Abstract
The effects of changes in CO2 and pH on biomass productivity and carbon uptake of Pleurochrysis carterae and Emiliania huxleyi in open raceway ponds and a plate photobioreactor were studied. The pH of P. carterae cultures increased during day and decreased at night, whereas the pH of E. huxleyi cultures showed no significant diurnal changes. P. carterae coccolith production occurs during the dark period, whereas in E. huxleyi, coccolith production is mainly during the day. Addition of CO2 at constant pH (pH-stat) resulted in an increase in P. carterae biomass and coccolith productivity, while CO2 addition lowered E. huxleyi biomass and coccolith production. Neither of these algae could grow at less than pH 7.5. Species-specific diurnal pH and pCO2 variations could be indicative of significant differences in carbon uptake between these two species. While E. huxleyi has been suggested to be predominantly a bicarbonate user, our results indicate that P. carterae may be using CO2 as the main C source for photosynthesis and calcification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The large-scale culture of microalgae as a CO2 “sink” for bioremediation of increased atmospheric CO2 levels has been proposed by several workers (Benemann 1997; Herzog and Drake 1996). Microalgae have a higher productivity than other photosynthetic organisms such as trees and also have the potential to be grown using saline water and on land which cannot be used for agriculture. The coccolithophorid algae (Haptophyta) have the further potential advantage in that they fix carbon not only into organic biomass high in lipids and hydrocarbons (Fernandez et al. 1994; Riebesell et al. 2000) but also produce CaCO3 in the form of small plates called coccoliths (Paasche 2002). This would allow the fixed C to be buried (“fossilized”) or, alternatively, the lipids and hydrocarbons can be used as a renewable fuel or as an energy source by direct co-firing (Wu et al. 1999).
Large-scale algae cultures are generally carbon limited, and the addition of CO2 enhances growth and productivity (Borowitzka 1998). However, the addition of CO2 also causes acidification of the medium. Recently, several studies have examined the effects of increased CO2 on Emiliania huxleyi (Leonardos and Geider 2005; Nielsen 1995; Riebesell et al. 2000; Zondervan et al. 2002). Leonardos and Geider (2005) found that elevated CO2 can result in increasing organic carbon fixation by E. huxleyi while grown at low N:P and in high light. Feng et al. (2008) also showed that doubling the pCO2 can result in reduced particulate inorganic carbon (PIC) in E. huxleyi at 400 μmol photons m−2 s−1. However, they did not detect any change in the particulate organic carbon (POC) between high and low pCO2. Thus, increasing CO2 was found to result in a decrease in calcification and an increase in organic carbon, at least in nutrient-limited E. huxleyi. While there have been extensive studies on the effect of pH and elevated pCO2 on E. huxleyi, there is very limited data on the effect of elevated pCO2 on the productivity and calcification of Pleurochrysis carterae.
We have demonstrated that the coccolithophore P. carterae can be reliably grown outdoors in open raceway ponds for extended periods of up to at least 1 year (Moheimani and Borowitzka 2006a). We have also studied the limits to growth and productivity of this alga when grown outdoors in raceway ponds (Moheimani and Borowitzka 2006b). As part of an examination of the factors limiting growth and coccolith formation in the outdoor cultures of P. carterae, we examined the effects of CO2 addition and compared P. carterae with E. huxleyi.
Materials and methods
The coccolithophorid algae P. carterae Braarud et Fagerland CCMP 647 and E. huxleyi Lohmann CCMP 371 were obtained from the Centre for Culture of Marine Phytoplankton, Bigelow Laboratory, Boothbay Harbor, ME, USA. P. carterae and E. huxleyi cultures were maintained in modified f/2 and f/50 medium, respectively (Guillard and Ryther 1962). The media were modified by omitting Mo and Si from the original recipe and by adding 0.06 μM SeO2.
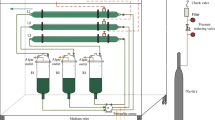
The algae were grown either in a plate-type photobioreactor (Fig. 1) or an outdoor raceway pond (Moheimani and Borowitzka 2006a). The plate photobioreactor had a culture volume of 6 L (W × H × L (cm) = 10 × 35 × 26). The base of the reactor was V-shaped with an air tube at the bottom of the V to promote the suspension or flotation of the relatively heavy coccolithophorid cells. The plate photobioreactor was chemically sterilized by using 12% sodium hypochlorite, for 2 h, rinsed 12 times in sterile deionized water, and dried in a 70 °C oven. The cultures were grown in semicontinuous mode with light provided by 12 cool white fluorescent tubes arranged at both sides of the reactor giving an average irradiance of 320 μmol photons m−2 s−1 (measured at 24 spots on the surface of reactor) with a 12:12-h light:dark cycle. The growth temperature was 23 ± 1.5 °C.
The outdoor cultures were carried out in September 2003 in two 1-m2 surface area fiberglass paddle wheel raceway ponds operated at 16 cm depth. The four-paddle paddlewheel, operating at a rotation speed of about 28 rpm, generated a flow rate of 20 cm s−1. The cultures were maintained in semicontinuous mode by daily harvesting of a part of the biomass and replacing the harvested medium with fresh medium. The ponds were located at Murdoch University, Perth, Western Australia (31°57 S; 115°52 E). The culture medium for the ponds was chemically sanitized (Moheimani and Borowitzka 2006a). CO2 was added to the pond using a 0.06-m2 floating CO2 injector, based on the design of Becker (1994), and positioned 10 cm downstream from the paddlewheel. In both systems, pH was controlled (±0.4 pH units) by CO2 addition using a pH controller and a solenoid switch connected to a CO2 gas cylinder. The carbon chemistry of the cultures, grown in the plate photobioreactor, was calculated from temperature, salinity, phosphate, total alkalinity, and pH of the medium using CO2sys software (Lewis and Wallace 1998). For total alkalinity, medium was filtered with syringe filter (0.45 μm) to remove cells and other particles. Total alkalinity was determined according to Strickland and Parsons (1972) . Media for the plate reactor were buffered to pH 7.50 (total alkalinity, pCO2, and total carbon were 2,453.8 μmol kg−1, 1,756.9 μatm, and 2,378.6 μmol kg−1, respectively). Samples were taken daily for measuring growth rates, organic biomass, lipid content, and calcium carbonate production using the methods described in Moheimani and Borowitzka (2006a).
Results
Preliminary experiments in batch cultures showed that in 300 mL cultures of P. carterae CCMP 647 and another Pleurochrysis sp., the medium pH rose from pH 8.2 to pH 9.5 by the end of the exponential phase, and then declined to pH 8.2 after about 5 days in stationary phase. In contrast, pH in E. huxleyi culture did not change throughout the growth period (data not shown). The effects of CO2 addition and pH were then examined in plate photobioreactor under controlled conditions of light and temperature and then also outdoors in open raceway ponds.
Plate photobioreactors
The pH of the cultures was initially unregulated, i.e., no CO2 was added. In the P. carterae culture, this resulted in a pH increase from pH 8.3 to pH 9.5 during the light period and then a decrease to pH 8.3 by the end of the subsequent dark period (Fig. 2a). In the E. huxleyi culture, the pH remained between pH 8.1 and 8.4 in both the light and dark periods (Fig. 2b). Culturing P. carterae under unregulated pH resulted in a reduction in pCO2, whereas E. huxleyi grown under the same unregulated condition increased the pCO2 (Table 1).
The pH of the culture medium was then regulated by addition of CO2 using the pH stat system. Between pH 7.9–8.1 and pH 7.6–7.9 for P. carterae and between pH 7.7 and 7.9 for E. huxleyi, the cultures continued to grow well (Fig. 2). When the pH was reduced to pH 7.4 for P. carterae and to pH 7.2 for E. huxleyi, the algal cells started sticking to the photobioreactor walls and also began to clump, and semicontinuous culture could not be maintained. Increasing the pH of the culture medium to the previous higher value significantly reduced clumping in both species.
The growth rate and productivities of both species at the different pH values are shown in Table 1. P. carterae showed the highest specific growth rate of 0.76 day−1 and maximum dry weight productivity of 0.51 g L−1 day−1 at pH 8. The growth rate and dry weight productivities of P. carterae were greater in pH 8 than pH 7.7, and unregulated pH and the total lipid and CaCO3 content also followed the same pattern. In P. carterae, pCO2 was higher at pH 7.7 than pH 8 and unregulated pH (Table 1). When grown at controlled pH, total alkalinity, pCO2, and total carbon declined in both strains in the afternoon (Table 1). In E. huxleyi, growth rate and all productivities were highest in the unregulated pH treatment, even though less pCO2 and total carbon was available to the cells when grown at pH 7.8 (Table 1).
Cell lipid per total dry weight remained constant between 21% and 24% of dry weight in P. carterae, whereas in E. huxleyi, the lipid content increase from 19% to 26% of total dry weight between the unregulated pH (pH 8.1–8.3) and pH 7.8. The highest amount of CaCO3 per total dry weight (11%) was at pH 8 in P. carterae, whereas in E. huxleyi, CaCO3 per total dry weight remained at 12% in both the unregulated pH and pH 7.8 cultures.
Raceway pond
Two raceway ponds of P. carterae operated in parallel were set up and starting on 28 September 2003. One pond had no pH control, and in the other, the pH was controlled by CO2 addition using a pH-stat. The light profile and medium temperature of cultures grown in raceway ponds have been reported previously by Moheimani and Borowitzka (2006a). The changes in cell density and pH variations in the raceway ponds are shown in Fig. 3, and the effects of different pH on growth rates and productivities are summarized in Table 2. The pH in both ponds was unregulated between 1 and 15 October 2003 (see Fig. 3). During this period, the pH increased during the day from pH 8.3 to pH 10.9 and decreased to the initial pH of 8.3 during the night. The pH decreased 2 pH units after each dilution and then reached the maximum daily pH less than 2 h thereafter. There was no difference in maximum cell concentration, growth rate, and productivity between the control and experimental raceway ponds during the unregulated pH period (see Table 2).
Between 16 October and 9 November 2003, the pH was set to pH 9.6 in the experimental pond, while the pH in the control pond remained unregulated (Fig. 3). This resulted in a significantly higher growth rate, total dry weight productivity, lipid productivity, and CaCO3 productivity in the pH-regulated pond compared to the control pond (one-way ANOVA, P < 0.05; Table 2). There was no difference between the maximum cell density between the two ponds at this pH (one-way ANOVA, P > 0.05).
Between 10 November and 7 December 2003, the pH in the experimental pond was decreased to pH 9.0 (Fig. 3b). While no difference was observed in growth rate and maximum cell concentration between the two ponds (one-way ANOVA, P > 0.05), the pH-regulated pond achieved significantly higher dry weight, lipid, and CaCO3 productivities (one-way ANOVA, P < 0.05; Table 2).
Between 8 and 26 December 2003, the pH was further decreased to pH 8.5 in the experimental pond (Fig. 3). Growing P. carterae at this pH resulted in reduction of cell number from 7 × 105 to 3 × 105 cells mL−1 in less than 12 days (Fig. 3b). Due to this reduction, dilution of the experimental pond was not possible (Fig. 3b). However, the control pond (unregulated pH) could be diluted five times during the same period of time (Fig. 3a). Shifting the pH from 8.5 back to pH 9.6 in the experimental pond resulted in a recovery of growth of P. carterae (Fig. 3b).
The pH changes by CO2 addition did not affect the cell lipid and CaCO3 content. Total lipid was between 32% and 34% of total dry weight, and CaCO3 was between 9.9% and 10.2% of total dry weight in both ponds.
Diurnal cycle (biomass and pH)
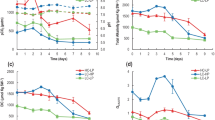
Over a diurnal cycle, the pH of the culture medium remained constant in the range of pH 8.3 to 8.5 in the E. huxleyi culture (Fig. 4c). On the other hand, in the P. carterae culture, the pH of the culture medium increased during the light period from pH 7.8 to 10.1 in the plate photobioreactor and from pH 8.2 to 11 in the raceway pond (Fig. 4a, b). During the dark period, the pH decreased to pH 8.0 ± 0.2 by the end of the night (Fig. 4a, b). In E. huxleyi, the coccolith concentration increased from the start of light period, and there appeared to be little decalcification during the night (Fig. 4c). In P. carterae cultures, the coccolith concentration increased from 2 × 105 to 9 × 105 coccoliths mL−1 during the light period (Fig. 4a, b). During the first 2 h of the dark period, coccolith number declined to 3 × 105 coccoliths mL−1 (Fig. 4a, b). This loss in the number of coccoliths was most likely due to decalcification in the first 5 h of the dark period together with a decrease in the pH of the culture medium (Fig. 4a, b). Coccolith numbers then started to increase so that by sunrise, the coccolith number was >50% of that reached during the day. In contrast, there was a very much smaller decline in coccolith numbers in the dark period in E. huxleyi, and coccolith numbers only increased after the onset of light (Fig. 4c). The cell dry weight of both P. carterae and E. huxleyi decreased by 55% to 75% during the dark period followed by an increase during the light period (data not shown).
Changes in cell concentration (squares), coccolith concentration (circles), and pH of the culture medium (triangles) over 24 h for aPleurochrysis carterae grown in a raceway pond (mean ± SE, n = 5), bP. carterae grown in a plate photobioreactor (mean ± SE, n = 5), and cE. huxleyi grown in a plate photobioreactor (mean ± range, n = 3)
Pearson product–moment correlation indicated a significant association between the pH of the culture medium and the coccolith concentration in the culture of P. carterae (r = −0.69, df = 24, P < 0.05), whereas there was no correlation between pH and coccolith concentration in the cultures of E. huxleyi (r = −0.31, df = 24, P > 0.05).
Discussion
Regulating the pH at pH 8 by the addition of CO2 increased both the growth rate and organic and CaCO3 productivity in P. carterae. Lowering the pH further to pH 7.7 reduced the growth rate, but the organic and CaCO3 productivity remained higher than when the cells were grown under unregulated pH (pH 8.3–9.5) conditions. The E. huxleyi cultures, however, had the highest growth rate and organic and CaCO3 productivities under unregulated pH (pH 8.1–8.3) conditions. These results suggest that the high pH values reached in the unregulated cultures of P. carterae lead to carbon limitation, as at pH 10, there is no free CO2, some HCO −3 , and the bulk of the Ci is in the form of CO 2−3 . The observed pH changes during a diurnal cycle in actively growing cultures of P carterae and E. huxleyi indicate significant differences in carbon uptake and metabolism between these species. During the light period, P. carterae significantly increased the pH of the medium (up to pH 11 in the outdoor cultures), whereas in E. huxleyi, the pH did not change. Israel and Gonzalez (1996) and Crenshaw (1964) observed the same differences in the pattern in pH during growth of P. carterae and E. huxleyi.
Growth of both strains, when grown under controlled pH, was accompanied by a concomitant decrease in total carbon and pCO2. While total carbon utilization was the same between P. carterae and E. huxleyi when grown under uncontrolled pH, there was a completely different pattern in pCO2 and pH for these two strains. E. huxleyi decreased the pH and increased the pCO2 between morning and afternoon, while the opposite was observed in P. carterae. The inorganic carbon system is the main buffering system in the ocean. Alkalinization of the medium is observed in many photosynthesizing algae and aquatic plants as a result of either CO2 uptake (with or without an external carbonic anhydrase) and/or HCO −3 uptake with concurrent OH− efflux (Borowitzka 1982; Brewer and Goldman 1976). The precipitation of CaCO3, on the other hand, can lead to acidification (Gattuso et al. 1995), and this was observed in our E. huxleyi culture. The interaction between photosynthesis and calcification, and the concomitant C fluxes has been extensively studied in E. huxleyi and, to a much lesser extent, in P. carterae and not at all in other coccolithophorid algae (Berry et al. 2002; Borowitzka 1989; Brownlee and Taylor 2004; Paasche 2002). There is substantial evidence that the bulk of the carbon for photosynthesis in E. huxleyi comes from bicarbonate (Buitenhuis et al. 1999; Sikes and Wheeler 1982), and it has been suggested that the H+ produced during CaCO3 formation is used to offset any cytoplasmic alkalinization resulting from HCO −3 utilization for photosynthesis and the action of carbonic anhydrase in the chloroplast (Berry et al. 2002; Quiroga and Gonzalez 1993).
It has been shown that E. huxleyi has a membrane anion exchange protein which is involved in active HCO −3 transport into the cells (Herfort et al. 2002). At low external Ci concentrations and in stationary phase E. huxleyi cells, extracellular carbonic anhydrase activity has also been detected (Herfort et al. 2002; Nimer et al. 1994, 1996, 1997). In contrast, Israel and Gonzalez (1996) have demonstrated external carbonic anhydrase activity at both high and low Ci concentrations in a Pleurochrysis sp. E. huxleyi is also a bicarbonate user which may explain there was no CA activity detection at high Ci concentration by Herfort et al. (2002). On the other hand, P. carterae, as inferred from the observed pH shifts in the current study, could be predominantly a CO2 user and thus requires an active external carbonic anhydrase. However, this hypothesis remains to be tested. The apparent differences in C uptake between E. huxleyi and P. carterae shown here may also help to explain the differences in the 18O stable isotopic composition of the coccoliths of these algae observed by Dudley et al. (1986). If the C for coccolith formation were not only provided by HCO −3 taken up from the seawater but were also provided by HCO −3 derived from CO2 in the cytoplasm, then this could account for the observed depletion in 18O of the coccolith CaCO3. Carbonic anhydrase, respiration, and other metabolic processes are known to discriminate against 18O (Guy et al. 1989, 1993; Miller et al. 1997).
In E. huxleyi, various studies have found that bicarbonate is used for calcification (Paasche 1964; Sikes et al. 1980) and that CO2 from intracellularly converted bicarbonate is the major “C” source for photosynthesis (Dong et al. 1993; Nimer and Merrett 1992; Sikes et al. 1980). The net change in the inorganic carbon in the medium is the product of inorganic “C” uptake by coccolithophorids subtracted from the respiratory CO2 excreted by cell. Nimer and Merrett (1993) showed that in E. huxleyi, when bicarbonate is the main “C” source in media, the stoichemistry between photosynthesis and calcification is 1:1 (measured using 14CO2). This means that the same amount of “C” is used for calcification and photosynthesis. The photosynthesis to calcification ratios (P:C) of P. carterae and E. huxleyi are summarized in Table 3. For E. huxleyi, the P:C ratio is between 0.52 to 1.53, while this ratio is at least tenfold higher in P. carterae. This is compatible with the observation of an absence of pH changes in the medium of actively growing E. huxleyi observed by us in this study. However, the large alkalinization of the medium in actively photosynthesizing P. carterae cultures means that CO2 uptake must significantly exceed HCO −3 uptake and implies that this CO2 is the main C source for photosynthesis, and possibly also for calcification, in this species. Comparative studies of carbon uptake and use in photosynthesis and calcification in Pleurochrysis and other coccolithophorid algae and compared with the extensively studied Emiliania are clearly required.
Neither P. carterae nor E. huxleyi could grow at a pH of less than about pH 7.5, with E. huxleyi appearing to be slightly more sensitive to low pH. This inhibition could be due to the inability of these algae to generate sufficient OH− to neutralize the H+ produced by calcification (Nimer and Merrett 1993; Sciandra et al. 2003) and thus prevent acidification of the cytoplasm or due to a direct effect of a more acidic cytoplasm.
Apparent decalcification during the night as observed in this study in P. carterae has also been reported for E. huxleyi (Balch et al. 1996; Linschooten et al. 1991; Paasche 1964; Sekino and Shiraiwa 1994). This decalcification is probably due to localized acidification caused by respiratory CO2 production, resulting in a partial dissolution of the coccoliths.
This study was part of a larger study examining the suitability of large-scale cultures of coccolithophorid algae for CO2 bioremediation (Moheimani and Borowitzka 2006a, b). This study showed that P. carterae CCMP647 could be grown in outdoor raceway cultures for periods of up to 10 months in semicontinuous culture, whereas E. huxleyi cultures could not be maintained in this system (Moheimani and Borowitzka 2006a). The results presented here show that CO2 addition used to maintain the culture pH between pH 8.1 and 9.3 increases the specific growth rate and productivity of P. carterae. P. carterae has shown to be a reliable microalga when grown in a semicontinuous mode and at a constant pH in both plate photobioreactor and raceway ponds. The ability to grow the alga in semicontinuous culture is very important as this reduces the overall cost of producing the algae (Borowitzka 1999).
This study has also provided some evidence that P. carterae seems to markedly differ from E. huxleyi in its carbon uptake system and carbon concentrating mechanism. An interesting question raised here is whether this difference is reflected in the evolution of the Haptophyta (Pleurochrysis is classified in the Coccolithales whereas Emiliania is in the Isochrydales (Edvardsen et al. 2000)) or in structural differences in coccolithogenesis (Hawkins and Lee 2001; Paasche 2002). The implication of this to our understanding of coccolithophorid calcification and photosynthesis and to the potential effects of ocean acidification due to increases in atmospheric CO2 requires further study.
References
Balch WM, Fritz JJ, Fernandez E (1996) Decoupling of calcification and photosynthesis in the coccolithophore Emiliania huxleyi under steady-state light-limited growth. Mar Ecol Prog Ser 142:87–97
Becker EW (1994) Microalgae: biotechnology and microbiology. Cambridge University Press, Cambridge, p 293
Benemann J (1997) CO2 mitigation with microalgae systems. Energy Convers Manage 38:475–479
Berry L, Taylor AR, Lucken U, Ryan KP, Brownlee C (2002) Calcification and inorganic carbon acquisition in coccolithophores. Funct Plant Biol 29:289–299
Borowitzka MA (1982) Mechanisms in algal calcification. Prog Phycol Res 1:137–177
Borowitzka MA (1989) Carbonate calcification in algae—initiation and control. In: Mann S, Webb J, Williams RJP (eds) Biomineralization: chemical and biochemical perspectives. VCH, Weinheim, pp 63–94
Borowitzka MA (1998) Limits to growth. In: Wong YS, Tam NFY (eds) Wastewater treatment with algae. Springer, Berlin, pp 203–218
Borowitzka MA (1999) Economic evaluation of microalgal processes and products. In: Cohen Z (ed) Chemicals from microalgae. Taylor & Francis, London, pp 387–409
Brewer PG, Goldman JC (1976) Alkalinity changes generated by phytoplankton growth. Limnol Oceanogr 21:108–117
Brownlee C, Taylor A (2004) Calcification in coccolithophores: a cellular perspective. In: Thierstein HR (ed) Coccolithophores: from molecular processes to global impact. Springer, Berlin, pp 31–49
Buitenhuis ET, de Baar HJW, Veldhuis MJW (1999) Photosynthesis and calcification by Emiliania huxleyi (Prymnesiophyceae) as a function of inorganic carbon species. J Phycol 35:949–959
Crenshaw MA (1964) Coccolith formation by two marine coccolithophorids, Coccolithus huxleyi and Hymenomonas sp. Ph.D. thesis, Duke University
Dong LF, Nimer NA, Okus E, Merrett MJ (1993) Dissolved inorganic carbon utilization in relation to calcite production in Emiliania huxleyi (Lohmann) Kamptner. New Phytol 123:679–684
Dudley WC, Blackwelder P, Brand L, Duplessy J (1986) Stable isotopic composition of coccoliths. Mar Micropaleontol 10:1–8
Edvardsen B, Eikrem W, Green JC, Andersen RA, Moon-Van der Staay SY, Medlin LK (2000) Phylogenetic reconstructions of the Haptophyta inferred from 18S ribosomal DNA sequences and available morphological data. Phycologia 39:19–35
Fabry, VJ (2007) Calcium Carbonate produced by coccolithophorid algae in long term, Carbon Dioxide Sequestration. pp 23–25
Feng Y, Warner ME, Zhang Y, Jun S, Fei-Xue F, Rose JM, Hutchins DA (2008) Interactive effects of increased pCO2, temperature and irradiance on the marine coccolithophore Emiliania huxleyi (Prymnesiophyceae). Eur J Phycol 43:87–98
Fernandez E, Balch WM, Maranon E, Holligan PM (1994) High rates of lipid biosynthesis in cultured, mesocosm and coastal populations of the coccolithophore Emiliania huxleyi. Mar Ecol Prog Ser 114:13–22
Gattuso JP, Pichon M, Frankignoulle M (1995) Biological control of air-sea CO2 fluxes—effect of photosynthetic and calcifying marine organisms and ecosystems. Mar Ecol Prog Ser 129:307–312
Guillard RRL, Ryther JH (1962) Studies on marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Guy RD, Berry JA, Fogel ML, Hoering TC (1989) Differential fractionation of oxygen isotopes by cyanide-resistant and cyanide-sensitive respiration in plants. Planta 177:483–491
Guy RD, Fogel ML, Berry JA (1993) Photosynthetic fractionation of the stable isotopes of oxygen and carbon. Plant Physiol 101:37–47
Hawkins EK, Lee JJ (2001) Architecture of the Golgi apparatus of a scale-forming alga: biogenesis and transport of scales. Protoplasma 216:227–238
Herfort L, Thanke B, Roberts J (2002) Acquisition and use of bicarbonate by Emiliania huxleyi. New Phytol 156:427–436
Herzog HJ, Drake EM (1996) Carbon dioxide recovery and disposal from large energy systems. Ann Rev Energ Env 21:145–166
Israel AA, Gonzalez EL (1996) Photosynthesis and inorganic carbon utilization in Pleurochrysis sp (Haptophyta), a coccolithophorid alga. Mar Ecol Prog Ser 137:243–250
Leonardos N, Geider RJ (2005) Elevated atmospheric carbon dioxide increases organic carbon fixation by Emiliania huxleyi (Haptophyta), under nutrient-limited high-light conditions. J Phycol 41:1196–1203
Lewis ER, Wallace DWR (1998) Program developed for CO2 system calculations. BNL-61827 informal. Oak Ridge National Laboratory, Oak Ridge
Linschooten C, Vanbleijswijk JDL, Vanemburg PR, Devrind JPM, Kempers ES, Westbroek P, De Vrind-De Jong EW (1991) Role of the light-dark cycle and medium composition on the production of coccoliths by Emiliania huxleyi (Haptophyceae). J Phycol 27:82–86
Miller AG, Salon C, Espie GS, Canvin DT (1997) Measurement of the amount of isotopic composition of the CO2 released from the cyanobacterium Synechococcus UTEX 625 after rapid quenching of the active CO2 transport system. Can J Bot 75:981–987
Moheimani NR, Borowitzka MA (2006a) The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J Appl Phycol 10:703–712
Moheimani NR, Borowitzka MA (2006b) Limits to productivity of the alga Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. Biotech Bioeng 96:27–36
Nielsen MV (1995) Photosynthetic characteristics of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae) exposed to elevated concentrations of dissolved inorganic carbon. J Phycol 31:715–719
Nimer NA, Merrett MJ (1992) Calcification and utilization of inorganic carbon by the coccolithophorid Emiliania huxleyi Lohmann. New Phytol 121:173–177
Nimer N, Merrett MJ (1993) Calcification rate in Emiliania huxleyi Lohmann in response to light, nitrate and availability of inorganic carbon. New Phytol 123:673–677
Nimer NA, Guan Q, Merrett MJ (1994) Extra- and intra-cellular carbonic anhydrase in relation to culture age in a high-calcifying strain of Emiliania huxleyi Lohmann. New Phytol 126:601–607
Nimer NA, Merrett MJ, Brownlee C (1996) Inorganic carbon transport in relation to culture age and inorganic carbon concentration in a high-calcifying strain of Emiliania huxleyi (Prymnesiophyceae). J Phycol 32:813–818
Nimer NA, Iglesias Rodriguez MD, Merrett MJ (1997) Bicarbonate utilization by marine phytoplankton species. J Phycol 33:625–631
Paasche E (1964) A tracer study of the inorganic carbon uptake during coccolith formation and photosynthesis in the coccolithophorid Coccolithus huxleyi. Physiol Plant Suppl 3:5–82
Paasche E (2002) A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation and calcification-photosynthesis interactions. Phycologia 40:503–509
Quiroga O, Gonzalez EL (1993) Carbonic anhydrase in the chloroplast of a coccolithophorid (Prymnesiophyceae). J Phycol 29:321–324
Riebesell U, Revill AT, Holdsworth G, Volkman JK (2000) The effects of varying CO2 concentration on lipid composition and carbon isotope fractionation in Emiliania huxleyi. Geochim Cosmochim Acta 64:4179–4192
Sciandra A, Harlay J, Lefèfre D, Lemée R, Rimmelin P, Denis M, Gattuso J-P (2003) Response of coccolithophorid Emiliania huxleyi to elevated partial pressure of CO2 under nitrogen limitation. Mar Ecol Prog Ser 261:111–127
Seki M, et al (1995) Effect of CO2 concentration on growth and carbon fixation rate of Pleurochrysis carterae. J Chem Eng Jap 28:474–476
Sekino K, Shiraiwa Y (1994) Accumulation and utilization of dissolved inorganic carbon by a marine unicellular coccolithophorid, Emiliania huxleyi. Plant Cell Physiol 35:353–361
Sikes CS, Wheeler AP (1982) Carbonic anhydrase and carbon fixation in coccolithophorids. J Phycol 18:423–426
Sikes CS, Roer RD, Wilbur KM (1980) Photosynthesis and coccolith formation: Inorganic carbon sources and net inorganic reaction of deposition. Limnol Oceanogr 25:248–261
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis, 2nd edn. Fisheries Research Board of Canada Bulletin 167. Fisheries Research Board of Canada, Ottawa, pp 24–38
Wu Q, Dai J, Shiraiwa Y, Sheng G, Fu J (1999) A renewable energy source—hydrocarbon gases resulting from pyrolysis of the marine nanoplanktonic alga Emiliania huxleyi. J Appl Phycol 11:137–142
Zondervan I, Rost B, Riebesell U (2002) Effect of CO2 concentration on the PIC/POC ratio in the coccolithophore Emiliania huxleyi grown under light-limiting conditions and different daylengths. J Exp Mar Biol Ecol 272:55–70
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moheimani, N.R., Borowitzka, M.A. Increased CO2 and the effect of pH on growth and calcification of Pleurochrysis carterae and Emiliania huxleyi (Haptophyta) in semicontinuous cultures. Appl Microbiol Biotechnol 90, 1399–1407 (2011). https://doi.org/10.1007/s00253-011-3174-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3174-x