Abstract
Oil and phenolics were extracted from Descurainia sophia (Sophia) seeds by a supercritical CO2 system. Extractions were conducted in two sequential steps, first using 100 % CO2 and then adding 10 % ethanol as co-solvent. The extracts were collected in each step using two separate collectors operating at different pressures. The extraction run was 3 and 4 h for the first period, and 2 h for the second period. The majority of the oil was collected in the first extraction period while phenolic compounds were obtained in the second extraction period. A combined mode of static/dynamic extraction (3 h running and 1 h soaking in CO2) was also used in the first extraction period, which enhanced the total extraction yield (29.3 ± 0.5 %) and was comparable to the 4 h extraction yield (31.4 ± 0.1 %). The total fatty acid (FA) content of oil in collector 1 (0.94 g) was nearly twice that in collector 2 (0.60 g). The oil contained 14 FAs with α-linolenic being predominant (48.5 %), with a total 91.1 % unsaturated FAs, a ω3/ω6 ratio of 2.7, and an erucic acid content of 6.2 %. More than 10 phenolic compounds were detected by HPLC in the Sophia seed extracts of which sinapic acid was the dominant compound. Sophia seed extracts showed high levels of antioxidant activity. These results suggest that Sophia seed oil and phenolics have the potential for functional food and pharmaceutical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Descurainia sophia L. Webb. ex Prantl (Sophia), commonly known as flixweed, belongs to the Brassicaceae family (mustards). Sophia can be distinguished from other close relatives including Sisymbrium species (tumble mustard), Descurainia pinnata (tansy mustard), and Descurainia richardsonii (grey tansy mustard) by its hair herbage branches and siliques/leaves/fruits shape and colour [1]. It is found throughout Canada and is one of the most abundant weeds in the Prairie Provinces and is well adapted to the climate of the Canadian prairies [1]. Sophia has traditionally been used as a folk medicine in many countries including China, India, and Iran [2]. The seed is edible, raw or cooked, and contains 28 % protein, 33 % oil, and 4 % ash [3]. Sophia seed and other seed from the Brassicaceae family have been considered as a potential source of seed oil for industrial utilization [4]. However, its application in the food industry has not been studied in detail and limited information is available. Sophia seed oil mainly consists of polyunsaturated α-linolenic (ω3) and linoleic (ω6) fatty acids [5], and so it can be considered a healthy or functional oil. Genetic and environmental factors can significantly affect the oil content and fatty acid composition of different Sophia varieties [4].
Hexane (and other organic solvents) is a good solvent for extracting free nonpolar lipids such as triglycerides, but is not efficient (a poor solvent) for polar lipids such as phospholipids, free fatty acids, and also bound lipid unless a pre-hydrolysing step is applied [6]. Furthermore, large amounts of organic solvents are used in the liquid–liquid extraction of fats which require subsequent evaporation prior to transesterification and analysis. Additionally, these solvents present potential hazards to both personnel and the environment. Supercritical fluid extraction (SFE) has recently been investigated as an alternative to organic-based extraction methods, especially supercritical CO2 (SC-CO2) owing to its effectiveness, low toxicity, reasonable cost, and reduced extraction time compared to organic solvents [7]. SC-CO2 is especially useful in the food and pharmaceutical industry where toxicity of the extraction medium, solvent entrapment, and thermal stability of the materials are major concerns. SC-CO2 is also a unique class of non-aqueous media in which the selectivity of extractable materials can be altered by changing the density of the SC-CO2. Therefore, the solvating power of the SC-CO2 is highly dependent on its temperature and pressure. CO2 can be applied at near room temperatures owing to its low critical temperature (31.1 °C), which minimizes the heat requirement and thus thermal damage to bioactive compounds. Raventós et al. [8] have reviewed the commercial applications and recent developments illustrating the different possibilities of SC-CO2 in industrial food processes.
Phenolic compounds are specialized metabolites found in most fruits and vegetables. They are involved in many environmental activities of the plant [9], contributing towards the colour and sensory characteristics of fruits and vegetables. They are involved in attracting pollinators as well as providing protection against pests and pathogens. Moreover, there is considerable interest in adding antioxidants from plants and herbs to processed food as an alternative to synthetic antioxidants, e.g. butylhydroxytoluene (BHT) and butylhydroxyanisole (BHA) [9].
Plant phytochemicals are conventionally extracted using organic solvents; however, the use of organic solvent has raised safety concerns for consumers. Supercritical fluid extraction (SFE) has been widely employed as an alternative to organic solvent extraction [7, 10–14]. Polar compounds, such as flavonoids and phenols, show low solubility in pure SC-CO2. The addition of an organic/polar solvent such as methanol, acetone, acetonitrile, and especially ethanol as co-solvent has been widely used to enhance the extraction efficiency of SFE. The extraction of plant natural compounds such as tocopherols, alkylresorcinols, and phenolics using organic co-solvent SC-CO2 has been developed during the last three decades [12].
There is limited information on the applications of Sophia seed as a potential source of bioactive compounds. Therefore, the objectives of this research were to (1) investigate the effect of time and combined static/dynamic mode of SFE on the oil and phenolics yield of Sophia seed extracted by SC-CO2, (2) analyse the fatty acid composition of extracted oil using GC-FID of FAMEs, (3) analyse the phenolics composition of SC-CO2 extract, meal, and whole seed using HPLC–PDA (photodiode array detector), and (4) evaluate the antioxidant activity of the SC-CO2 extract, meal, and whole seed.
Materials and Methods
Materials
The Sophia seeds were purchased from a local market in Ottawa, ON. Plants were grown from these seeds and positively identified as D. sophia by Brassicaceae expert Dr. Sara Martin (Eastern Cereal and Oilseed Research Centre, Agriculture and Agri-Food Canada, Ottawa, Canada) using key morphological features and DNA barcoding. The plants were positively identified as D. sophia by DNA barcoding using chloroplast markers matk, psbA, and rbcL, and the nuclear internal transcribed spacer (ITS). These DNA barcoding markers are able to distinguish D. sophia from D. californica and D. pinnata. The D. sophia specimen used here was deposited in the National Collection of Vascular Plants, Agriculture and Agri-Food Canada, Ottawa under herbarium accession number DAO 896491 and DNA barcode number 01-01000592773. Prior to chemical analyses, seeds were cleaned and inspected for external and contaminating substances. A mixture of 37 fatty acid methyl ester standards was purchased from Sigma (Supelco™ 37 component FAME Mix, Oakville, ON, Canada) for use in the GC-FAME analysis. Mono-and dibasic potassium phosphate, fluorescein, trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), rutin (quercetin 3β-d-rutinoside trihydrate), AAPH (2,2′-azobis(2-methylpropionamidine) dihydrochloride), β-carotene, Folin–Ciocalteau reagent, and SFE co-solvent (ethanol) were obtained from Sigma (Oakville, ON, Canada). Phenolic acid and flavonoid standards were purchased from Sigma-Fluka Analytical (Oakville, ON, Canada). The solvents used for HPLC, methanol and acetonitrile, were HPLC grade (Fisher Scientific Co., Ottawa, ON). All the other chemicals used were analytical grades.
SC-CO2 Extraction Method
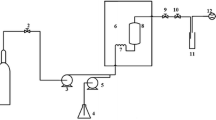
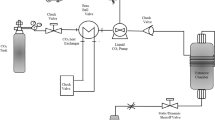
The extraction of oil and phenolics from Sophia seed was conducted using an SFE-1000F-2-FMC50 system (Thar Technology Inc., Pittsburgh, PA, USA). This apparatus consisted of one extraction vessel (500 mL), two collector vessels (500 mL each), a CO2 pump (P-200), a co-solvent pump (P-50A), an automated back pressure regulator (ABPR-20), and a cooling/heating recirculating chiller system (Accel 500 LC, Thermo Scientific, Newington, NH, USA), Fig. 1. The temperatures of vessels, fluids flow rate, and extraction vessel pressure were controlled by SuperChrom SFC Suite software (version 5.9, Thar Technologies Inc.). The temperature and pressure values for extraction were selected on the basis of SC-CO2 extraction of different oilseeds reported by Seal et al. [15]. A preliminary test was performed to study the kinetics of oil extraction. The extract was collected at 30-min intervals for 4 h. From those results the final parameters were selected as illustrated in Fig. 2. The first extraction period was performed using 100 % CO2, followed by the second extraction period by adding 10 % (wt%) ethanol as co-solvent. A combined static and dynamic mode of batch extraction was also tested in which an extraction run of 90 min (dynamic) followed by 1 h static period (the CO2 flow was stopped, but not depressurized), and then the extraction continued for 90 min (dynamic). For each extraction period, two collectors operating at different temperatures and pressures were used. By changing the solubility and transporting properties of fluid, the aim was to optimally fractionate the extracted materials. Four extract fractions were collected under these conditions, which were labelled E1-C1 and E1-C2 for the first extraction period and E2-C1 and E2-C2 for the second extraction period. The co-solvent (ethanol) was evaporated and extracts were stored at −20 °C for further analyses. Each extraction treatment was performed in triplicate (three repeats each using a fresh sample of the Sophia seed).
Esterification and GC-FID Analysis of Fatty Acid Composition
Lipid hydrolysation and methyl esterification of fatty acids as well as GC analysis of SC−CO2 oil fractions were performed as described by Li-Beisson et al. [16]. Briefly, 30.0 mg of oil extract was mixed in a 10-mL Teflon-lined screw-capped glass tube with 2 mL of 5 % (v/v) sulphuric acid in methanol, 25 µL of 0.2 % BHT solution, 5 mg of C17:0 TAG (triheptadecanoin) as internal standard, and 300 µL of toluene as co-solvent. The mixture was heated at 85 °C for 90 min with intermittent vortexing (speed 10 of a vortex mixer version 1.9.302.530, Fisher Scientific, Waltham, MA, USA), cooled down, and then 3 mL of 0.9 % NaCl and 2 mL of hexane added to extract FAMEs. After centrifugation, the organic phase was transferred to a GC vial and FAMEs analysed on a Varian GC-450 system equipped with autosampler, FID detector, and a polar column DB23 (30 m × 0.25 mm, 0.25 µm film; J&W Scientific, Folsom, CA, USA). The instrument was controlled by Galaxie software (Galexie Chromatography Data System, Varian Inc., Palo Alto, CA, USA) and the run conditions were helium (carrier gas) at a flow rate of 1.0 mL/min; split mode injection (1:40); injector and detector temperatures, 260 °C; oven temperature program 150 °C for 3 min, then increasing at 10 °C/min to 240 °C, and then held at this temperature for an additional 5 min. A mixture of 37 FAME standards was run under the same conditions for identification and quantification of sample peaks. To validate the peak identification, several individual FAME standards were injected singly under the same conditions. Also, the elution order of standards was compared to the results of Lee et al. [17] who analysed the same standards with the same column type and similar conditions.
Phenolics Extraction Using Acidified Ethanol
An acidified methanol extraction method was used to determine the phenolics composition of Sophia seed and meal. Ground whole Sophia seed and Sophia meal (residue after SFE) were subjected to acidified (1 N HCl) ethanol (15 % acid/85 % ethanol, v/v) with a sample to solvent ratio of 1:15 (g/mL), stirred for 6 h at RT, and followed by centrifugation at 2500 g at RT. The supernatant was kept at −20 °C for further analyses. Each treatment was performed in triplicate.
HPLC–PDA Analysis of Phenolics Composition
Phenolic acids and flavonoids from different extraction samples (whole seed, meal, and SFE extracts) were analysed using a Water Alliance HPLC system (Waters e2695) equipped with a PDA and EmPower 3 software (Waters, Milford, Massachusetts, USA). A reversed-phase column, Synergi Max-RP, 250 × 4.6 mm, 4 μm (Phenomenex, Torrance, CA, USA), with temperature set at 30 °C was used for separation. Phenolic acids and flavonoids were separated in a single run using a gradient elution program [18]. The mobile phases were 0.01 % formic acid/Milli-Q water (A) and 100 % acetonitrile (B) at a flow rate of 1.0 mL/min and a linear gradient increase of 10–50 % of solvent B in 35 min. Selected wavelengths used for qualitative and quantitative analysis were 280 nm for phenolic acids and 280 and 320 nm for flavonoids for all HPLC samples. Since some other compounds such as peptides and indoles also show absorbance at 280 nm, spectral data from 200–700 nm was also recorded for all sample and standard runs. The identification of phenolic compounds in the samples was performed by comparing the spectra of available standards with the spectra obtained for the samples. Five different concentrations of each phenolic acid were prepared in a mixture of 11 phenolic acids and the standard curve of each phenolic acid was plotted using the corresponding peak area. The regression value for the standard curves ranged between 0.9956 and 1.0000. The same procedure was followed for a mix of 10 flavonoids in a separate run and the regression value for the flavonoid standard curves ranged between 0.9959 and 1.0000. All samples were analysed in triplicate and the concentration of each phenolic acid and flavonoid was calculated using a standard curve equation and expressed in milligrams per gram. The identification of compounds with very close retention times (e.g. p-hydroxybenzoic acid and epicatechin) was confirmed using the whole spectra comparison of sample peak with the corresponding standard.
Antioxidant Activity Measurement
Antioxidant capacity of the extracted phenolics was evaluated by measuring total phenolic content (TPC), oxygen radical absorbance capacity (ORAC), and with a β-carotene bleaching assay as described previously [19]. All analyses were conducted in triplicate.
Statistical Analyses
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Analysis of variance (ANOVA) was used to determine if there was a significant difference (P < 0.05) among treatments including oil yield, fatty acids composition, phenolic composition, and antioxidant activity. Significant treatments were further analysed with Duncan’s multiple range test (P < 0.05).
Results and Discussion
SC-CO2 Extraction Yield
Figure 3 depicts the kinetics of Sophia seed oil extraction. The extraction curve is also presented by plotting cumulative yields against time. The extraction yield showed an increase for collector 1 in the first hour and a slight decrease up to 3 h, and showed an increase again in the last hour of extraction, whereas the extraction yield for collector 2 was maximum in the last hour of extraction. An extraction curve of solid material in dynamic mode usually consists of an initial period where the cumulative extraction increases with time until the curve reaches a plateau [7]. In our study, the cumulative yield increased up to 150 min, followed by a gradual increase. However, there was a second period of increased material being extracted, which occurred in the last hour of extraction (from 180 to 240 min). About 60 % of total oil exhaustion was completed after 3 h; therefore, for the next step two extraction times of 3 and 4 h were selected. Also, a combined static/dynamic extraction mode was selected. This would allow enough time for the extractable compounds to dissolve in the CO2.
Table 1 shows the extraction yields of Sophia seed samples. Different extraction times during the first period resulted in significantly (P < 0.001) different yields. The extractability of SC-CO2 depends on its density. At pressures near the critical point, a moderate temperature increase can cause a large decrease in CO2 density resulting in a decrease in solute solubility. However, at much higher pressures (e.g. 350 bar), the CO2 becomes less compressible and an increase in temperature causes a much less dramatic decrease in density [7]. We found that increasing the first extraction time period resulted in significantly higher extract yields for fractions E1-C1 and E1-C2, which are fractions enriched in oil as a result of extraction with neat SC-CO2 fluid (a nonpolar solvent). The majority of oil in the first extraction period was collected in collector 1 (E1-C1) operating at 40 °C and 120 bar pressure, and the oil yield % in collector 1 decreased as the first extraction period increased (92.8, 82.5, and 72.1 % for 3 h, 3 h and 1 h static, and 4 h treatments, respectively). These results confirmed that a longer extraction time not only provided more extracted oil but also facilitated the fractionation of extracted oil into two collectors. Collector 2 was operated at 40 °C and 50 bar pressure. The density of SC-CO2 was around 0.7 kg/L and 0.1 kg/L for collector 1 and 2, respectively.
The different extraction period in the first step influenced the second extraction which was the same for all treatments. The treatment with higher yield in the first period (4 h) showed also higher yield for the second period. The results showed a higher yield for collector 2 (E2-C2) compared to collector 1 (E2-C1) for all treatments (Table 1). The extracts collected in the second extraction period, which were identified as crude phenolic fractions, were between 4.5 and 9.3 g per 100 g Sophia seed. These correspond to 17.8–31.7 % of the total extraction. The 4 h treatment provided a longer time during the first extraction period which could also cause a better extraction of polar compounds in the second period.
The combined static/dynamic extraction treatment facilitated the extraction and resulted in about 32 % higher yield for the first extraction period compared to a 3 h dynamic extraction mode. Static extraction particularly provided a significant improvement in the phenolic extraction yield in the second period. The extraction of natural material using SFE is a complex process which involves SC-CO2 penetration into solid matter, swelling of the cell membranes, dissolving the extracted components in the fluid, transporting extracted materials to the surface of the solid matter, crossing the cellular membrane, and finally transporting to the bulk of the supercritical fluid [13]. Addition of a static period to the extraction enhanced the yield by allowing enough time for the dissolution and diffusion of extractable materials into the bulk of fluid. On the other hand, in dynamic extraction mode, there is a limited amount of extractable compounds that the CO2 is able to dissolve in the residence time it is allowed inside the extraction vessel giving longer extraction periods. The treatment for 3 h and 1 h static required 900 g less CO2 for extraction compared to the 4 h treatment, while total extraction yield was very close for those two treatments (29.3 and 31.4 % for the 3 h and 1 h static and the 4 h treatments, respectively).
To compare the SC-CO2 extraction method with a traditional organic solvent method, we also extracted the Sophia seed oil using hexane (1:20 ratio, w/v, for 2 h, twice). The yield after drying the solvent was 27.4 % ± 0.6. This value is comparable to the pure oil yield of the first extraction period (neat CO2) in the 4 h treatment, which indicates that the chosen parameters for SC-CO2 extraction were adequate. To the best of our knowledge, there is no published report of extracting Sophia seed oil using SC-CO2. However, several researchers have reported the oil yield of this seed by traditional organic solvent extraction, which ranged from 22 to 44 % [3–5, 20, 21]. This wide range of oil yield could be the result of different seed varieties grown in different areas as well as different extraction conditions used. Bekker et al. [20] used benzene at 70–80 °C and obtained a yellowish-brown oil with 22 % yield. Peng et al. [4] compared Sophia species grown in three different regions in China and showed an oil yield of 32, 35, and 44 % for seeds from Guengyuan, Chengchen, and Hangyuen counties, respectively. Chao [5] studied four different oil extraction methods and reported that petroleum ether was the best solvent for extracting fatty oil from Sophia seed, and ultrasound-assisted extraction significantly changed the physical and chemical properties of the oil (including specific gravity, refractive index, acid value, saponification value, peroxide value, and iodine value). The Sophia seed used in this study was golden brown colour and the 1000 seed weight was 0.14 g ± 0.01. Peng et al. [4] reported a range of 0.11–0.20 g of 1000 seed weight for different Sophia species.
Fatty Acid Profile of Sophia Seed Oil
The fatty acid compositions of Sophia oil seed extracted under different conditions and collected in two collectors are presented in Table 2. The composition of FAs was very similar for all three extraction modes as well as in the two different collectors except for fraction E1-C2 from the 3 h treatment. It can be concluded that increasing the extraction time, which resulted in higher oil yield, did not change the principle FA composition. Fourteen FAs were detected in the Sophia oil of which α-linolenic (C18:3 ω3) was by far the predominant FA (48.5 % on the average). This is much higher than the amount reported by Chao [5] (26.1 %) and Bekker et al. [20] (34.7 %), but closer to the results (40.9 %) published by Luo et al. [21] using organic solvent extraction.
The amount of erucic acid in Sophia seed oil was in the range of 5.15 % ± 0.45 to 6.59 % ± 0.07, which is much lower compared to rapeseed oil (20–54 %) and Sinapis alba (white mustard) oil (36–42 %) [22]. This range for erucic acid content is very close to the maximum of 5 % permitted in Canada for a cooking oil, salad oil, margarine, and shortening according to Food and Drug Regulations (B.09.022, 2014, http://laws-lois.justice.gc.ca).
The FA composition of Sophia seed in our study are in agreement with the reported values in the literature with slightly different values for α-linolenic, eicosenoic, erucic, and oleic fatty acids [20], but it should be noted that the FA composition reported by those authors also varied. Peng et al. [4] analysed the oil of Sophia seeds from three different regions in China and obtained the following composition range; 36.1–40.9 % (18:3), 15.5–18.4 % (18:2), 10.8–12.9 % (20:1), 9.0–12.1 % (22:1), and 11.4–14.9 % (18:1). Bekker et al. [20] reported the following FA composition for the Sophia seed oil: 37.1 % (18:3), 15.9 % (18:2), 12.1 % (20:0), 10.9 % (22:1), and 10.2 % (18:1); although we believe that the 20:0 reported in this study was in fact 20:1 as it is then consistent with our study and that of Peng et al. [4]. This diversity of FA composition could be explained by genetic and environmental effects as well as extraction and esterification methods.
Table 3 compares the fatty acid profile of Sophia seed oil extracted under different extraction conditions. Like the oil content results, the total FA content increased by prolonging the extraction period. However, the amount of total FAs in 1 g of oil collected in the first collector (E1-C1) was more concentrated that the Sophia oil in the second collector (E1-C2). This finding demonstrated that Sophia oil was fractionated by the different collecting conditions. The higher SC-CO2 density fluid in the first collector resulted in higher total FAs in the oil. Considering the high yield of oil in this fraction, the SC-CO2 extraction technique gives highly pure triglycerides (TAG) from Sophia seed.
The Sophia seed oil contains about 9 % saturated, 22 % monosaturated (MUFA), and 69 % polyunsaturated (PUFA) FAs. Figure 4 compares the fatty acid profile of the Sophia oil with other highly unsaturated oils. Among the four oilseeds from the Brassicaceae family members in Fig. 4 (S. alba or white mustard, canola, Camelina sativa, and Sophia), Sophia possesses the highest ω3, comparable to flaxseed oil ω3 FA levels.
Oil profile comparison of different plant seed with regard to saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and polyunsaturated fatty acid (ω6 and ω3). Data for oil illustrated with asterisk obtained from our lab, and data for other oil are from Dubois et al. [22]
The ratio of ω3/ω6 FAs measured in the Sophia seed oil was 2.7 and 2.8 for all treatments except for 3 h treatment, E2-C2 fraction which was 2.1 (Table 3). The oil from Sophia seed can be introduced as a new source of highly valuable ω3 oil with a considerably lower amount of ω6.
The second extraction period fractions (E2-C1 and E2-C2) were also analysed for FA composition (data not shown) and 240 mg total FAs was calculated per 1 g of the extract in each fraction. The FA composition was quite similar to that from the first extraction period except for two FAs; α-linolenic acid content was lower (45 %) and erucic acid content was higher (8.1 %).
Sophia Seed Phenolic Extract Composition
Twelve phenolic compounds were identified in the whole Sophia seed and Sophia meal extracted by acidified ethanol (Table 4). In the chromatogram, some unidentified peaks were detected close to known peaks; these peaks were assigned as unknown phenolics and their quantities were analysed on the basis of a sinapic acid standard curve (sinapic acid equivalent). Sinapic and protocatechuic acids were phenolic acids with the highest concentrations in both whole and Sophia meal samples. Sinapic acid has also been reported to be the main phenolic acid in rapeseed [23, 24]. Pyrogallol, quercetin, and catechin were in higher concentrations in the whole Sophia seed extract; however, in the meal extract samples, their quantities declined, especially quercetin and ferulic acid. Quercetin and ferulic acid content decreased about 83.9 and 78.9 %, respectively, in the Sophia meal compared to the whole seed. Interestingly, the content of quercetin-3-β-glucoside was significantly (P < 0.001) higher in the Sophia meal samples as were caffeic acid and chlorogenic acid. The total phenolics content calculated by HPLC in the Sophia meal extracts was converted to obtain the total phenolics content based on the original whole Sophia seed. In this regard, the total phenolics for 3 h, 3 h and 1 h static, and 4 h Sophia meal were 5.91, 5.38, and 4.93 mg/g original seed, all of which were lower than 6.37 mg/g for whole Sophia seed. These results were expected since sample with less depleted material in SFE (3 h treatment) should have a meal with more phenolics to be extracted using acidified ethanol.
The flavonoids and phenolic acids in Sophia seed have been isolated and identified by Wang et al. [25]. They reported 12 principle compounds including quercetin-3-O-β-d-glucopyranosyl-7-O-β-gentiobioside, kaempferol-3-O-β-d-glucopyranosyl-7-O-β-gentiobioside, isorhamnetin-3-O-β-d-glucopyranosyl-7-O-β-gentiobioside, quercetin-7-O-β-gentiobioside, kaempferol-7-O-β-gentiobioside, isorhamnetin-7-O-β-gentiobioside, quercetin-3,7-di-O-β-d-glucopyranoside, kaempferol-3,7-di-O-β-d-glucopyranoside, isorhamnetin-3,7-di-O-β-d-glucopyranoside, kaempferol-3-O-β-d-glucopyranosyl-7-O-(2-O-trans-sinnapoyl)-β-d-glucopyranosyl(1 → 6)-β-d-glucopyranoside, sinapic acid ethyl ester, and 3,4,5-trimethoxyl cinnamic acid. Our results are in agreement with their findings except for kaempferol which was not detected in our study.
Also, two lactones, descurainolide A and B, and a new descurainin compound were found by Sun et al. [26] in Sophia seed. They claimed that all three new compounds are sinapic acid derivatives since they showed the same 4-hydroxy-3,5-dimethoxyphenyl structure. In our study, we were not able to confirm the presence of these compounds because of unavailability of the standards.
HPLC was also applied to analyse the phenolic composition of SFE extracts; the fractions as produced in the second extraction period (10 % ethanol as co-solvent), Table 5. For all treatments, the second collector (E2-C2) had significantly (P < 0.001) higher amounts of phenolics per gram of extract compared to the first collector (E2-C1). Only sinapic and p-coumaric acids were detected in the extracts in which the former was in relatively higher quantities.
The treatment for 3 h and 1 h static resulted in higher phenolic amounts for all detected peaks. These results demonstrate that soaking samples with SC-CO2 (1 h static extraction) facilitates the subsequent ethanol-assisted SC-CO2 extraction probably by interactions between fluid and matrix and providing better dissolving and transporting of phenolics in the second extraction period. The total phenolic amounts were calculated on the basis of the amount of phenolics in each collector and their yield ratios. The treatment for 3 h and 1 h static had significantly (P < 0.001) the highest phenolic amounts analysed by HPLC–PDA.
The first extraction fractions (E1-C1 and E1-C2) were also analysed to evaluate if any phenolic compounds were extracted in that period. Very low amounts of p-coumaric acid, sinapic acid, and ferulic acid were detected along with some other unknown phenolic peaks (data not shown). The total phenolics were about 0.009 and 0.04 mg/g extract for collector 1 and 2, respectively, with no significant differences between the different treatments. Comparing the first and second extraction periods, there was a negligible amount of detected phenolics in the first period. SC-CO2 is a good solvent for extraction of nonpolar or low polar natural products. However, organic solvent-assisted SC-CO2 (polar co-solvent) increases the solvent strength to extract more polar compounds, such as polyphenols, from plant tissues.
Overall, compared to the total phenolic compounds detectable in the Sophia seed (whole seed, Table 4), the SFE extracts showed much lower phenolics content and these findings were also confirmed by analysing the phenolics in the meal showing that significant amounts of phenolics remained in the meal. Ethanol-assisted SC-CO2 method has been reported for extraction of polyphenols from grape waste at much higher pressure (500 bar) compared to our extraction conditions [27]. Total phenols from passion fruit were also extracted at much higher ethanol addition of 23 % (wt%) and higher temperature (60 °C) [28]. Another important factor is the time, the co-solvent can be added at the beginning without having a pre-step of neat CO2 extraction.
Bioactivity of Sophia Seed
There is no single test that accurately represents all antioxidant capacities, especially in such complex systems as food stuffs; therefore in this study three assays were used to evaluate the antioxidant capacity of the Sophia seed. Table 6 illustrates the bioactivity of whole Sophia seed and the residues after SC-CO2 extraction. TPC is an estimation of active phenolics based on their reaction with Folin–Ciocalteau reagent. TPC was the highest for the whole Sophia seed and lowest for the residue from 3 h treatment. These results correlate with the results obtained for phenolic composition.
The ORAC values showed the same trend as TPC for the sample extracts and indicated that Sophia seed is a promising source of phenolics with high antioxidant activity. The beneficial effects of polyphenols are related to their ability to scavenge reactive oxygen species [9]. Genetic and environmental factors would affect the phenolics concentration of different Sophia varieties and their antioxidant activities. The extraction methodology may also affect yields and antioxidant activities. High antioxidant activity of Sophia meal along with many bioactive phenolics detected with HPLC demonstrated that the Sophia meal can be considered as a promising source of antioxidant after extraction of oil. Szydłowska-Czerniak et al. [24] also reported similar antioxidant activity for rapeseed meal (99.74 µmol TE/g meal) and noted that the effect of solvent polarity on the antioxidant activity was 3.6 times greater than the effect of processing temperature.
The IC50 values in the β-carotene bleaching assay showed the required concentration of compound to inhibit oxidation by 50 %; the lower the IC50, the higher the antioxidant activity. The β-carotene results showed a different trend in which whole seed still had the highest antioxidant capacity, and the residue of the 3 h treatment showed higher antioxidant activity compared to the other treatments. This can be explained by the lower oil yield for this treatment. This means that more lipophilic antioxidants might remain in the defatted Sophia or could also show more leftover pigment, which can be detected by the β-carotene assay since this assay measures the ability of the extract to inhibit oxidation of linoleic acid followed by decolourisation of β-carotene. Lazze et al. [29] also reported different protective behaviours for polyphenolic compounds extracted from grape waste using SCF extraction. In their study, the bioactive compounds showed strong anti-radical activity in vitro using the 2,2-diphenyl-1-picrylhydrazyl radical assay (DPPH) and protects against reactive oxygen species production in human colon adenocarcinoma cells (Caco-2).
Table 6 illustrates the TPC and ORAC results for the extracts collected by ethanol-assisted SC-CO2. By increasing the extraction time, the TPC value significantly increased for extracts in collector 1; however, the trend was not observed for extracts in collector 2. Considering the extract yield in both collectors, the total TPC value was similar for the 4 h and the 3 h and 1 h static treatments and both gave higher amounts compared to the 3 h treatment.
The same trend as TPC was obtained using ORAC for extracts in collector 1; however, extracts in collector 2 showed a different trend in which the 3 h and 1 h static treatment showed the highest ORAC value (Table 6). Since each antioxidant assay is more sensitive to a particular special bioactive compound, our results demonstrated that ethanol-assisted SC-CO2 extraction resulted in fractionation of phenolic compounds in terms of their antioxidant activity.
In order to evaluate the relevancy of sinapic acid as the main reported phenolic acid in Sophia seed with its bioactivity, correlations were calculated between sinapic acid content and TPC values as well as ORAC values of the extracts. The following equations were obtained for TPC and ORAC:
There was a highly significant (P < 0.001) positive correlation between sinapic acid concentration and ORAC and TPC values with correlation coefficients of 0.707 and 0.732, respectively.
There is no information about the antioxidant activity of Sophia seed in the literature. However, Lee et al. [17] identified and characterized 14 bioactive compound in Sophia seed in which a cardenolide glycoside named helveticoside showed potent cytotoxicity (IC50 values ranging from 0.034 to 0.596 µM) against all human cancer cell lines tested and was identified as the main active cytotoxic constituent of Sophia seed.
Conclusion
This study was conducted to evaluate the oil constituent profile and phenolic compositions of Descurainia sophia seed extracted by SFE. We demonstrate that SFE enables effective extraction and fractionation of the Sophia seed oil, and also extracts and fractionates phenolic compounds by addition of ethanol as co-solvent. The seed oil is rich in unsaturated FAs (69 %), especially ω3, and has promising potential as a healthy/functional oil. Sophia seed is also a good source of phenolic compounds. These features make Sophia seed a potentially interesting material for incorporation into functional foods or possible therapy for the prevention of some diseases.
References
Best KF (1977) The biology of Canadian weeds: 22. Descurainia sophia (L.) Webb. Can J Plant Sci 57:499–507
Khan M, Xiao Y, Yu B, Wang N, Rasul A, Yi F et al (2012) Artabotryside A, a constituent from Descurainia sophia (L.) induces cell death in U87 glioma cells through apoptosis and cell cycle arrest at G2/M phase. J Med Plants Res 6:3754–3765
Who (1997) Medicinal plants in China. World Health Organization Regional Publications, Manila
Peng L, Yi Y, Fu-Li G, Ze-Qü L (1997) A preliminary study on the introduction of Descurainia sophia, an oil plant species for industrial uses. Acta Bot Sin 39:477–479
Chao L (2009) Studies on the extraction, isolation and identification of the seed oil of Descurainia sophia (L.). Master thesis R284, Chemistry, Zhengzhou University, Zhengzhou, China
Eller FJ, King JW (1998) Supercritical CO2 extraction of fat: comparison of gravimetric and GC–FAME methods. J Agric Food Chem 46:3657–3661
Brunner G (2013) Gas extraction: an introduction to fundamentals of supercritical fluids and the application to separation processes. Softcover reprint of the original 1st (1994) edn. Steinkopff-Verlag, Darmstadt
Raventós M, Duarte S, Alarcón R (2002) Application and possibilities of supercritical CO2 extraction in food processing industry: an overview. Food Sci Technol Int 8:269–284
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Kwon KT, Uddin MS, Jung GW, Sim JE, Chun BS (2010) Supercritical carbon dioxide extraction of phenolics and tocopherols enriched oil from wheat bran. Int J Biol Life Sci 6:117–122
Murga R, MaT Sanz, Beltrán S, Cabezas JL (2002) Solubility of some phenolic compounds contained in grape seeds, in supercritical carbon dioxide. J Supercrit Fluids 23:113–121
Rawson A, Tiwari BK, Brunton N, Brennan C, Cullen PJ, O’donnell CP (2012) Application of supercritical carbon dioxide to fruit and vegetables: extraction, processing, and preservation. Food Rev Int 28:253–276
Reverchon E, De Marco I (2006) Supercritical fluid extraction and fractionation of natural matter. J Supercrit Fluids 38:146–166
Yang B, Ahotupa M, Määttä P, Kallio H (2011) Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res Int 44:2009–2017
Seal CE, Kranner I, Pritchard HW (2008) Quantification of seed oil from species with varying oil content using supercritical fluid extraction. Phytochem Anal 19:493–498
Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD et al (2013) Acyl-lipid metabolism. The Arabidopsis Book 11:e0161. doi:10.1199/tab.0161
Lee YJ, Kim NS, Kim H, Yi JM, Oh SM, Bang OS et al (2013) Cytotoxic and anti-inflammatory constituents from the seeds of Descurainia sophia. Arch Pharm Res 36:536–541
Gunenc A, Hadinezhad M, Farah I, Hashem A, Hosseinian F (2015) Impact of supercritical CO2 and traditional solvent extraction systems on the extractability of alkylresorcinols, phenolic profile and their antioxidant activity in wheat bran. J Funct Foods 12:109–119
Hadinezhad M, Duc C, Han NF, Hosseinian F (2013) Flaxseed soluble dietary fibre enhances lactic acid bacterial survival and growth in kefir and possesses high antioxidant capacity. J Food Res 2:152–163
Bekker NP, Ul’chenko NT, Glushenkova AI (2005) Lipids from Descurainia sophia seeds. Chem Nat Compd 41:346–347
Luo P, Lan ZQ, Gao HB, Ye DP (1999) Descurainia sophia—a neglected cruciferous plant resource. Crucif Newsl Eucarpia 21:15–16
Dubois V, Breton S, Linder M, Fanni J, Parmentier M (2007) Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Technol 109:710–732
Khattab R, Eskin M, Aliani M, Thiyam U (2010) Determination of sinapic acid derivatives in canola extracts using high-performance liquid chromatography. J Am Oil Chem Soc 87:147–155
Szydłowska-Czerniak A, Amarowicz R, Szłyk E (2010) Antioxidant capacity of rapeseed meal and rapeseed oils enriched with meal extract. Eur J Lipid Sci Technol 112:750–760
Wang AQ, Wang XK, Li JL, Cui XY (2004) Isolation and structure identification of chemical constituents from the seeds of Descurainia sophia (L.) Webb ex Prantl. Acta Pharm Sin 39:46–51
Sun K, Li X, Li W, Wang J, Liu J, Sha Y (2004) Two new lactones and one new aryl-8-oxa-bicyclo[3,2,1]oct-3-en-2-one from Descurainia sophia. Chem Pharm Bull 52:1483–1486
Fiori L, De Faveri D, Casazza AA, Perego P (2009) Grape by-products: extraction of polyphenolic compounds using supercritical CO2 and liquid organic solvent—a preliminary investigation. CyTA J Food 7:163–171
Takeuchi TM, Leal PF, Favareto R, Cardozo-Filho L, Corazza ML, Rosa PTV et al (2008) Study of the phase equilibrium formed inside the flash tank used at the separation step of a supercritical fluid extraction unit. J Supercrit Fluids 43:447–459
Lazze MC, Pizzala R, Gutierrez Pecharroman FJ, Gaton Garnica P, Antolin Rodriguez JM, Fabris N et al (2009) Grape waste extract obtained by supercritical fluid extraction contains bioactive antioxidant molecules and induces antiproliferative effects in human colon adenocarcinoma cells. J Med Food 12:561–568
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). We thank Connie Sauder and Dr. Sara Martin of Agriculture and Agri-Food Canada for D. sophia species identification. We also acknowledge Eloise Debussy and Jerry Wu for their assistance during this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
HadiNezhad, M., Rowland, O. & Hosseinian, F. The Fatty Acid Profile and Phenolic Composition of Descurainia sophia Seeds Extracted by Supercritical CO2 . J Am Oil Chem Soc 92, 1379–1390 (2015). https://doi.org/10.1007/s11746-015-2693-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2693-5