Abstract
We applied DNA barcoding analysis to the red alga Grateloupia because it is one of the most complicated taxa in the family Halymeniaceae. We used two DNA barcoding markers, the 5′ end of the cytochrome c oxidase I gene and the universal plastid amplicon of the 23S rRNA gene, to define levels of genetic diversity and species relationships within rbcL phylogeny. We detected nine species of Grateloupia, four species of Pachymeniopsis, and one Kintokiocolax in Korea: G. angusta, G. asiatica, G. catenata, G. divaricata, G. imbricata, G. jejuensis, G. kurogii, G. subpectinata, G. turuturu, P. elliptica, P. gargiuli, P. lanceolata, a new species, and K. aggregato-cerantha. The COI-5P barcode successfully differentiated species, with interspecific divergence values ranging between 3.7 and 14 %. Barcoding data provided a rapid and accurate methodology for species-level identification and for taxonomic explorations when used in combination with morphological data. We describe Pachymeniopsis volvita sp. nov. based on DNA barcoding analysis. Fourteen species detected in four different subclades with strong bootstrap support within rbcL phylogenetic tree.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The red algal genus Grateloupia C. Agardh is one of the most taxonomically complex one in the family Halymeniaceae. The generitype Grateloupia filicina (Lamouroux) C. Agardh is characterized by a finely pinnate branching pattern, numerous marginal proliferations, laxly constructed medullary structure, and four-celled carpogonial branches and three-celled auxiliary branches (De Clerck et al. 2005). Recently, the genus Pachymeniopsis Yamada was resurrected from the genus Grateloupia based on the reproductive anatomy and postfertilization development, including molecular data (Gargiulo et al. 2013; Kim et al. 2014). The generitype Pachymeniopsis lanceolata (Okamura) Yamada ex Kawabata has six-celled carpogonial branches, five-celled auxiliary branches, and upwardly and downwardly directed branched nutritive filaments (Gargiulo et al. 2013). Two genera have ecologically important roles in temperate and tropical coastal waters (Kawaguchi et al. 2001). Some of the species have invaded geographic regions beyond their previous ranges and have become serious impacts (Inderjit et al. 2006), e.g., Grateloupia imbricata in waters of the Canary Islands (Garcia-Jiménez et al. 2008), Grateloupia turuturu in France, and the Gulf of Maine, USA (Simon et al. 2001; Mathieson et al. 2008), and P. lanceolata (as Grateloupia lanceolata) in California, France, and New Zealand (Miller et al. 2009; Verlaque et al. 2005).
Grateloupia is a source of bioactive materials for foods and medicines. Wang et al. (2007) examined the structure and antiretroviral (HIV-1) activity of native sulfated galactans extracted from Grateloupia filicina and Grateloupia longifolia. G. turuturu may have potential in (i) the production of valuable molecules (R-phycoerythrin), (ii) enzymatic degradation, and (iii) antifouling activity (Denis et al. 2009a; 2010). There is a long history of human seaweed consumption in Asian nations (Dawczynski et al. 2007). G. turuturu and G. asiatica (as G. filicina) are commonly used dietary items (Denis et al. 2009b). However, algal chemical composition varies by species, geography, season, and environmental conditions (Denis et al. 2010); this variability is of relevance with respect to nutritional and pharmacological considerations.
Approximately 25 Grateloupia species occur in the northwestern Pacific water; some of these represent a substantial resource of unexploited red algal materials (Denis et al. 2009a, b, 2010). Several of the congeners have been subjected to morphological, anatomical, geographic, and taxonomic analyses (D’Archino et al. 2007; Kawaguchi 1997; Lin et al. 2008). Nevertheless, the identification of Grateloupia species is difficult using only traditional morphological traits because terete to blade-like thalli are highly variable in structure. The taxonomic status of congeners belonging to closely related genera is confused, particularly in East Asia (Wang et al. 2000; Zhao et al. 2012; Faye et al. 2004; Wang et al. 2001). For example, it had been necessary to transfer G. lanceolata and G. elliptica from the genus Pachymeniopsis (Kawaguchi 1997), but Gargiulo et al. (2013) resurrected again the genus Pachymeniopsis. The morphology and rbcL sequences of Grateloupia catenata were reexamined by Wang et al. (2000). Grateloupia subpectinata has been resurrected to full specific status; previously, it had been reduced to synonymy under G. asiatica and G. prolongata (Faye et al. 2004). Wilkes et al. (2005) has stressed the need to establish robust criteria for taxonomic discrimination. Therefore, reliable species identification will be required to take advantage of the bioindustrial potential of Grateloupia. DNA barcoding facilitates disentanglement of taxonomic confusion in such large and complex genus.
The DNA barcoding technique uses short, standardized DNA sequences from designated regions of the genome. The sequences function as tags for rapid and accurate species-level identification and biodiversity explorations (Hebert et al. 2003). Two DNA barcode markers, cytochrome c oxidase I (COI) and UPA, have been proposed as standard markers for cataloging red algal biodiversity and resolving differences between closely related species (Kucera and Saunders 2012). COI-5P (ca. 650 bp) is a protein-coding gene in the 5′ region of the mitochondrial cytochrome c oxidase I gene that has been investigated extensively as a taxonomic tool (Saunders 2005; Le Gall and Saunders 2010). This marker requires specific amplification primers for the various red algal lineages (Saunders 2008). UPA (ca. 400 bp) is the universal plastid amplicon in domain V of the 23S rRNA gene, and it has been amplified from numerous species in algal lineages using a single set of primers (Sherwood and Presting 2007). DNA barcoding has been applied previously to two species of Grateloupia (Yang et al. 2013): two congeners had a sequence divergence of 3.7–4.6 % in COI, but among conspecific individuals, sequence divergences were only 0.3 % in P. elliptica (as G. elliptica) and 1.0 % in P. lanceolata (as G. lanceolata), respectively (Yang et al. 2013).
The chloroplast-encoded large subunit of the Rubisco gene (rbcL) is frequently used for the phylogenetic analysis within the red algae at diverse taxonomic levels (Bellorin et al. 2008; Kim et al. 2008; Geraldino et al. 2009; Kim et al. 2010). Although molecular analyses have been used to clarify the taxonomic status of Grateloupia (Faye et al. 2004; D’Archino et al. 2007; Zhao et al. 2012), no DNA barcoding determination of the level of genetic variation has been performed. In addition, Kintokiocolax aggregato-cerantha Tanaka & Nozawa, characterized by a small and clustered morphology, was first described as a parasite of Grateloupia angusta (Tanaka and Nozawa 1960), but this species has not been confirmed yet by molecular analysis. In the present study, we applied DNA barcoding to Grateloupia, Pachymeniopsis, and Kintokiocolax using COI and UPA markers to define levels of genetic diversity and to discriminate species in Korea. Concurrently, we reviewed the phylogenetic relationships available from the sequence analysis of rbcL gene to make recommendations on the direction of the future studies.
Materials and methods
Collections of samples were conducted between 2009 and 2013. Each specimen was prepared as a herbarium voucher, and a small portion was removed for DNA analysis. Portions for molecular work were dried in silica gel and stored at −20 °C. A complete list of specimens is presented in Table 1. Samples used in morphological observations were preserved in 5 % formalin/seawater or pressed on herbarium sheets. Voucher specimens are housed at the herbaria of Jeju National University (JNUB), Jeju, Korea, and the National Institute of Biological Resources (NIBR), Incheon, Korea. Sections were produced by hand or with the aid of a freezing microtome. Sections on slides were stained with 1 % aqueous aniline blue acidified with a drop of 1 % HCl and mounted in 30 % Karo corn syrup. Photomicrographs were captured with a QIMAGING 1,394 camera (QImaging, Canada) attached to a BX50 microscope (Olympus, Japan). All images were imported into the Adobe PhotoShop 5.5 (Adobe Systems Inc., USA) for plate assembly.
Total genomic DNA was extracted following the protocol of a DNeasy Plant Mini Kit (Qiagen, Germany) modified by incubating the disrupted samples with AP1 buffer for a minimum of 1 h at 63 °C. AccuPower PCR Premix (Bioneer, Daejeon, Korea) was used according to the manufacturer’s recommendations for all PCR reactions. The PCR procedure for COI-5P followed the outline of Saunders (2005, 2008) except that we used an annealing temperature of 50 °C in reactions that involved primers. The PCR procedure for UPA followed Sherwood and Presting (2007). The PCR procedure for rbcL was as follows: initial denaturation at 96 °C for 4 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 50 °C for 1 min, an extension at 72 °C for 2 min, and a final extension at 72 °C for 7 min. PCR products were cleaned using an AccuPrep PCR Purification Kit (Bioneer, Korea). Sequencing was performed by a commercial contractor (Macrogen, Seoul, Korea).
Sequences obtained from COI, UPA, and rbcL genes were edited using Chromas version 1.45 software (Technelysium Pty Ltd, Australia); multiple sequence alignments were made in BioEdit (Hall 1999) and aligned visually. None of the alignments posed a problem since no gaps were observed. The COI-5P alignment consisted of 86 isolates and 661 nucleotides. The UPA alignment consisted of 59 isolates and 386 nucleotides. The rbcL alignment consisted of 69 isolates and 1,440 nucleotides. We assessed the level of variation in the sequences of the three genes; uncorrected (p) pair-wise genetic distances were estimated with the MEGA 4.0 software (Tamura et al. 2007). The clustering tree for COI was constructed with the MEGA 4.0 software using a neighbor-joining (NJ) algorithm based on the Kimura-2-parameter (K2P) distance method.
We used the rbcL dataset to evaluate the phylogenetic relationships of newly acquired genetic species groups, including the three outgroups Polyopes affinis, Polyopes constrictus, and Polyopes lancifolius. Maximum likelihood (ML) analysis was performed using the RAxML version 7.2.6 software (Stamatakis 2006) using the GTR + Γ evolutionary model. To identify the best tree, we constructed 200 independent tree inferences using the -# option with default -I (automatically optimized subtree pruning-regrafting rearrangement) and -c (25 distinct rate categories) software options. We performed 1,000 replications using the same software and settings to generate bootstrap values for the phylogeny.
Results
Molecular analyses
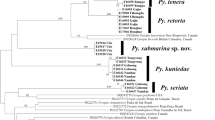
We report here on a total of nine species of Grateloupia, four species of Pachymeniopsis, and one Kintokiocolax based on the molecular analyses (Fig. 1); the taxa are listed in the Table 1. Among 101 collections, we amplified COI-5P sequences of 60 specimens selected, and neighbor-joining tree is depicted in Fig. 2. Genetic variation within groups ranged from 0 to 1.6 %, with a mean of 0.41 %. Interspecific genetic variation ranged from 3.7 to 14 %, with a mean of 9.3 %. Interspecific genetic variation between Pachymeniopsis volvita sp. nov. and other taxa ranged from 7.2–7.5 % (with P. elliptica) to 9.8–10 % (with HQ422590 G. filicina). Interspecific divergence between P. gargiuli and P. lanceolata, which are sister taxa, ranged from 3.7 to 4.4 %. G. jejuensis from Japan was 0.2 % divergent from six conspecific specimens collected in Korea. Intraspecific divergence reached 0.7 % among Korean specimens of G. divaricata. Japanese specimens of G. asiatica were 0.7 % divergent from Korean conspecifics. Seven samples of G. imbricata from Korea formed a clade with G. hawaiiana (HQ422635); interspecific genetic variation in this clade reached 0.5 %. Hawaiian samples identified as G. catenata were genetically linked to Korean collections of the same species; maximum sequence divergence in this group reached 1.6 %.
Nine species of Grateloupia, four of Pachymeniopsis, and one of Kintokiocolax used in this study. a Grateloupia angusta with the parasite, Kintokiocolax (enlargement), Jeju, Korea (5 May 2012). b G. asiatica, Gangneung, Korea (19 May 2012). c G. catenata, Jeju, Korea (1 June 2012). d G. divaricata, Gangneung, Korea (19 May 2012). e P. elliptica, Uljin, Korea (28 April 2012). f G. imbricata, Namhaedo, Korea (20 May 2012). g G. jejuensis, Namhaedo, Korea (19 May 2012). h G. kurogii, Daesambudo, Korea (25 July 2012). i P. lanceolata, Namhaedo, Korea (19 May 2012). j G. subpectinata, Pohang, Korea (21 July 2012). k G. turuturu, Ulsan, Korea (20 July 2012). l Pachymeniopsis volvita sp. nov., Jeju, Korea (22 May 2013). m P. gargiuli, Jeju, Korea (16 May 2012). Scale bars: a, c, e, g, i, j = 3 cm; b, d, k = 5 cm; f, h, l = 2 cm
We sequenced UPA marker in 46 samples, and neighbor-joining analyses revealed few differences in the resolution of genetic species groups by comparison with COI-5P and rbcL (tree not shown). In particular, intraspecific and interspecific variability were overlapped in UPA analysis. Intraspecific divergences ranged from 0 to 0.9 %, with a mean of 0.28 %. A high intraspecific divergence was 0.9 % between specimens of G. catenata collected in Korea and Hawaii. We measured intraspecific variation value of 0.6 % in Korean G. divaricata and P. lanceolata. Intraspecific divergences in G. subpectinata and G. imbricata reached 0.3 %. Interspecific variation ranged from 0.6 to 4.6 %, with a mean of 2.6 %. We detected an interspecific divergence of 0.6 % between G. angusta and G. kurogii.
We successfully sequenced rbcL gene in 30 specimens, and maximum likelihood estimation for this gene is represented in Fig. 3. Intraspecific divergences among our specimens ranged from 0 to 0.9 %, with a mean of 0.22 %. Interspecific divergences ranged from 1.0 to 9.9 %, with a mean of 6.44 %. The highest intraspecific divergence (0.9 %) was detected among collections of G. subpectinata from Korea, Japan, and France (as G. filicina var. luxurians). We found nine species of Grateloupia, four species of Pachymeniopsis, and one of Kintokiocolax in four different subclades of the rbcL phylogenetic tree with strong bootstrap values. ML analysis produced an unknown Pachymeniopsis species, P. volvita sp. nov. We measured an interspecific divergence of 1.0–1.2 % between P. gargiuli and P. lanceolata (100 % bootstrap value) and 1.9–2.3 % between the sequences of P. volvita sp. nov. and P. elliptica (100 % bootstrap value). Intraspecific divergence among specimens of P. volvita sp. nov. reached 0.2 %. G. imbricata specimens from Korea and Japan were sister to G. crispate from Japan (0.2–0.3 % interspecific divergences). Kintokiocolax aggregato-cerantha fell in the Grateloupia clade and grouped with G. cornea and G. chiangii from Japan (2.8–3.6 % divergences; low bootstrap value: 52 %) in the ML analysis.
Taxonomic results
Pachymeniopsis volvita M.Y. Yang et M.S. Kim sp. nov. (Fig. 4)
Pachymeniopsis volvita sp. nov. a Holotype (JN-HAL073), a tetrasporophyte collected from Siheung, Jeju, Korea, on 30 August 2013. b Specimens showing to be rolled toward ventral when detached from substrate. c Ventral surface with single holdfast. d Transverse section of the thallus showing cortex. e Transverse section of the thallus showing medulla with granular protoplasts. f Transverse section of the thallus showing tetrasporangia cruciately divided (arrows). g Formation of auxiliary cell ampulla. h Mature cystocarp. Scale bars: a, b = 2 cm; c = 1 cm; d = 50 μm; e = 200 μm; f, g = 30 μm; h = 100 μm
Description
Thallus solitary, up to 6 cm tall, flattened, circle to peltate or reniform, dark brown, fleshy coriaceous and slippery in texture, lacking a stipe, arising from single discoid holdfast 0.7 cm in diameter, rolling toward the ventral surface when detached from the substratum; margins entire or irregularly curved, sometimes crenulate. Cortex composed of six to nine layers of outer moniliform cells and one or two layers of polygonal shaped inner cells; medulla refractive filaments with granular protoplasts. Tetrasporophytes and carposporophytes isomorphic. Tetrasporangia forming from the cortical cell layer, cruciately divided when mature, 43–52 μm long, 13–16 μm wide. Carposporangial ampullae not observed, auxiliary cell ampullae abundant; first-order ampullar filaments initiating from subbasal inner cortical cell, more than 13 cells long and curved, grows apically; the sixth cell of the primary filament enlarged, a function as the auxiliary cell producing a simple second-order filament. Mature cystocarp 135–220 μm in diameter; carposporangia rounded, 10–17 μm in diameter.
Holotype
JN-HAL073 (tetrasporophyte, Fig. 4a) collected from Siheung-ri, Jeju Province, Korea (33° 28′ 30′′ N, 126° 54′ 41′′ E) on 30 August 2013, deposited in JNUB (Herbarium of the Department of Biology, Jeju National University, Korea).
Isotypes
JN-HAL085 ∼ 087 (JNUB), NIBRAL0000138753 ∼ 55 (NIBR).
Etymology
The specific epithet (volvita) was chosen to describe the ventrally oriented thallus rolling of specimens following detachment from the substratum.
Habitat
Pachymeniopsis volvita was collected at 10–15 m depth where plants grew on rocky substrata or conch shells. Reproductive thalli were collected mostly during summer. A hitherto endemic in Jeju Island, Korea.
Other specimens examined
JN-HAL082, vegetative thallus (Gapado, Jeju on 4 Mar. 2013); JN-HAL080, vegetative thallus (Taeheung, Jeju on 5 Mar. 2013); JN-HAL071, vegetative thallus (Mureung, Jeju on 15 Mar. 2013); JN-HAL073, vegetative thallus (Siheung, Jeju on19 Mar. 2012); JN-HAL083, tetrasporophyte (Gangjeong, Jeju on 3 Jun. 2013); JN-HAL075, tetrasporophyte (Seopsum, Jeju on 4 May 2013); JN-HAL056, female gametophyte (Oedo, Jeju on 6 Aug. 2012).
Morphology
Thallus is solitary, flattened, circle to peltate or reniform, and up to 6 cm in length (Fig. 4a). Blades are dark brown in color, fleshy coriaceous, and slippery in texture. Margins are entire or irregularly curved, sometimes crenulate, rolling toward the ventral surface when detached from the substratum (Fig. 4b). Thallus is arising from single discoid holdfast, 0.7 cm in diameter and lack stipe (Fig. 4c). In the cross section, thallus is 660–775 μm in thickness; it comprises a compact cellular cortex and a filamentous medulla. The cortex comprises six to nine layers of outer moniliform cells measuring 2–6 × 4–12 μm and one or two layers of polygonal shaped inner cells (Fig. 4d). The medulla comprises stellate cells and refractive filamentous cell, sometimes with granular protoplasts, 400–454 μm in diameter (Fig. 4e).
Tetrasporangia occur scattered over the entire thallus and form from cortical cells. Mature tetrasporangia are narrowly ellipsoidal and cruciately divided, measuring 43–52 μm in length and 13–16 μm in width (Fig. 4f).
Gonimoblasts are scattered over the thallus and are embedded between the cortex and medulla of fertile blades. Carpogonial ampullae were not observed in the specimens examined; auxiliary cell ampullae were abundant in all female plants. Initials of the first-order ampullar filaments are cut off from the subbasal inner cortical cells. The first-order filament is more than 13 cells long and curved and grows apically by transverse divisions (Fig. 4g). The sixth cell of the primary filament is enlarged and a function as the auxiliary cell, and produces a simple second-order filament toward the thallus surface. Mature cystocarps are 135–220 μm in diameter (Fig. 4h). Carposporangia are rounded and 10–17 μm in diameter (Fig. 4h). Spermatangial thalli were not found in our collections.
Discussion
The difficulties encountered in attempting to identify red macroalgae may be greatly mitigated by procedures of molecular-assisted alpha taxonomy (Saunders 2008), such as DNA barcoding. DNA barcoding has advantages in the ease of sequencing and aligning the relatively short fragments to provide of additional evidence by complementing morphological traits (Hebert et al. 2003; Saunders 2005). To determine the level of genetic diversity among Grateloupia species in Korea, we performed analyses of DNA barcoding: 661 bp of COI-5P (60 specimens) and 386 bp of UPA (46 specimens). Yang et al. (2013) had previously barcoded two Grateloupia species. COI and UPA have been officially accepted as DNA barcodes for marine red algal taxa (Kucera and Saunders 2012). Our analysis allowed us to clearly discriminate nine species of Grateloupia, four species of Pachymeniopsis, and one Kintokiocolax in Korea. COI provided valuable data and may be a more useful barcode than UPA for accurate identification of Grateloupia species. According to Hebert et al. (2003), COI-5P is highly variable, especially in the third codon position, which makes it an effective element for discriminating between even closely related species. Although a total of 14 species examined were relatively variable in morphology, intraspecific divergence in COI was 1.6 %, and interspecific divergences ranged from 3.7 to 14 %. This clear barcode gap between intraspecific and interspecific divergences in Grateloupia and Pachymeniopsis is similar to patterns in other red algal taxa (Kucera and Saunders 2012).
An intraspecific divergence of >2 % is generally regarded as adequate for the discrimination of red algal species (Le Gall and Saunders 2010). As an alternative, Hebert et al. (2004) proposed a standard sequence threshold divergence for species discrimination that is tenfold greater than the mean intraspecific variation within the group. In Pachymeniopsis volvita sp. nov., we examined 0.66 % intraspecific divergence. Hence, the standard sequence threshold divergence for species discrimination would be 6.6 %. We detected a minimum interspecific divergence of 7.6 % (based on COI gene) in P. gargiuli and P. volvita sp. nov. Therefore, COI barcode is now available for the identification of Pachymeniopsis species. Kucera and Saunders (2012) compared molecular markers for use as species identification tools among members of the Bangiales. They demonstrated that COI-5P had the highest level of variability and therefore the greatest taxonomic resolving power. However, they found that significant effort was required to develop appropriate primers. They also identified an intron (4–5 kb) in COI-5P of the Bangiales. We did not detect introns in our study of Grateloupia and were able to demonstrate the highest levels of intraspecific and interspecific divergence in this barcode. Levels of intraspecific and intraspecific variation overlapped in UPA (unlike COI-5P), indicating reduced taxonomic resolving power for this second barcode. Hence, UPA is unsuitable as a marker for species identification in the genus Grateloupia, as in the Bangiales (Kucera and Saunders 2012).
We also confirmed the presence of nine species of Grateloupia, four species of Pachymeniopsis, and one of Kintokiocolax in Korea using rbcL phylogeny. The interspecific divergence among these species ranged from 1.1 to 9.9 %; 14 species were detected in four subclades. Subclade I included eight species: P. lanceolata, P. elliptica, P. gargiuli, P. volvita sp. nov. G. kurogii, G. angusta, G. imbricata, and Kintokiocolax aggregato-cerantha. Yang et al. (2013) recently reassessed the relationships between P. elliptica and P. lanceolata (as Grateloupia); they used molecular identification to more accurately define genetic diversity in these morphologically confused taxa, which share blade-like thalli with leathery textures (Kawaguchi 1997; Lee and Lee 1993). In our study, COI, UPA, and rbcL analyses detected a cryptic species, Pachymeniopsis volvita sp. nov. with morphology very similar to young specimens of G. kurogii, which have circular blades with refractive cells (Kawaguchi 1990), but the interspecific divergence of two species is 3.8–4.2 % (Table 2). RbcL gene sequence of P. volvita sp. nov. diverged from that of P. elliptica by 1.9–2.3 %. G. imbricata Holmes was first described in Japan and recently introduced into the Canary Islands (Garcia-Jiménez et al. 2008). In our COI and UPA analyses, G. hawaiiana from Hawaii (HQ422635) fell among specimens of G. imbricata from Korea (Sherwood et al. 2010). G. angusta was first described by Harvey as a variety of Gymnogongrus ligulatus Harvey from specimens collected at Shimoda, Japan; many nomenclatural changes have been made since that time. Finally, Wang et al. (2001) transferred Cryptonemia angusta Okamura into Grateloupia angusta based on the texture of blades and the positions of reproductive structures. We found that Kintokiocolax aggregato-cerantha is genetically grouped with G. chiangii and G. cornea, with 2.9–3.7 % interspecific divergences.
Our subclade II included G. jejuensis, G. asiatica, and G. divaricata. Kim et al. (2013) reported G. jejuensis as a new species and described diagnostic characteristics, including a caespitose and flattened habit with discoid holdfasts, cartilaginous texture, and a blunt or bifid axis. We found that G. jejuensis differed by 27–29 bp (2.28–2.45 %) from G. elata in rbcL gene and by 63 bp (5.48 %) from G. cornea. G. asiatica was proposed as a new species from the northwestern Pacific by Kawaguchi and Wang (Kawaguchi et al. 2001). G. asiatica may be distinguished by its compressed to narrowly flattened axes with numerous pinnate proliferations. G. divaricata was first recognized as Carpopeltis divaricata by Okamura (1934) and characterized by compressed thalli with cuneate bases, repeatedly dichotomously branching in one plane, and rounded axils that result in a flabellate shape (Kawaguchi 1989).
Our subclade III included G. subpectinata and G. turuturu. Faye et al. (2004) reinstated G. subpectinata based on rbcL sequences and morphological features, including fleshy texture, thick axes, long proliferations, and oblong auxiliary cells. Their sequences for G. subpectinata from Japan (AB114208) diverged by 0.6–0.9 % from those of three conspecific specimens from Korea. Our rbcL analyses also showed that G. subpectinata is genetically grouped with G. luxurians from France and Australia (AJ868492 and AY435175). G. luxurians, however, is currently regarded as a taxonomic synonym of G. subpectinata Holmes (Wilkes et al. 2005; De Clerck et al. 2005). G. turuturu Yamada was originally described from Hokkaido, Japan, and is distinguished by the anticlinal arrangement of medullary filaments, a thin cortex of roundish cells and an abrupt transition between cortex and medulla (Gavio and Fredericq 2002). G. turuturu has been the focus of much research as it has invaded many parts of the world (Verlaque et al. 2005; Saunders and Withall 2006).
Our subclade IV included G. catenata, which was first described by Yendo from Hokkaido, in northern Japan. It has subsequently been reduced to synonymy of several other taxa (Sheng et al. 2012). Wang et al. (2000) clarified the taxonomic status of western Pacific G. catenata, which has a hollow axis, numerous short proliferations, and a tendency for reproductive structures to be restricted to the proliferations. In our COI analyses, Korean specimens of G. catenata fell among Hawaiian specimens, with a 1.6 % intraspecific divergence value. UPA sequences also demonstrated a similar topology in samples from Hawaii (Sherwood et al. 2010).
In conclusion, we have demonstrated for the first time that COI and UPA barcoding markers are effective for species identification in the genus Grateloupia and Pachymeniopsis, but COI gene had the greatest utility at the species level. Within- and between-species divergence values were lower for UPA than for COI-5P. UPA may lack resolving power among closely related species, which could lead to underestimations of species diversity (Clarkston and Saunders 2010). COI-5P and rbcL markers in combination provide a powerful tool for species identification, discovery of cryptic species, and phylogenetic reconstruction (Kucera and Saunders 2012). We confirmed the presence of a cryptic species: Pachymeniopsis volvita sp. nov.
References
Bellorin AM, Buriyo A, Sohrabipour J, Oliveira MC, Oliveira EC (2008) Gracilariopsis mclachlanii sp. nov. and Gracilariopsis persica sp. nov. of the Gracilariaceae (Gracilariales, Rhodophyceae) from the Indian Ocean. J Phycol 44:1022–1032
Clarkston BE, Saunders GW (2010) A comparison of two DNA barcode markers for species discrimination in the red algal family Kallymeniaceae (Gigartinales, Florideophyceae), with a description of Euthora timburtonii sp. nov. Botany 88:119–131
D’Archino RD, Nelson WA, Zuccarello GC (2007) Invasive marine red alga introduced to New Zealand waters: first record of Grateloupia turuturu (Halymeniaceae, Rhodophyta). N Z J Mar Freshw Res 41:35–42
Dawczynski C, Schubert R, Jahreis C (2007) Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem 103:891–899
De Clerck O, Gavio B, Fredericq S, Bárbara I, Coppejans E (2005) Systematics of Grateloupia filicina (Halymeniaceae, Rhodophyta), based on rbcL sequence analyses and morphological evidence, including the reinstatement of G. minima and the description of G. capensis sp. nov. J Phycol 41:391–410
Denis C, Jeune HL, Gaudin P, Fleurence J (2009a) An evaluation of methods for quantifying the enzymatic degradation of red seaweed Grateloupia turuturu. J Appl Phycol 21:153–159
Denis C, Morancais M, Gaudin P, Fleurence J (2009b) Effect of enzymatic digestion on thallus degradation and extraction of hydrosoluble compounds from Grateloupia turuturu. Bot Mar 52:262–267
Denis C, Morancais M, Li M, Deniaud E, Gaudin P, Wielgosz-Collin G, Barnathan G, Jaouen P, Fleurence J (2010) Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem 119:913–917
Faye ET, Wang HW, Kawaguchi S, Shimada S, Masuda M (2004) Reinstatement of Grateloupia subpectinata (Rhodophyta, Halymeniaceae) based on morphology and rbcL sequences. Phycol Res 52:59–68
Garcia-Jiménez P, Geraldino PJL, Boo SM, Robaina RR (2008) Red alga Grateloupia imbricata (Halymeniaceae), a species introduced into the Canary Islands. Phycol Res 56:166–171
Gargiulo GM, Morabito M, Manghisi A (2013) A re-assessment of reproductive anatomy and postfertilization development in the systematic of Grateloupia (Halymeniales, Rhodophyta). Cryptogam Algol 34:3–35
Gavio B, Fredericq S (2002) Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora. Eur J Phycol 37:349–360
Geraldino PJL, Yang EC, Kim MS, Boo SM (2009) Systematics of Hypnea asiatica sp. nov. (Hypneaceae, Rhodophyta) based on morphology and nrDNA SSU, plastid rbcL, and mitochondrial cox1. Taxon 58:606–616
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc Lond B 270:313–321
Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM (2004) Identification of birds through DNA barcodes. PLoS Biol 2:1657–1663
Inderjit CD, Ranelletti M, Kaushik S (2006) Invasive marine algae: an ecological perspective. Bot Rev 72:153–178
Kawaguchi S (1989) The genus Prionitis (Halymeniaceae, Rhodophyta) in Japan. J Fac Sci Hokkaido Univ Series V (Botany) 16:193–257
Kawaguchi S (1990) Grateloupia kurogii, a new species of the Halymeniaceae (Rhodophyta) from Japan. Jap J Phycol 38:135–146
Kawaguchi S (1997) Taxonomic notes on the Halymeniaceae (Gigartinales, Rhodophyta) from Japan, III. Synonymization of Pachymeniopsis Yamada in Kawabata with Grateloupia C. Agardh. Phycol Res 45:9–21
Kawaguchi S, Wang HW, Horiguchi T, Sartoni G, Masuda M (2001) A comparative study of the red alga Grateloupia filicina (Halymeniaceae) from the northwestern Pacific and Mediterranean with the description of Grateloupia asiatica, sp. nov. J Phycol 37:433–442
Kim MS, Yang EC, Kim SY, Hwang IK, Boo SM (2008) Reinstatement of Gracilariopsis chorda (Gracilariaceae, Rhodophyta) based on plastid rbcL and mitochondrial cox1 sequences. Algae 23:209–217
Kim MS, Kim SY, Nelson W (2010) Symphyocladia lithophila sp. nov. (Rhodomelaceae, Ceramiales), a new Korean red algal species based on morphology and rbcL sequences. Bot Mar 53:233–241
Kim SY, Han EG, Kim MS, Park JK, Boo SM (2013) Grateloupia jejuensis (Halymeniales, Rhodophyta): a new species previously confused with G. elata and G. cornea in Korea. Algae 28:233–240
Kim SY, Manghisi A, Morabito M, Yang EC, Yoon HS, Miller KA, Boo SM (2014) Genetic diversity and haplotype distribution of Pachymeniopsis gargiuli sp. nov. and P. lanceolata (Halymeniales, Rhodophyta) in Korea, with notes on their non-native distributions. J Phycol. Doi:10.1111/jpy.12218-13-242
Kucera H, Saunders GW (2012) A survey of Bangiales (Rhodophyta) based on multiple molecular markers reveals cryptic diversity. J Phycol 48:869–882
Le Gall L, Saunders GW (2010) DNA barcoding is a powerful tool to uncover algal diversity: a case study of the Phyllophoraceae (Gigartinales, Rhodophyta) in the Canadian flora. J Phycol 46:374–389
Lee HB, Lee IK (1993) A taxonomic study on the genus Pachymeniopsis Halymeniaceae, Rhodophyta in Korea. Korean J Phycol 8:55–65
Lin SM, Liang HY, Hommersand MH (2008) Two types of auxiliary cell ampullae in Grateloupia (Halymeniaceae) including G. taiwanensis sp. nov. and G. orientalis sp. nov. from Taiwan based on rbcL gene sequence analysis and cystocarp development. J Phycol 44:196–214
Mathieson AC, Dawes CJ, Pederson J, Gladych RA, Carlton JT (2008) The Asian red seaweed Grateloupia turuturu (Rhodophyta) invades the Gulf of Maine. Biol Invasions 10:985–988
Miller KA, Hughey JR, Gabrielson PW (2009) First report of the Japanese species Grateloupia lanceolata (Halymeniaceae, Rhodophyta) from California, USA. Phycol Res 57:238–241
Okamura K (1934) Icones of Japanese algae, vol 7 pp. 19–48 (English), 17–44 (Japanese). Plates CCCXI-CCCXXV, Tokyo
Saunders GW (2005) Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philos Trans R Soc Lond B 360:1879–1888
Saunders GW (2008) A DNA barcode examination of the red algal family Dumontiaceae in Canadian waters reveals substantial cryptic species diversity. 1. The foliose Dilsea–Neodilsea complex and Weeksia. Botany 86:773–789
Saunders GW, Withall RD (2006) Collections of the invasive species Grateloupia turuturu (Halymeniales, Rhodophyta) from Tasmania, Australia. Phycologia 45:711–714
Sheng YW, Zhang W, Zhao D, Wang HW (2012) A morphological and molecular assessment of the genus Sinotubimorpha (Halymeniaceae, Rhodophyta). J Syst Evol 50:146–152
Sherwood AR, Presting GG (2007) Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. J Phycol 43:605–608
Sherwood AR, Kurihara A, Conklin KY, Sauvage T, Presting GG (2010) The Hawaiian Rhodophyta biodiversity survey (2006–2010): a summary of principal findings. BMC Plant Biol 10:1–29
Simon C, ArGall E, Deslandes E (2001) Expansion of the red alga Grateloupia doryphora along the coasts of Brittany (France). Hydrobiologia 443:23–29
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood- based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA 4: molecular evolutionary genetic analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tanaka T, Nozawa Y (1960) One red algal parasite from Japan. Mem Fac Fish Kagoshima Univ 9:107–113
Verlaque M, Brannock PM, Komatsu T, Villalard-Bohnsack M, Marston M (2005) The genus Grateloupia C. Agardh (Halymeniaceae, Rhodophyta) in the Thau Lagoon (France, Mediterranean): a case study of marine plurispecific introductions. Phycologia 44:477–496
Wang HW, Kawaguchi S, Horiguchi T, Masuda M (2000) Reinstatement of Grateloupia catenata (Rhodophyta, Halymeniaceae) on the basis of morphology and rbcL sequences. Phycologia 39:228–237
Wang HW, Kawaguchi S, Horiguchi T, Masuda M (2001) A morphological and molecular assessment of the genus Prionitis J. Agardh (Halymeniaceae, Rhodophyta). Phycol Res 49:251–262
Wang SC, Bligh SWA, Shi SS, Wang ZT, Hu ZB, Crowder J, Branford-White C, Vella C (2007) Structural features and anti-HIV-1 activity of novel polysaccharides from red algae Grateloupia longifolia and Grateloupia filicina. Int J Biol Macromol 41:369–375
Wilkes RJ, McIvor LM, Guiry MD (2005) Using rbcL sequence data to reassess the taxonomic position of some Grateloupia and Dermocorynus species (Halymeniaceae, Rhodophyta) from the north-eastern Atlantic. Eur J Phycol 40:53–60
Yang MY, Han EG, Kim MS (2013) Molecular identification of Grateloupia elliptica and G. lanceolata (Rhodophyta) inferred from plastid rbcL and mitochondrial COI genes sequence data. Genes Genom 35:239–246
Zhao D, Wang HW, Sheng YW, Lu JZ, Luan RX (2012) Morphological observation and rbcL gene sequences studies of two new species, Grateloupia dalianensis H.W. Wang et D. Zhao, sp. nov. and G. yinggehaiensis H.W. Wang et R.X. Luan, sp. nov. (Halymeniaceae, Rhodophyta) from China. Acta Oceanol Sin 31:109–120
Acknowledgments
We thank Mr. J.C. Kang for collecting samples from the subtidal and other members of the molecular phylogeny of marine algae laboratory at Jeju National University. This study was supported by a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR No. 2013-02-001 for collecting samples and NIBR No. 2012-02-042 for molecular phylogeny of Korean major taxa).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, M.Y., Kim, M.S. Taxonomy of Grateloupia (Halymeniales, Rhodophyta) by DNA barcode marker analysis and a description of Pachymeniopsis volvita sp. nov.. J Appl Phycol 27, 1373–1384 (2015). https://doi.org/10.1007/s10811-014-0432-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0432-1