Abstract
The marine red algae Grateloupia is the largest genus in the family Halymeniaceae and widely distributed from tropical to warm temperate regions of the world. In the genus Grateloupia, especially G. elliptica and G. lanceolata have common features of bladelike thalli with leather in texture and cruciately divided tetrasporangia. Due to this similar morphology, G. elliptica and G. lanceolata are frequently confused and resulted in considerable difficulty distinguishing these two taxa. We have reassessed the relationships between two species using molecular identification including plastid rbcL and mitochondrial COI genes to more accurately define their genetic diversity owing to the confusion of identification. As a result, the chloroplast-encoded rbcL sequence analyses support the distinction of two species, G. elliptica and G. lanceolata collected from Jeju Island, Korea and Japan at the species level, with interspecific divergence of 3.7-4.6 %. The genetic diversity of COI gene within species are estimated to be 0–0.3 % in G. elliptica and 0–1.0 % in G. lanceolata, respectively. The effectiveness of mtDNA COI barcoding in the identification for two species demonstrates in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The red algal genus Grateloupia C. Agardh belongs to the family Halymeniaceae and grows a wide region in temperate and tropical waters (Kawaguchi et al. 2001). Grateloupia is one of the most taxonomically complex genus because they are very variable in gross morphology, such as overall habit, texture, cortex structure, and the location of reproductive structures (De Clerck et al. 2005; Wilkes et al. 2005; Garcia-Jiménez et al. 2008). Although species of the genus are considered difficult to define, recent molecular analyses are beginning to elucidate the taxonomic status of some morphologically similar species. For examples, Grateloupia filicina from the eastern Asian entity was described as a new species, Grateloupia asiatica, based on rbcL sequences (Kawaguchi et al. 2001). Grateloupia turuturu and Grateloupia imbricata, native to Japan and Korea, were considered to be invasive in Western Europe, North America, and Tasmania by molecular analysis of rbcL and cox2–3 sequences (Gavio and Fredericq 2002; D’Archino et al. 2007; Garcia-Jiménez et al. 2008). Faye et al. (2004) reinstated the taxonomic entity of the north-western Pacific species of Grateloupia subpectinata, which has been placed into synonymy under G. asiatica or Grateloupia prolongata in previous reports.
Wilkes et al. (2005) have shown that further study of foliose Grateloupia is required in the Mediterranean. In the Northwest Pacific Asia, there are reported six foliose Grateloupia species: Grateloupia elliptica, G. imbricata, G. kurogii, G. lanceolata, G. sparsa, G. turuturu (Yoshida 1998; Lee 2008). Especially G. elliptica and G. lanceolata have common features of bladelike thalli with leather in texture, and a small fusion cell arising gonimoblasts. Due to this similar morphology, G. elliptica and G. lanceolata are frequently confused and resulted in difficulty distinguishing these two taxa. G. elliptica was originally described by Holmes (1896) based on the specimen collected at Enoshima, Japan. Kawabata (1957) described profusely branched auxiliary cell ampullae and transferred G. elliptica to the genus Pachymeniopsis. Moreover, P. elliptica (as G. elliptica) is very similar with Pachymeniopsis yendoi Kawabata in gross morphology. These morphological similarities strongly suggest that these species are conspecific in culture study of P. yendoi (Kawaguchi 1997) and therefore, P. yendoi was synonymous with P. elliptica, as did Lee and Lee (1993). G. elliptica is a commonly reported as an intertidal alga throughout Pacific Asia only (Yoshida 1998; Lee et al. 2009). Grateloupia lanceolata was described by Okamura (1934) based on the specimen collected at Enoshima, Japan, as Aeodes lanceolata. Kawabata (1954) proposed to combine A. lanceolata with the new genus Pachymeniopsis, as P. lanceolata. However, the overall anatomy and the spore development pattern of P. lanceolata did not support the generic segregation of Pachymeniopsis. P. lanceolata was, therefore, considered to be a species within the genus Grateloupia (Kawaguchi 1997). G. lanceolata is reported on the Northeast Pacific Asia (Lee 2008), North America (Miller et al. 2009), Mediterranean Sea (Verlaque et al. 2005) and Atlantic Ocean (Garcia-Jiménez et al. 2008). Although anatomical evidence has been used to identify two foliose similar species, this classical method is inadequate to distinguish two species because of the morphological similarity and variability (Gabrielson 2008).
The chloroplast-encoded large subunit of the Rubisco gene (rbcL) has been very commonly used for identification and phylogeny at the various levels of taxonomic ranks of red algae (Bellorin et al. 2008; Kim et al. 2008; Geraldino et al. 2009; Kim et al. 2010a). On the other hand, DNA barcoding is a diagnostic species identification technique that uses a short, standardized DNA region for genetic variation between species, providing a rapid and efficient method of species-level research (Saunders 2008; Kim et al. 2010b). The 5′-region of the mitochondrial cytochrome c oxidase subunit I (COI) gene has been used to examine intraspecific variation and has proven to be useful for resolving differences between closely related species (Le Gall and Saunders 2010). Although molecular analyses for Halymeniaceae have been conducted to clarify the taxonomic status (Faye et al. 2004; D’Archino et al. 2007; Lee et al. 2009), there is no study on determining the level of genetic variation for species boundaries of two species, G. elliptica and G. lanceolata, using DNA barcoding.
In the present study, we clarify the taxonomic position of these two entities and review the phylogenetic evidence based on the sequence analysis of chloroplast-encoded rbcL gene to discuss the direction further studies of the taxonomy of Grateloupia. At the same time, we performed DNA barcoding of mitochondrial COI gene of G. elliptica and G. lanceolata specimens to define the level of genetic diversity and to specify species identification for two similar foliose species.
Materials and methods
Sample collection and morphological observation
Eighteen samples of G. elliptica and twenty-eight samples of G. lanceolata were collected from Korea and Japan (Table 1). Specimens for morphological analyses were fixed in 5 % formalin/seawater and pressed as herbarium sheets, with exception of a small piece of thallus to be used for molecular study. The voucher specimens were deposited in the herbarium of Jeju National University (JNUB). Photographs were taken using a μ-Tough-8000 digital camera (Olympus, Tokyo, Japan) and plates were edited using Photoshop 7.0.1 (Adobe, San Jose, CA, USA). Voucher images of representative isolates of each entity are presented in Fig. 1.
Grateloupia elliptica Holmes and Grateloupia lanceolata (Okamura) Kawaguchi. A–F Grateloupia elliptica: A GM03; Geomundo, Korea (12 June 2010), B MI43; Misaki, Japan (30 April 2010), C MI46; Misaki, Japan (30 April 2010), D HAL013; Uljin, Korea (28 April 2012), E G112; Jeju, Korea (29 May 2010), F HAL014; Jeju, Korea (23 March 2012). G–K Grateloupia lanceolata: G EN17; Enoshima, Japan (29 April 2010), H HAL023; Jeju, Korea (21 March 2012), I HAL025; Jeju, Korea (27 March 2012), J HAL035; Namhaedo, Korea (19 May 2012), K MI06; Misaki, Japan (30 April 2010). Scale bars: 5 cm
DNA extraction
Field-collected samples were transported live to the laboratory. The piece of cleaned thallus was air-dried and desiccated with silica gel for DNA extraction. We ground the silica gel-dried thallus in liquid nitrogen and extracted the total genomic DNA using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany), and following the manufacturer’s instructions. The extracted DNA was stored at −20 °C and used to amplify rbcL and COI. Extracts were dissolved in 20 μL of distilled water for amplification.
RbcL and COI region amplification and sequencing
For amplification and sequencing of rbcL gene, the following specific primer pairs were used: rbcLF7-rbcLR753 and rbcLF645-rbcS start (Gavio and Fredericq 2002). PCR reaction of rbcL gene was carried out with an initial denaturation at 96 °C for 4 min, followed by 35 cycles of amplification (denaturation at 94 °C for 1 min, annealing at 50 °C for 1 min and extension at 72 °C for 2 min) with a final extension at 72 °C for 7 min. COI region was amplified using the following primer pairs: GazF1-GazR1 (Saunders 2005) and GHalF-COX1R1 (Saunders 2008). PCR reaction of COI was carried out with an initial denaturation at 96 °C for 4 min, followed by 40 cycles of amplification (denaturation at 94 °C for 30 s, annealing at 45 °C for 30 s and extension at 72 °C for 1 min) with a final extension 72 °C for 7 min. All PCR amplifications were carried out using Swift MaxPro thermal cyclers (ESCO, Singapore). The PCR products were purified using the AccuPrep PCR Purification Kit (Bioneer, Daejeon, Korea) and then sequenced commercially (Macrogen, Seoul, Korea).
Alignment and phylogenetic analysis
Both electropherogram outputs from each sample were edited using Chromas version 1.45 (Queensland, Australia). Total rbcL and COI sequences were organized using the multiple-sequence editing program BioEdit (Hall 1999) and aligned visually. To assess the level of variation in the sequences of rbcL and COI, uncorrected (p) pair-wise genetic distances were estimated with PAUP* v4.0b10 (Swofford 2002). Maximum likelihood (ML) analysis was performed using PAUP 4.0 (Swofford 2002). We determined the best model for the individual data sets using Modeltest 3.4 software (Posada and Crandall 1998). The best model was a general time reversible (GTR) evolutionary model with gamma correction for among-site variation (Γ) and the proportion of invariable sites (I). To estimate ML tree, we used a heuristic search with 100 random addition sequence replicates and tree bisection and reconnection (TBR) branch swapping. To test node stability, we performed bootstrap analyses with 1,000 replicated ML searches, using the same program and settings. Bayesian analyses (BA) were performed using MrBayes v.3.1.2 (Ronquist and Heulsenbeck 2003) using a GTR + I + Γ model. Posterior probabilities were estimated using a Metropolis coupled Markov chain Monte Carlo approach with sampling according to the Metropolis–Hastings algorithm. For each matrix, one million generations of two independent runs were performed with four chains and trees were sampled every 100 generations. Clustering tree on COI was performed in MEGA 4.0 (Tamura et al. 2007) using the Neighbor-joining (NJ) algorithm based on Kimura-2-parameter (K2P) distance method. To compare other data, we contained six COI sequences from GenBank. NJ tree was used to provide a visual display of COI variation within and between species.
Results
Molecular phylogeny
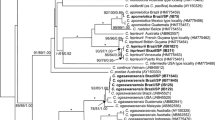
The phylogenetic relationships of Grateloupia species were determined through ML and BA analyses of the rbcL sequence data. The phylogenetic trees of ML and BA (data not shown) produced the same topology from a dataset (Fig. 2). We determined a total of 54 taxa for rbcL genes including 35 published sequences; 19 sequences collected from Korea and Japan, and two genera, Cryptonemia and Halymenia, as an outgroup. In total, 1,336 base pair (bp) of rbcL were aligned; 330 positions (24.7 %) were variable and 251 positions (18.7 %) were phylogenetically informative. Eight G. elliptica sequences from Korea (6) and Japan (2) were identical, although specimens from Kochi (AB055476) and Miyazaki (AB038605) of Japan differed by relatively high nucleotide divergences (2.4 %) from those of other sites. The 19 specimens of G. lanceolata from Korea (11) and Japan (8) were almost identical, with moderate divergence (1.0–1.2 %) in two specimens from Korea (GU168560, GT068). The interspecific sequence divergence values ranged 3.7–4.6 % between G. elliptica and G. lanceolata. The rbcL region within Grateloupia ranged from 0.7 % between G. filicina var. luxurians from Spain and G. subpectinata from Japan to 8.5 % between G. lanceolata and G. ramosissima. The most closely related genus, Cryptonemia and Halymenia, differed by 8.5–11.7 % from Grateloupia. In the phylogenetic tree (Fig. 2), specimens of G. elliptica and G. lanceolata formed a monophyletic clade with strong support (100 % for ML and BA in G. elliptica, 98 % for ML and 100 % for BA in G. lanceolata) and formed a sister group with G. kurogii and G. angusta.
Maximum likelihood phylogenetic tree of the red algal genus Grateloupia species estimated using rbcL sequence data. Numbers above each clade represent maximum likelihood bootstrap values and Bayesian posterior probabilities, respectively. Species name of the boldface type are shown the specimens collected in this study. Scale bar: substitutions/site
DNA barcoding
We obtained the fragment at the 5′-end of COI sequences for 37 specimens of G. lanceolata and G. elliptica from Korea and Japan, in addition to six sequences of Grateloupia from GenBank. No previously determined COI sequences were available through GenBank for G. elliptica and G. lanceolata. The length of the amplified region after editing was 616 bp; 138 positions (22.4 %) were variable and 116 positions (18.8 %) were phylogenetically informative. The neighbor-joining (NJ) tree using K2P model based on these sequences illustrated the levels of divergence within and between morphologically identified species (Fig. 3). Thirty-seven individuals resolved into two expected clusters that were assignable to G. lanceolata (n = 23; 15 isolates from Korea and 8 from Japan), and G. elliptica (n = 14; 12 from Korea and 2 from Japan). The within-species variation of Grateloupia was generally between 0 and 1.0 % divergence in G. lanceolata, and 0.3 % in G. elliptica. Within the clade of G. lanceolata, there is the highest genetic variation between specimen from Namhaedo (HAL035) and Jeju (G040). Within the clade of G. elliptica, there is relatively lower genetic variation than G. lanceolata. The sequence divergence (uncorrected distance) between different species ranged from 8.1 % (between G. lanceolata and G. elliptica) to 12.5 % (between G. lanceolata and G. phuquocensis).
Discussion
The comparative rbcL and COI sequence analyses point to significant differences between G. elliptica and G. lanceolata. RbcL sequences of G. elliptica from Korean populations analyzed were identical to two materials from Misaki, Japan, where is about 20 km away from type locality, Enoshima in Japan, even though other two specimens (AB055476 and AB038605 in Kawaguchi et al. 2001) from Japan separated from that clade by 2.4 % intraspecific divergences. On the other hand, eleven samples of G. lanceolata from Korea and eight ones from Japan including specimens from type locality, Enoshima in Japan were identical, and other samples from two localities differed from these sequences by 1.0–1.2 % intraspecific divergences. The DNA barcode fragment used here allowed the clear distinction between two species in the genus Grateloupia. We analyzed 616 bp of the COI gene for 23 specimens of G. lanceolata and 14 samples of G. elliptica from Korea and Japan for the first time. Although two species individuals were quite variable in morphology (Fig. 1), the intraspecific divergence of COI is 1.0 % (G. lanceolata) and 0.3 % (G. elliptica) with producing a monophyletic group each other. Two species were also clearly distinguishable from all published sequences (Sherwood et al. 2010) of Grateloupia in the COI phylogenetic tree (Fig. 3).
Grateloupia is the most species-rich genus of the red algal family Halymeniaceae, with at present over 50 species recognized (Guiry and Guiry 2011). Based on the morphological differences, species boundaries have been considered problematic because of the individual variation in gross morphology (De Clerck et al. 2005). Reliance on purely external morphological criteria has led to confusion with regard to two species, G. elliptica and G. lanceolata, which have similar morphology in the vegetative and reproductive structures of fully developed thalli (Kawaguchi 1997). However, this study has shown that many of these populations are in fact taxonomically distinct entities as Kawaguchi (1997) mentioned. The large thalli of G. elliptica can reach length of up to 30 cm and 800 μm thick in Korea (Fig. 1), in contrast to populations in Japan, which reach 40 cm and 1,300 μm thick (Kawaguchi 1997). The texture of both entities is basically leathery, but G. elliptica is somewhat thicker (18–20 cells of cortex) than G. lanceolata (10–20 cells of cortex). They can also be separable based on their basal structures: the discoid holdfast of G. elliptica is located on the undersurface of the thallus, whereas in G. lanceolata, it is having a short stipe (Kawaguchi 1997). These morphological differences are supported by our rbcL sequence analysis.
The phylogenetic trees obtained from ML analysis clearly revealed that G. elliptica is remote from G. lanceolata, with sequence divergences well within the interspecific values observed within Grateloupia (Garcia-Jiménez et al. 2008; Lee et al. 2009). Isolates of G. lanceolata from Misaki, Enoshima, Fukuoca, Hokkaido of Japan form a well-supported clade (98 % for ML and 100 % for BA), with two specimens from Jeju Island, Korea in 1.2 % intraspecific divergences. G. elliptica constitutes a distinct monophyletic subclade within the large Grateloupia clade together with the specimens from Miyazaki (AB038605 in Wang et al. 2000) and Kochi (AB055476 in Kawaguchi et al. 2001), Japan. In G. elliptica specimens from Korea, all had identical rbcL sequences from the Japanese samples (collected at “Misaki” MI43, MI46), except for two specimens from Japan (AB038605 and AB055476) having 2.4 % genetic variations unusually. This level of divergence is considerably higher intraspecific variation than other Grateloupia species observed (Wang et al. 2000, 2001; Gavio and Fredericq 2002; Faye et al. 2004; De Clerck et al. 2005; Mateo-Cid et al. 2005; Wilkes et al. 2005). Thus it is the need to confirm the identity of these specimens as G. elliptica. Values of interspecific rbcL sequence difference in the genus Grateloupia usually vary from 1.4 to 8.5 %, with the lowest divergence in 0.7 % (AJ868492 and AB114208).
To define the level of genetic diversity, we used DNA barcode COI which is officially accepted as the DNA barcode for marine red-algal groups (Saunders 2005). The benefit of COI barcoding is the ease of sequencing and aligning a relatively short fragment, and the supply of additional evidence to identification by complementing morphological characteristics (Le Gall and Saunders 2010). Our results indicate that COI can be valid and useful barcodes for accurate identification of two species, G. lanceolata and G. elliptica. Intraspecific variation of COI is 1.0 % in G. lanceolata and 0.3 % in G. elliptica. Interspecific one ranged from 8.1 % (G. lanceolata and G. elliptica) to 12.5 % (G. lanceolata and G. phuquocensis). Therefore, the clear barcode gap between intra- and interspecific divergences exists in this study of the genus Grateloupia as other red algal group, Gracilariaceae and Amansieae (Kim et al. 2010b; Sherwood et al. 2010). Generally, an intraspecific divergence of more than 2 % appears to be adequate to discriminate between species of red algae (Saunders 2008; Clarkston and Saunders 2010; Le Gall and Saunders 2010). Consequently, COI sequencing can now be used for identification of Grateloupia species providing better resolution and support for current taxonomy.
Grateloupia has been the focus of many researches as the major invasive genus in the marine ecosystem: for example, G. turuturu in Atlantic, the Mediterranean Sea, Australia and New Zealand (Verlaque et al. 2005; Saunders and Withall 2006; D’Archino et al. 2007), G. imbricata in the Canary Islands (Garcia-Jiménez et al. 2008), and G. lanceolata from nearly all tropical to cold-temperate regions, making it one of the most widespread species of red algae (Verlaque 2001; Miller et al. 2009). Although we did not have the information as an introduced species on G. elliptica, it is also possible to discover from all parts of the world because of its similar gross morphology with leathery thalli. A recent cryptic introduction could explain this observation. Taxon sampling in this study is too limited to fully appreciate the distribution of two Asian species. Additional detailed morphological and molecular studies of Grateloupia taxa will be induced the results of discovery and identification about invasive species from the worldwide.
References
Bellorin AM, Buriyo A, Sohrabipour J, Oliveira MC, Oliveira EC (2008) Gracilariopsis mclachlanii sp. nov. and Gracilariopsis persica sp. nov. of the Gracilariaceae (Gracilariales, Rhodophyceae) from the Indian Ocean. J Phycol 44:1022–1032
Clarkston BE, Saunders GW (2010) A comparison of two DNA barcode markers for species discrimination in the red algal family Kallymeniaceae (Gigartinales, Florideophyceae), with a description of Euthora timburtonii sp. nov. Botany 88:119–131
D’Archino R, Nelson WA, Zuccarello GC (2007) Invasive marine red alga introduced to New Zealand waters: first record of Grateloupia turuturu (Halymeniaceae, Rhodophyta). NZ J Mar Freshwater Res 41:35–42
De Clerck O, Gavio B, Fredericq S, Bárbara I, Coppejans E (2005) Systematics of Grateloupia filicina (Halymeniaceae, Rhodophyta), based on rbcL sequence analyses and morphological evidence, including the reinstatement of G. minima and the description of G. capensis sp. nov. J Phycol 41:391–410
Faye ET, Wang HW, Kawaguchi S, Shimada S, Masuda M (2004) Reinstatement of Grateloupia subpectinata (Rhodophyta, Halymeniaceae) based on morphology and rbcL sequences. Phycol Res 52:59–68
Gabrielson PW (2008) Molecular sequencing of Northeast Pacific type material reveals two earlier names for Prionitis lyallii, Prionitis jubata and Prionitis sternbergii, with brief comments on Grateloupia versicolor (Halymeniaceae, Rhodophyta). Phycologia 47:89–97
Garcia-Jiménez P, Gerladino PJL, Boo SM, Robaina RR (2008) Red alga Grateloupia imbricata (Halymeniaceae), a species introduced into the Canary Islands. Phycol Res 56:166–171
Gavio B, Fredericq S (2002) Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora. Eur J Phycol 37:349–360
Geraldino PJL, Yang EC, Kim MS, Boo SM (2009) Systematics of Hypnea asiatica sp. nov. (Hypneaceae, Rhodophyta) based on morphology and nrDNA SSU, plastid rbcL, and mitochondrial cox1. Taxon 58:606–616
Guiry MD, Guiry GM (2011) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. Accessed 08 Dec 2011
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Holmes EM (1896) New marine algae from Japan. J Linn Soc Bot 31:248–260, pls VII–XII
Kawabata S (1954) On the structure of the frond, and the reproductive organ of Pachymeniopsis lanceolata Yamada (Aeodes lanceolata Okam.). Jpn J Phycol (Sôrui) 2:67–71
Kawabata S (1957) On the structure of the frond and the reproductive organ of Pachymeniopsis yendoi Yamada (Syn. Pachymenia carsnosa Yendo, non J. Ag.). Bull Jpn Soc Phycol 5:8–13
Kawaguchi S (1997) Taxonomic notes on the Halymeniaceae (Gigartinales, Rhodophyta) from Japan, III. Synonymization of Pachymeniopsis Yamada in Kawabata with Grateloupia C. Agardh. Phycol Res 45:9–21
Kawaguchi S, Wang HW, Horiguchi T, Sartoni G, Masuda M (2001) A comparative study of the red alga Grateloupia filicina (Halymeniaceae) from the northwestern Pacific and Mediterranean with the desciption of Grateloupia asiatica, sp. nov. J Phycol 37:433–442
Kim MS, Kim M, Terada R, Yang EC, Boo SM (2008) Gracilaria parvispora is the correct name of the species known as G. bursa-pastoris in Korea and Japan. Taxon 57:231–237
Kim MS, Kim SY, Nelson W (2010a) Symphyocladia lithophila sp. nov. (Rhodomelaceae, Ceramiales), a new Korean red algal species based on morphology and rbcL sequences. Bot Mar 53:233–241
Kim MS, Yang MY, Cho GY (2010b) Applying DNA barcoding to Korean Gracilariaceae (Rhodophyta). Cryptogam Algol 31:387–401
Le Gall L, Saunders GW (2010) DNA barcoding is a powerful tool to uncover algal diversity: a case study of the Phyllophoraceae (Gigartinales, Rhodophyta) in the Canadian flora. J Phycol 46:374–389
Lee Y (2008) Marine algae of Jeju. Academy Publication, Seoul, pp i–xvi, 1–177, map
Lee HB, Lee IK (1993) A taxonomic study on the genus Pachymeniopsis (Halymeniaceae Rhodophyta) in Korea. Korean J Phycol 8:55–65
Lee JI, Kim HG, Geraldino PLJ, Hwang IK, Boo SM (2009) Molecular classification of the genus Grateloupia (Halymeniaceae, Rhodophyta) in Korea. Algae 24:231–238
Mateo-Cid LE, Mendoza-González AC, Gavio B, Fredericq S (2005) Grateloupia huertana sp. nov. (Halymeniaceae, Rhodophyta), a peculiar new prostrate species from tropical Pacific Mexico. Phycologia 44:4–16
Miller KA, Hughey JR, Gabrielson PW (2009) First report of the Japanese species Grateloupia lanceolata (Halymeniaceae, Rhodophyta) from California, USA (Research note). Phycol Res 57:238–241
Okamura K (1934) Icons of Japanese Algae, vol 7, No 5. Kazamashobo, Tokyo. pp 39–48, pls 321–325
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Ronquist F, Heulsenbeck JP (2003) Mrbayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Saunders GW (2005) Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philos Trans R Soc Lond B 360:1879–1888
Saunders GW (2008) A DNA barcode examination of the red algal family Dumontiaceae in Canadian waters reveals substantial cryptic species diversity. 1. The foliose Dilsea-Neodilsea comnplex and Weeksia. Botany 86:773–789
Saunders GW, Withall RD (2006) Collections of the invasive species Grateloupia turuturu (Halymeniales, Rhodophyta) from Tasmania, Australia. Phycologia 45:711–714
Sherwood AR, Kurihara A, Conklin KY, Sauvage T, Presting GG (2010) The Hawaiian Rhodophyta Biodiversity Survey (2006–2010): a summary of principal findings. BMC Plant Biol 10:258
Swofford DL (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA 4: molecular evolutionary genetic analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Verlaque M (2001) Checklist of the macroalgae of Thau Lagoon (Hérault, France), a hot spot of marine species introduction in Europe. Oceanol Acta 24:29–49
Verlaque M, Brannock PM, Komatsu T, Villalard-Bohnsack M, Marston M (2005) The genus Grateloupia C. Agardh (Halymeniaceae, Rhodophyta) in the Thau Lagoon (France, Mediterranean): a case study of marine plurispecific introductions. Phycologia 44:477–496
Wang HW, Kawaguchi S, Horiguchi T, Masuda M (2000) Reinstatement of Grateloupia catenata (Rhodophyta, Halymeniaceae) on the basis of morphology and rbcL sequences. Phycologia 39:228–237
Wang HW, Kawaguchi S, Horiguchi T, Masuda M (2001) A morphological and molecular assessment of the genus Prionitis J. Agardh (Halymeniaceae, Rhodophyta). Phycol Res 49:251–262
Wilkes RJ, McIvor LM, Guiry MD (2005) Using rbcL sequence data to reassess the taxonomic position of some Grateloupia and Dermocorynus species (Halymeniaceae, Rhodophyta) from the north-eastern Atlantic. Eur J Phycol 40:53–60
Yoshida T (1998) Marine algae of Japan. Uchida Rokakuho Publishing Co., Ltd., Tokyo, pp 1–2, 1–25, 1–1222
Acknowledgments
This work was supported by the National Institute of Biological Resources (NIBR No. 2012-02-042).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, M.Y., Han, E.G. & Kim, M.S. Molecular identification of Grateloupia elliptica and G. lanceolata (Rhodophyta) inferred from plastid rbcL and mitochondrial COI genes sequence data. Genes Genom 35, 239–246 (2013). https://doi.org/10.1007/s13258-013-0083-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-013-0083-7