The growth-saturating light intensity for our Acutodesmus sp. was quite high when compared to most of the previously reported strains of this alga (Liu et al. 2012; Gris et al. 2014). The growth rate was maximum at a light intensity of 500 μmol photons m−2 s−1, and decreased at sub-optimal and supra-optimal light conditions. Photoinhibition above the saturating light level can occur due to disruption of the Photosystem II function and inactivation of enzymes involved in CO2 fixation (Franklin et al. 2003; Juneja et al. 2013). However, our strain of Acutodesmus appeared to be tolerant of light intensities of up to 700 μmol photons m−2 s−1 without a significant decrease in growth rate.
Thermotolerance of microalgae is a promising attribute in the enhancement of biomass production, especially by the reduction of the risk of contamination by pathogens and the lowering of the cooling costs of flue gases, if they are used for cultivation. This strain showed a broad range of temperature tolerance. However, the maximum growth rate occurred at 37 °C, presumably reflecting the usual impacts of temperature on metabolic processes, with increasing temperatures increasing rates of biological processes to an optimal point, beyond which higher temperatures begin to adversely affect protein structure and enzyme activity. Most of the reported strains of the genus Scenedesmus have been grown in a temperature range of 25–32 °C (Ho et al. 2012; Wang et al. 2013; Welter et al. 2013; Mandotra et al. 2014), except for a very few thermo-tolerant strains such as those reported by Basu et al. (2013) and Onay et al. (2014). The excellent temperature tolerance ability of our strain makes it suitable for reduction in cooling costs, when treated with direct hot flue gases.
Another important factor in algae cultivation is pH. It determines the solubility and availability of CO2 and other nutrients, and significantly affects algal metabolism. The impact of pH was studied in the range 4–11. Most previous studies are based on only manipulating the initial pH (Watanabe et al. 1992; Yue and Chen 2005). However, the pH increases sharply as a consequence of CO2 depletion by photosynthesis and, in some cases, due to nitrate assimilation (Varshney et al. 2015). Hence, we used biological buffers to avoid the pH change and measured growth by changes in OD at 720 nm. The growth rate was optimal at pH 8.0 and was suppressed above pH 9.0, possibly due to limitation in the availability of carbon from CO2. At a higher pH, the majority of dissolved inorganic carbon is found in the form of bicarbonate and carbonate and the availability of free CO2 decreases (Juneja et al. 2013). Declining growth at pH values above 9 suggest that the species of Acutodesmus we used had a poor ability to utilize bicarbonate ions from the medium, though neutrophilic Scenedesmus species can use bicarbonate well (Thielmann et al. 1990). Also, at high or low pH, cells may have to expend energy to maintain the internal pH within the range necessary for cell function, which in turn results in reduced growth rates. Similar tests were also performed by Vidyashankar et al. (2013), in which Scenedesmus dimorphus was grown in the pH range of 5–11 and showed a good growth profile under the pH range 9–11, but formed aggregates and flocculated at pH 11. This property is important and favorable when using industrial stack gases for mass cultivation of algae.
In addition, our Acutodesmus strain showed relatively higher CO2 tolerance and higher carbohydrate and protein content when compared with most of the strains of the genus Scenedesmus examined (Table 2). Carbohydrate is a primary energy reservoir of green algal cells. It is mainly present in the form of starch in the chloroplast and polysaccharides such as cellulose, pectins, and glucans, in the cell walls (Domozych et al. 2012). Under ambient conditions, the growth rate was relatively low, and cells might have experienced CO2 limitation, which resulted in low biomass accumulation and, thereby, low carbohydrate, protein, and lipid content per L of culture. It is evident from the results that varying CO2 treatments not only affected the microalgal growth rate but also altered its macromolecular composition. Generally, when the alga is in the early stages of growth, the cells have a low CHO and lipid content, but high protein content. As the cells reach the stationary phase of growth, more carbon is incorporated into storage, either as CHO or as lipid, and the protein level declines (Gatenby et al. 2003). In our case, both the protein and CHO content of cells declined from 72 to 120 h. However, the lipid content showed a pattern that was similar to that which has been reported in previous studies. The maximum carbohydrate content of this strain was comparable to the results reported by Miranda et al. (2012) and Wang et al. (2013), but was higher than the values reported by Ho et al. (2012) and Basu et al. (2013). When treated with 5 % CO2, this strain gave a protein content of around 71.5 % of dry biomass in its late stationary phase, which is much higher than those reported in previous studies (Basu et al. 2013; Zhang et al. 2014). High protein content makes it an efficient source of organic fertilizers, animal feed, and human nutrition supplements. Lipids, especially neutral lipids, accumulate under stressed conditions and the process of lipid accumulation is directly proportional to the decline in intracellular protein content of cells (Wang et al. 2013). This study does not account for the quantitative measurement of total lipids; rather, we used Nile Red staining to determine the neutral lipid component. Results show that the maximum amount of neutral lipid accumulation was found at 10 % CO2 concentration when compared to ambient and other CO2 treatments. However, it seems that higher CO2 levels (15–20 %) decreased the accumulation of lipids. These data corresponded well with the trend reported by Basu et al. (2013), who obtained 30 % higher total lipid in 13.8 ± 1.5 % CO2-treated cells with respect to control (ambient CO2) cells. In contrast, Acutodesmus (Scenedesmus) obliquus SJTU-3 showed a continuous increasing trend of total lipids under different CO2 concentrations ranging from 0.03 to 50 % (Tang et al. 2011). Total lipid extraction and quantification by the gravimetric method and fatty acid profiling by GC-MS for Acutodesmus sp. is the subject of current work.
In conclusion, the screening for high-temperature, intense-light, and high-CO2-tolerant microalgal strains was successful. The results presented here for one of these strains, Acutodesmus sp., are very promising for its exploitation in mass culture for CO2 remediation. In terms of biomass, the most favorable CO2 concentration was 10 %. However, no significant decrease in the biomass was observed at up to 20 % CO2. Major macromolecular components of alga, including carbohydrates, protein, and neutral lipids, were examined under different CO2 treatments. The gross composition of the microalga changes depending on the type or severity of stress, the growth stage, and the type of species. Understanding this gross composition is very important in determining the nutritional quality of the microalga.
It is evident that the algal culture for CO2 sequestration/remediation requires a large amount of nutrients, with nitrogen and phosphorus playing a central role (Jia et al. 2015). Wastewater discharged from CO2-emitting industries may contain some or all of these. Hence, using this strain, future attempts can be made to combine the process of removing CO2 from flue gas and nutrients from wastewater, by which an economically feasible process can be developed (Kastánek et al. 2010). In addition, the tolerance of this strain should be tested against other flue gas compounds such as nitrogen oxides (NOx) and sulfur oxides (SOx).
References
Al-qasmi M, Member NR, Talebi S, Al-Rajhi S, Al-Barwani T (2012) A review of effect of light on microalgae growth. In: Proceedings of the World Congress on Engineering. London, U.K., pp 608–10
Bachu S (2008) CO2 storage in geological media: role, means, status and barriers to deployment. Prog Energy Combust Sci 34:254–273
Basu S, Roy AS, Mohanty K, Ghoshal AK (2013) Enhanced CO2 sequestration by a novel microalga: Scenedesmus obliquus SA1 isolated from bio-diversity hotspot region of Assam, India. Bioresour Technol 143:369–377
Ben-Amotz A, Avron M (1990) The biotechnology of cultivating the halotolerant alga Dunaliella. Trends Biotechnol 8:121–126
Centi G, Perathoner S (2011) CO2-based energy vectors for the storage of solar energy. Greenh Gas Sci Technol 35:21–35
Cheah WY, Show PL, Chang J-S, Ling TC, Juan JC (2015) Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour Technol 184:190–201
Chen W, Zhang C, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile Red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77:41–47
Cheng L, Zhang L, Chen H, Gao C (2006) Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep Purif Technol 50:324–329
de Morais MG, Costa JAV (2007) Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energy Convers Manag 48:2169–2173
Doan TTY, Obbard JP (2011) Improved Nile Red staining of Nannochloropsis sp. J Appl Phycol 23:895–901
Domozych DS, Ciancia M, Fangel JU, Mikkelsen MD, Ulvskov P, Willats WG (2012) The cell walls of green algae: a journey through evolution and diversity. Front Plant Sci 3:1–7
Doria E, Longoni P, Scibilia L, Iazzi N, Cella R, Nielsen E (2012) Isolation and characterization of a Scenedesmus acutus strain to be used for bioremediation of urban wastewater. J Appl Phycol 24:375–383
DuBois M, Gilles KA, Hamilton J, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Franklin L, Osmond CB, Larkum AWD (2003) Photoinhibition, UV-B and algal photosynthesis. In: Larkum AWD, Douglas SE, Raven JA (eds) Photosynthesis in algae. Springer, Dordrecht, pp 351–384
Gatenby CM, Orcutt DM, Kreeger DA, Parker BC, Jones VA, Neves RJ (2003) Biochemical composition of three algal species proposed as food for captive freshwater mussels. J Appl Phycol 15:1–11
Glibert PM, Azanza R, Burford M et al (2008) Ocean urea fertilization for carbon credits poses high ecological risks. Mar Pollut Bull 56:1049–1056
Gouveia L, Batista AP, Miranda A, Empis J, Raymundo A (2007) Chlorella vulgaris biomass used as colouring source in traditional butter cookies. Innov Food Sci Emerg Technol 8:433–436
Gris B, Morosinotto T, Giacometti GM, Bertucco A, Sforza E (2014) Cultivation of Scenedesmus obliquus in photobioreactors: effects of light intensities and light–dark cycles on growth, productivity, and biochemical composition. Appl Biochem Biotechnol 172:1–13
Hegewald E, Bock C, Krienitz L (2013) A phylogenetic study on Scenedesmaceae with the description of a new species of Pectinodesmus and the new genera Verrucodesmus and Chodatodesmus (Chlorophyta, Chlorophyceae). Fottea 13:149–164
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252
Jia Q, Xiang W, Yang F, Hu Q, Tang M, Chen C, Wang G, Dai S, Wu H, Wu H (2015) Low-cost cultivation of Scenedesmus sp. with filtered anaerobically digested piggery wastewater: biofuel production and pollutant remediation. J Appl Phycol. DOI: 10.1007/s10811-015-0610-9 1–10.
Juneja A, Ceballos R, Murthy G (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6:4607–4638
Kastánek F, Sabata S, Solcová O, Maléterová Y, Kastánek P, Brányiková I, Kuthan K, Zachleder V (2010) In-field experimental verification of cultivation of microalgae Chlorella sp. using the flue gas from a cogeneration unit as a source of carbon dioxide. Waste Manag Res 28:961–966
Kirk JTO (2010) Light and photosynthesis in aquatic ecosystems, 3rd edn. Cambridge University Press, Cambridge
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Malcata FX, van Langenhove H (2010) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380
Lara-Gil JA, Álvarez MM, Pacheco A (2014) Toxicity of flue gas components from cement plants in microalgae CO2 mitigation systems. J Appl Phycol 26:357–368
Lewis LA, Flechtner VR (2004) Cryptic species of Scenedesmus (Chlorophyta) from desert soil communities of Western North America. J Phycol 40:1127–1137
Liu J, Yuan C, Hu G, Li F (2012) Effects of light intensity on the growth and lipid accumulation of microalga Scenedesmus sp. 11–1 under nitrogen limitation. Appl Biochem Biotechnol 166:2127–2137
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mandotra SK, Kumar P, Suseela MR, Ramteke PW (2014) Fresh water green microalga Scenedesmus abundans: a potential feedstock for high quality biodiesel production. Bioresour Technol 156:42–47
Miranda JR, Passarinho PC, Gouveia L (2012) Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour Technol 104:342–348
Mondal MK, Balsora HK, Varshney P (2012) Progress and trends in CO2 capture/separation technologies: a review. Energy 46:431–441
Onay M, Sonmez C, Oktem HA, Yucel AM (2014) Thermo-resistant green microalgae for effective biodiesel production: isolation and characterization of unialgal species from geothermal flora of Central Anatolia. Bioresour Technol 169:62–71
Park JBK, Craggs RJ, Shilton AN (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol 102:35–42
Rahaman MSA, Cheng LH, Xu XH et al (2011) A review of carbon dioxide capture and utilization by membrane integrated microalgal cultivation processes. Renew Sustain Energy Rev 15:4002–4012
Ramaraj R, Tsai DDW, Chen PH (2014) Freshwater microalgae niche of air carbon dioxide mitigation. Ecol Eng 68:47–52
Ren HY, Liu BF, Ma C, Zhao L, Ren NQ (2013) A new lipid-rich microalga Scenedesmus sp. strain R-16 isolated using Nile red staining: effects of carbon and nitrogen sources and initial pH on the biomass and lipid production. Biotechnol Biofuels 6:143
Ritschard RL (1992) Marine algae as a CO2 sink. Water Air Soil Pollut 64:289–303
Seth JR, Wangikar PP (2015) Challenges and opportunities for microalgae-mediated CO2 capture and biorefinery. Biotechnol Bioeng 112:1281–1296
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Tang D, Han W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076
Thielmann J, Tolbert NE, Goyal A, Sneger H (1990) Two systems for concentrating CO2 and bicarbonate during photosynthesis by Scenedesmus. Plant Physiol 92:622–629
Treves H, Raanan H, Finkel OM, Berkowicz SM, Keren N, Shotland Y, Kaplan A (2013) A newly isolated Chlorella sp. from desert sand crusts exhibits a unique resistance to excess light intensity. FEMS Microbiol Ecol 86:373–380
Varshney P, Mikulic P, Vonshak A, Beardall J, Wangikar PP (2015) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol 184:363–372
Vidyashankar S, Deviprasad K, Chauhan VS, Ravishankar GA, Sarada R (2013) Selection and evaluation of CO2 tolerant indigenous microalga Scenedesmus dimorphus for unsaturated fatty acid rich lipid production under different culture conditions. Bioresour Technol 144:28–37
Vonshak A, Richmond A (1988) Mass production of the blue-green alga Spirulina: an overview. Biomass 15:233–247
Walker DA (2009) Biofuels, facts, fantasy, and feasibility. J Appl Phycol 21:509–517
Wang L, Li Y, Sommerfeld M, Hu Q (2013) A flexible culture process for production of the green microalga Scenedesmus dimorphus rich in protein, carbohydrate or lipid. Bioresour Technol 129:289–295
Watanabe Y, Ohmura N, Saiki H (1992) Isolation and determination of cultural characteristics of microalgae which functions under CO2 enriched atmosphere. Energy Convers Manag 33:545–552
Welter C, Schwenk J, Kanani B, Van Blargan J, Belovich JM (2013) Minimal medium for optimal growth and lipid production of the microalgae Scenedesmus dimorphus. AICHE 32:937–945
Yue L, Chen W (2005) Isolation and determination of cultural characteristics of a new highly CO2 tolerant fresh water microalgae. Energy Convers Manag 46:1868–1876
Yun Y-S, Lee SB, Park JM, Lee C-I, Yang J-W (1997) Carbon dioxide fixation by algal cultivation using wastewater nutrients. J Chem Technol Biotechnol 69:451–455
Zhang T-Y, Wu Y-H, Hu H-Y (2014) Domestic wastewater treatment and biofuel production by using microalga Scenedesmus sp. ZTY1. Water Sci Technol 69:2492
Acknowledgments
The authors are thankful to JSW Foundation, India, for providing financial assistance for this research. We are also grateful to Dr. Kumar M. Iyer of JSW Steel for his useful suggestions and discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varshney, P., Sohoni, S., Wangikar, P.P. et al. Effect of high CO2 concentrations on the growth and macromolecular composition of a heat- and high-light-tolerant microalga. J Appl Phycol 28, 2631–2640 (2016). https://doi.org/10.1007/s10811-016-0797-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0797-4


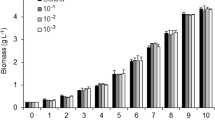


 )] and maximum dry biomass [DCW (
)] and maximum dry biomass [DCW ( )], at 120 h of Acutodesmus sp. under different CO2 treatments. Data represent mean ± SEM of three replicates
)], at 120 h of Acutodesmus sp. under different CO2 treatments. Data represent mean ± SEM of three replicates
