Abstract
The combined effect of temperature, light intensity, and NaNO3 concentration on lipid biosynthesis and fatty acid composition was investigated for the thermophilic cyanobacterium Gloeocapsa gelatinosa (Kützing 1843) isolated from a thermal spring in Tunisia. Under optimal growth conditions, the lipid content was 7.3 % DW. Fatty acid analysis revealed the predominance of 16:0 and 18:0 (23.7 and 18.2 %, respectively) as main straight carbon chains of saturated fatty acids. Unsaturated fatty acids were also identified with 18:1n9c (18.8 %) and 16:1n7 (5 %) being the predominant components. The effect of environmental factors on fatty acid composition was monitored by using principal component analysis and central composite design. Variation of light intensity (20 to 150 μmol photons m−2 s−1), temperature (20 to 60 °C), and nitrogen concentration (0 to 3 g L−1) induced a significant variation in the amount of fatty acid proportions, whereas lipid content was only slightly modified. Results showed that light intensity had the strongest effect on the composition of fatty acids. Temperature had a synergic effect with light intensity while nitrogen concentration had a trivial effect. The combined effect of high light intensity (150 μmol photons m−2 s−1) and high temperature (60 °C) increased the proportion of saturated 16:0 and 18:0 fatty acids along with long-chain fatty acids to 82 % which was twofold higher than that in optimal growth conditions. This induced fatty acid profile makes G. gelatinosa-based biofuels adaptable for higher energetic efficiency and higher oxidative stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When put into stressful environments (e.g., temperature, light intensity, and nutrient starvation), algae may switch carbon allocation from reproduction to oil production (Illman et al. 2000; Pruvost et al. 2009; Lv et al. 2010). This oil from algae can be extracted and turned into biodiesel through a chemical process called trans-esterification (Vasudevan and Briggs 2008).
The screening of microalgae strains suitable for biodiesel production has been the subject of several investigations since 1978 (Sheehan et al. 1998). Recently, some studies on the screening of oleaginous microalgae have been reported, focusing on optimizing culture conditions to increase lipid productivity and evaluation of the potential for biodiesel production (Rodolfi et al. 2009; Gouveia et al. 2009; Li et al. 2010). The main indexes determining the potential of microalgal strains as biodiesel feedstock were considered as growth rate, lipid content, and lipid productivity. It was found that many eucaryotic microalgae, such as Chlorophyta and diatoms, can accumulate oils at a large extent (of 15–77 % total dry weight) (Liang et al. 2006; Chisti 2007).
The properties of biodiesel are largely determined by the structure of its component fatty acid esters (Knothe 2005). Biodiesel produced from feedstocks that are high in PUFAs has good cold-flow properties. However, these fatty acids are particularly susceptible to oxidation. On the other hand, saturated fats produce a biodiesel with superior oxidative stability and a higher cetane number, but rather poor low-temperature properties (Stansell et al. 2011). It has been pointed out that microalgal fatty acid compositions react considerably to stress conditions imposed by chemical and physical stimuli, either acting individually or in combination (Illman et al. 2000; Liu et al. 2008).
Cyanobacteria have also been subjected to screening for lipid production (Cobelas and Lechado 1989; Basova 2005). Unfortunately, considerable amounts of total lipids have not been found in cyanobacteria species examined in the laboratory (Hu et al. 2008). Nevertheless, cyanobacteria have many significant advantages when compared to other microalgae. These advantages are rapid growth rate (Parmar et al. 2011), high metabolic plasticity, and rapid adaptation to environmental conditions through lipid class synthesis (Karatay and Dönmez 2011). Cyanobacteria could therefore constitute an excellent model in a production process to favorably manipulate fatty acid composition in a dynamic manner and to predict the effect of stressful environmental changes that may influence the biodiesel quality. Few publications address the issue of enhancing the fatty acid profile of cyanobacteria (Knothe 2013).
Nowadays, increased attention is being paid to the isolation and characterization of new algal strains from thermal springs. According to Vladimirova and Semenenko (1962) and Richmond (1986), organisms with high optimal growth temperature possess wider possibilities for adaptation. As reported in hot springs worldwide (Ward and Castenholz 2002; Debnath et al. 2009; Ionescu et al. 2010), cyanobacteria are the most common microbial group constituting the mats which occur in these ecosystems. Furthermore, the majority of cyanobacteria species isolated from thermal springs are facultative thermophiles (also called moderate thermophiles) which can thrive at a wide range of temperatures ranging from 0 to 75 °C (Castenholz 1969). This exceptional metabolic flexibility offers the potential to modify cyanobacterial fatty acid compositions using environmental variables and highlights the necessity of exploring thermophilic species as potentially excellent models for a lipid composition pathway to reveal the most prospective strains for further investigation. Such investigation should not then be applied separately and over a limited interval because different species respond to different variables in different ways (Guschina and Harwood 2006).
We have selected the hot spring cyanobacterium Gloeocapsa gelatinosa (Kützing 1843) as a model for the investigation of thermophilic microorganism lipids. The aim of this study was to investigate the lipid content and fatty acid variation of G. gelatinosa by exposing concentrated cultures to different combinations of stress conditions of temperature, light intensity, and NaNO3 concentration (nitrogen source), using an experimental design.
However, robust algal growth and high lipid production are usually mutually exclusive (Sheehan et al. 1998). High lipid contents produced under stress conditions are usually associated with relatively low biomass productivity and, consequently, low overall lipid productivity (Li et al. 2011). Based on these considerations, we suggest a two-phase cultivation strategy, with a first optimal-growth biomass production phase followed by a lipid induction phase under stress conditions. Instead of changing temperature, light intensity, and nitrogen concentration, this study describes an attempt to modulate the fatty acid composition of G. gelatinosa in the culture in phase II of the proposed two-phase process which is much more feasible and easier to operate.
Materials and methods
Samples were taken from “Aïn Atrous,” a hot spring (60 °C) located in the northern part of Tunisia (36° 49′ N, 10° 34′ E). Sampling materials were composed of microbial mats anchored to submerged stones. Mats collected were treated by filtration, centrifugation, and dilution techniques according to standard microbiological protocols (Stanier et al. 1971; Rippka et al. 1979). A purified strain was identified morphologically according to the keys and description established by Naz et al. (2004) as G. gelatinosa (Kützing, 1843). This cyanobacterium was initially subjected to different media and to different temperature and light intensity conditions to define the optimal growth conditions. These preliminary in-lab experiments indicated that the species showed the highest growth in modified BG11 liquid medium containing (in g L−1) 1.5 NaNO3, 0.08 K2HPO4, 0.15 MgSO4 7H2O, 0.072 CaCl2 2H2O, 0.012 C6H8O7 H2O, 0.012 FeSO4, 0.002 Na2EDTA 2H2O, and 0.04 Na2CO3 and 1 mL trace metal solution (g L−1): 2.86 H3BO3, 1.81 MnCl2 4H2O, 0.39 Na2MoO4 2H2O, 0.079 CuSO4 5H2O, 0.049 Co(NO3)2 6H2O, and 0.222 ZnSO4 7H2O (Stanier et al. 1971; Rippka et al. 1979) at 40 °C and 85 μmol photons m−2 s−1.
Growth condition culture
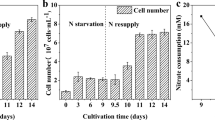
Pre-cultures were achieved under conditions that optimize the algal culture growth (temperature 40 °C, light intensity 85 μmol photons m−2 s−1, and NaNO3 concentration 1.5 g L−1). Cultivation was carried out, in triplicate, in 10-L sterilized flasks containing 5 L of modified BG11 medium and equipped with a device for aseptic removal of samples. Each culture was inoculated with an initial biomass concentration of 0.17 mg mL−1. Cultures were stirred continuously with air at a constant flow rate (0.1 v/v/min).
Biomass concentration was determined daily and expressed as g L−1 dry weight. The dry weight was obtained by placing the sample in a dry (105 °C for 24 h) tared crucible with a 1.5-μm pore size, 24-mm-diameter glass microfiber filter (Whatman, UK), rinsing with distilled water, and drying at 105 °C for 24 h. The change in the weight of the crucibles with the addition of the rinsed algae after drying off all of the water was considered the dry weight.
Lipid synthesis induction
Cultures were conducted in multiple batch reactors and exposed separately to different temperatures (20–40–60 °C), light intensities ranging from 20 to 120 μmol photons m−2 s−1, and sodium nitrate concentrations (N) (as nitrogen source) from 0 to 3 g L−1, as shown in Table 1.
Cells were harvested from pre-cultures at the end of the exponential growth phase (day 5), concentrated by centrifugation, and resuspended equally (0.71 mg mL−1) in 250-mL Erlenmeyer flasks containing 110 mL of the appropriate medium. Aliquots of each culture (50 mL) were harvested after 2 days of treatment, by centrifugation (at 4 °C, 4,000 rpm for 5 min). The pellet was washed three times with deionized water to eliminate medium salts. The obtained cells were freeze-dried and subsequently weighed and stored at −20 °C until lipid analysis.
All experimental cultures were conducted under controlled light in a temperature-programmable chamber with continuous illumination. Phyto-Claude halogen lamps (400 W) were used to illuminate chambers. The intensity of incident light was measured using the silicon sensor HD 8366.
Lipid extraction
For all samples, lipid analysis was conducted in triplicate. Pellets of each sample were added to 5 mL of a methanol/water/HCl (30:3:1, by vol) mixture into clean Teflon-lined screw-capped glass test tubes and held at 55 °C for 6 h (Rezanka et al. 2003). A total of 15 mL of cold water–hexane (2:1, by vol) mixture was added to the sample and vortex mixed for 20 s. The hexane layer was filtered and concentrated to dryness under a stream of nitrogen at 10 °C. The residue was extracted three times with 5 mL chloroform, then filtered and concentrated to dryness under a stream of nitrogen at 10 °C. The dried hexane and chloroform extracts were combined and dissolved in 1 mL of hexane–chloroform (1:1, by vol) mixture. The solvent was removed by being flushed with nitrogen, and the total lipid content was estimated gravimetrically (Rezanka et al. 2003).
The comparison of lipid content values was performed by using one-way analysis of variance (ANOVA), and the significance of the differences (P < 0.05) estimated by Tukey’s multiple comparison test.
Fatty acid analysis
To convert fatty acids to methyl esters, 0.5 mL of 14 % boron trifluoride in methanol was added to the dried whole lipids, and the mixture was refluxed for 1 h at 100 °C (Morrison and Smith 1964; Medina et al. 1992). Distilled water, 0.5 mL, was added, and fatty acid methyl esters (FAME) were extracted three times with hexane. The hexane extracts were rinsed twice with distilled water and evaporated under a stream of nitrogen at 10 °C.
FAMEs were detected using a Hewlett-Packard HP 5890, series II, a gas chromatograph fitted with a flame ionization detector (FID) and equipped with a 60 × 0.32-mm DB-23 polar column. High-purity nitrogen was used as the carrier gas. The initial column temperature was set at 150 °C and was subsequently raised to 250 °C at 2 °C min−1. The FID temperature was kept at 280 °C. The fatty acid content was determined by comparison of their integrated peak areas with that of the nonadecanoic acid methyl ester (19:0) as internal standard. The structures of FAMEs were confirmed by comparison of retention times and fragmentation patterns with those of standard FAMEs (PUFA-1 Marine Source, Supelco).
We used a fatty acid shorthand notation of the form A: Bnx, where A represents the number of carbon atoms, B is the number of double bonds, and nx is the position of the double bond closest to the terminal methyl group.
Principal component analysis of fatty acids
Principal components analysis (PCA) and cluster analysis of fatty acid responses were used to reduce the dimensionality of the data set while keeping as much as possible of the original information. This was achieved by transforming the original set of variables into a smaller set of linear combinations called principal components (Jolliffe 2002; Rencher 2002). Their values are the component scores, and the coefficients of linear combinations are the loadings. The score plot was used for examining the classification of the data. The loading plot was used for investigating the importance of variables to each component.
Two coordinates were described that accounted for the largest and second largest variance among the samples. This allowed a display of the major trends within the data set without significant loss of the total original variation.
Experimental design
The three independent factors, temperature, light intensity, and sodium nitrate concentration (as nitrogen source) were examined at three levels, chosen on the basis of the available literature and preliminary experiments. The range and levels of these factors are listed in Table 1.
A central composite face design (CCF) was applied to the factors using MODDE 7.0 software for experimental design and optimization (Umetrics AB, Sweden). A total of 18 experiments (14 points of the factorial design and 4 center points, representing the medians of the studied factor domains, to establish the experimental errors) were carried out (Table 3).
The parameters of the model were estimated by multiple linear regressions (MLR). The accuracy of the model fit was evaluated by the explained variation (R 2) and the model validity (lack of fit). The influences of the scaled and centered coefficients of equations were investigated at a confidence interval of 0.90. A coefficient was recognized as having an effect on the response if it was significantly different from zero. Insignificant terms were excluded from the model, and the coefficients were recalculated.
Results
Optimal growth condition culture investigation
The optimal growth curve of G. gelatinosa is shown in Fig. 1. Under this condition, the maximum biomass yield was 1.27 g L−1. Exponential growth was observed from day 3 to day 5 of culture time. Low levels of lipid content were observed initially during the exponential phase (<3.5 % of biomass dry weight). However, as the culture approached the stationary phase, lipid content increased and lipid accumulation was 7.3 to 8.1 % of biomass dry weight on days 5, 6, and 7 (Fig. 1).
The major fatty acids present in the log phase were hexadecanoic acid 16:0 and octadecanoic acid 18:0 (23.72 and 18.22 %, respectively) as the main saturated straight-carbon-chain fatty acids. Unsaturated fatty acids were also identified, with octadecenoic acid 18:1n9c (18.84 %) and hexadecenoic 16:1n7 (5.08 %) being the predominant components. Results also showed that G. gelatinosa had the ability to synthesize octadecatrienoic acid whose content was smaller and did not exceed 1.39 % (Table 2).
Effect of temperature, light intensity, and NaNO3 concentration on lipid biosynthesis
Lipid content variation
Results on the effects of different factor combinations (temperature, light intensity, and NaNO3 concentration) on the lipid content of Gloeocapsa cells are plotted in Fig. 2. For all experiments, the lipid content ranged between 4.11 and 7.89 % dry weight (DW). The highest lipid proportion was only 1.12 times greater than the optimal growth condition. Statistical analysis showed that the lipid content was not significantly affected by different factor treatments.
Fatty acid variation
Analysis of the amount variation of the G. gelatinosa fatty acids (Table 2) with respect to different experimental combinations of temperature, light intensity, and nitrogen concentration demonstrated a large difference in the amount of all fatty acids. For example, fatty acid proportions increased from 0.01 to 0.87 % for octadecatrienoic acid, from 0.02 to 074 % for tetradecenoic acid, from 0.14 to 2.36 % for octadecadienoic acid, and from 0.62 to 7.5 % for eicosapentaenoic acid.
To investigate the variation of fatty acid proportions with respect to different experimental combinations of temperature, light intensity, and NaNO3 concentration, a principal component analysis was carried out. This technique was employed using 28 fatty acids as variables and a combination of the three factors (temperature, light intensity, and NaNO3 concentration) as values.
Figure 3a shows the loading plot for the first two principal components which accounted for 62.5 % of the variance in the data set. The first PC accounted for 48.5 % of the variance and clearly separated fatty acids into two groups. The first one (G1) loaded negatively onto this axis and grouped short-chain saturated fatty acids of 10:0, 12:0, 13:0, 15:0iso and 16:0iso, and 16:0Me and also monounsaturated fatty acids such as 14:1, 16:1n7, and 18:1n9c. The second group (G2) loaded positively onto the PC1 axis and grouped fatty acids higher than 16 carbons (16:0, 17:0, 17:0iso, 18:0, 20:0, 20:1n9, 20:5n3, 22:5, 22:6).
Principal component analysis of fatty acid data from G. gelatinosa exposed to different combinations of temperature, light intensity, and NaNO3 concentration. Fatty acid parameters used were 10:0, 12:0, 13:0, 14:0, 14:1, 15:0, 15:0i, 15:0a, 15:1, 16:0, 16:0i, 16:0Me, 16:1n7, 17:0, 17:0Me, 17:0i, 17:1, 18:0, 18:1n9c, 18:1n7t, 18:3n3, 18:3n6, 20:0, 20:1n9, 20:5n3, 22:5, 22:6, and N.D. Groups were determined by cluster analysis. a Fatty acid parameter coefficients for PC1 and PC2. b Combination factor scores for PC1 and PC2
Figure 3b shows the scores of the temperature, light intensity, and NaNO3 concentration combinations plotted along the PC1 and PC2 axes. Factor combinations with high light values (150 μmol photons m−2 s−1) were grouped together on the positive side of the PC1. Inversely, factor combinations with low light intensities were grouped together on the negative side of the axis. The grouping of high light values on the positive segment of the axis was expected as high light induces a high level of G2 fatty acids. But, low light appeared to induce a high level of G1 fatty acids.
The PC2 axis accounted for only 14 % of the variance and grouped (17:0iso, 17:0 Me, 18:3n3, 18:3n6, 22:5) fatty acids on its positive side. The score matrix did not show a clear separation of parameter combination along this axis.
Experimental design
For a further investigation of the effect of combined experimental factors to the proportion of G2 fatty acids and G1 fatty acids, a response surface methodology (RSM) was used. The sums of fatty acid percentage of group 1 and group 2, distinguished by PCA analysis, were adopted as responses.
Table 3 lists the results of each group (G1 and G2) obtained for all the experiments corresponding to the matrix design. Results showed that fatty acid proportion values in G2 varied by more than a magnitude of ten in the experimental domain, and therefore, a transformation of the response was used (Draper and Cox 1969). The Box–Cox mathematical transformation of the response (Peltier et al. 1998) was chosen to achieve a simple and parsimonious model, and MODDE 7 software computed a log transformation for the values of this matrix.
The effects of each significant factor on each response and the results of the ANOVA are reported in Fig. 4. Adjusted plots are displayed as bar graphs for the selected factors and the confidence interval as error bars (Fig. 4). As indicated in Fig. 4, the models developed for the two groups of fatty acids had a good R 2 (R 2 ≥ 0.98 and 0.99, respectively) without any lack of fit. Therefore, these models appeared adequate to accurately fit the experimental data.
The values of process factor effects (computed as twice the MLR coefficients) on the G1 and G2 fatty acid groups. a G1—monounsaturated 14:1, 16:1n7, 18:1n9c, and short-chain fatty acids. b G2—saturated 16:0, 18:0, and long-chain fatty acids. Temp temperature, Light light intensity, N NaNO3 concentration. The ±90 % confidence interval is shown as error bars
As can be seen, the linear term of light intensity (Light) had the strongest effect in the two responses, positive in G1 fatty acids and negative in those of G2.
The effect of temperature (Temp) was less marked than light intensity in the two responses. Its quadratic term (Temp × Temp) indicated an optimum in the experimental interval. Its interactive term with light intensity (Light × Temp) indicated a synergic effect (positive) in the G1 response variable and an antagonist effect (negative) in those of G2. The effect of NaNO3 concentration was the least marked, with a positive contribution in G1 consolidated with its positive quadratic effect and negative contribution in G2 consolidated with its negative interaction with light intensity (Light × N) and temperature (Temp × N).
The predicted proportions of G1 and G2 fatty acids, as estimated by the two models, are represented in Fig. 5 for each combination of experimental factors. The optimal growth condition ratio for each group of fatty acids is also represented. Results showed that high light (150 μmol photons m−2 s−1) combined with high temperature (60 °C) and minimal NaNO3 concentration increased the proportion of saturated 16:0 and 18:0 and long-chain fatty acids (G2 fatty acids) to 82 % which was twofold higher than the optimal growth correspondent level. Conversely, in low light intensity (20 μmol photons m−2 s−1) combined with low or medium temperatures (20–40 °C) and maximal NaNO3 concentration, the G2 fatty acid proportion was equal or little enhanced compared to the optimal growth conditions.
Discussion
In this work, the cyanobacterium G. gelatinosa isolated from the thermal hot spring “Aïn Atrous” has the ability to survive in a large range of temperatures. In fact, this species can thrive not only at a low temperature (20 °C) but also at an extremely higher temperature (60 °C). This thermal flexibility is of crucial importance in exploratory processes not only in studying the variation of lipid and fatty acid composition according to environmental factors but also in manipulating this composition in a dynamic manner.
At optimal growth condition, the time course of lipid accumulation clearly revealed the capability of G. gelatinosa to increasingly accumulate lipids with advanced age. Low levels of lipid accumulation during the exponential phase can be explained by the strain fulfilling its biotic potential and diverting maximum carbon and energy into cell growth and reproduction.
Throughout the experimental process, an insignificant variation of lipid accumulation was observed, and the level of lipid content remained below 8 % of biomass dry weight. In fact, many cyanobacteria have been subjected to screening for lipid production, but no substantial lipid amounts have been found (Tedesco and Duerr 1989; Vargas et al. 1998; Sharma et al. 2011; Selvan et al. 2013).
Hu et al. (2008) found 9.8 % DW as the average of cyanobacterial lipid content under normal growth and stress conditions. This result is in agreement with our results. Although it is widely accepted that stress conditions increase total lipid content that can reach 42 % DW in many eukaryotic microalgae (i.e., Chlorella, Botryococcus) as storage form of carbon excess caused generally by growth limitation (Cobelas 1989; Hu et al. 2008), this adaptive way is still not confirmed in cyanobacteria species.
In our work, the analysis of Gloeocapsa fatty acids revealed a low amount of polyunsaturated fatty acids (2.36–18.33 %) and the total absence of hexadecadienoic acid, a characteristic advanced by Kenyon (1972) in Gloeocapsa. In addition, a high level of fatty acid 16:0 was observed which is commonly found in bacteria and cyanobacteria (Blumer et al. 1969; Piorreck et al. 1984; Vargas et al. 1998). Gloeocapsa also contained an interesting amount of octadecenoic acid (10–34.3 %) that Kenyon (1972) also reported in the thermophilic cyanobacterium Synechococcus. However, our results showed the presence of octadecatrienoic acid, eicosanoic acid, eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid none of which have been identified in other Gloeocapsa species (Kenyon 1972).
In this study, Gloeocapsa cultures exposed to factor combination investigation showed a large difference in the amount of some fatty acids. Principal compound analysis revealed the presence of two fatty acid groups, principally correlated to light intensities. It seems that thermophilic Gloeocapsa synthesize mainly monounsaturated 14:1, 16:1n7, and 18:1n9c fatty acids and saturated short-chain fatty acids, when it is exposed to low light intensity. Conversely, high light intensity promotes the synthesis of the saturated fatty acids, mainly 16:0 and 18:0 and long-carbon-chain fatty acids (17:0, 17:0iso, 18:0, 20:0, 20:1n9, 20:5n3, 22:5, 22:6).
Few researchers have considered light intensity as the main factor responsible for fatty acid modification (Cohen et al. 1987; Tedesco and Duerr 1989; Mühling et al. 2005) and particularly for fatty acid saturation. Significant increases in the production of saturated fatty acids at higher light intensities are indicative of interruptions of the biosynthetic processes of chain elongation and desaturation (Renaud et al. 2002). In more recent studies, Seyfabadi et al. (2011) reported that the production of long-chain saturated fatty acids may help in dissipating excess light energy and preventing photochemical damage of algal cells. Another study has also showed that long-chain fatty acids were swiftly accumulated in triacylglycerols as storage substance in microalgae to allow adaptation to environmental stress (Bigogno et al. 2002). In cyanobacterial cells, lipids are typically found only in the membranes (Murata and Wada 1995). Chintalapati et al. (2004) reported that long-chain fatty acids span the width of the bilayer more easily and promote acyl chain packing which makes the membrane environment more gel-like. In contrast, shorter chains cannot form hydrophobic interactions with other lipids and proteins and thus maintain the fluid state of the membrane.
Nevertheless, some authors have noted different effects of light intensity on fatty acid composition. In fact, Walsh et al. (1997) reported that monounsaturated fatty acids of the cyanobacterium Microcystis aeruginosa were increased under high irradiance and that polyunsaturated fatty acid concentrations were reduced. Whitelam and Codd (1986) considered that high light intensity and UV radiation increased polyunsaturated fatty acids, and the protective role of PUFA against the damage of high light intensity and UV radiation was suggested as a protective way against extreme environments. Sato and Murata (1981) also reported that strong illumination stimulated the desaturation of Anabaena variabilis fatty acids.
Experimental design analyses in this study have shown that temperature, as well, presented a significant and synergic effect with light intensity and that a combination between high light intensity and high temperature led to maximizing the amount of saturated and long-carbon-chain fatty acids. On the other hand, low light intensity combined with low temperature enhanced monounsaturated and short-carbon-chain fatty acids.
Thompson et al. (1992) studied the effect of temperature on fatty acid composition in eight microalgae. They only found a very weak inverse trend between polyunsaturated fatty acid content and temperature. Instead, Renaud et al. (2002) noted that an increase in the production of saturated fatty acids such as 16:0 and 18:0 was associated with high temperature (35 °C), which is much lower than the 60 °C tested in this work. Analogous results were reported by Colla et al. (2004) for the fatty acid variation of Spirulina platensis cultivated at 30 and 35 °C.
Another important environmental variable which has been studied is nitrogen starvation. Guschina and Harwood (2006) found that this limitation normally increased storage of triacylglycerols in many microalgae. Nevertheless, according to Piorreck et al. (1984) and Tedesco and Duerr (1989), the effects of nitrogen concentration on biomass lipid content and lipid composition in the cyanobacteria species were not significant. Our results confirm this trend. Indeed, no remarkable nitrogen restriction effects on Gloeocapsa fatty acids were observed.
The two most important properties of fatty acids that affect the fuel properties are the length of the carbon chain and the number of double bonds. Long chains and low degrees of unsaturation enhance high cetane number and high oxidative stability on a fuel against poor cold-flow properties and viscosities (Knothe 2009). Our study demonstrates that thermophilic G. gelatinosa contains mainly saturated (16:0 and 18:0) and long-chain fatty acids (82 % of the total fatty acids) when biomass is exposed to high light intensity (150 μmol photons m−2 s−1) and high temperature (60 °C). In these conditions, the induced fatty acid profile makes the G. gelatinosa-based biodiesel adaptable for oxidative stability and energetic efficiency (Chintalapati et al. 2004; Hu et al. 2008). The culture condition optimizing this required fatty acid profile is obviously not the condition necessary for Gloeocapsa optimum growth, and this makes our methodology a good solution to manipulate culture lipid composition and to overcome biomass productivity restrictions in a short time.
In conclusion, G. gelatinosa demonstrated an interesting set of properties to be used as a feedstock source in the synthesis of biodiesel. This cyanobacterium showed appropriate values of biomass, as well as fatty acid profiles which can be used successfully in the synthesis of biodiesel. More screening of hot spring cyanobacteria is necessary to provide a very broad spectrum of information, which could help to identify cyanobacterial species which are resistant to changing environmental conditions and have high biomass and high lipid productivity.
References
Basova MM (2005) Fatty acid composition of lipids in microalgae. Int J Algae 7:33–57
Bigogno C, Khozin-Goldberg I, Boussiba S et al (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60:497–503
Blumer M, Chase T, Watson SW (1969) Fatty acids in the lipids of marine and terrestrial nitrifying bacteria. J Bacteriol 99:366–370
Castenholz RW (1969) Thermophilic blue-green algae and the thermal environment. Bact Rev 33:476–504
Chintalapati S, Kiran MD, Shivaji S (2004) Role of membrane lipid fatty acids in cold adaptation. Cell Mol Biol 50:631–642
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Cobelas MA (1989) Lipids in microalgae. A review. II. Environment. Grasas y Aceites 40:213–223
Cobelas MA, Lechado JZ (1989) Lipids in microalgae. A review. I. Biochemistry. Grasas y Aceites 40:118–145
Cohen Z, Vonshak A, Richmond A (1987) Fatty acid composition of Spirulina strains grown under various environmental conditions. Phytochemistry 26:2255–2258
Colla LM, Bertolin TE, Costa JAV (2004) Fatty acids profile of Spirulina platensis grown under different temperatures and nitrogen concentrations. Z Naturforsch C 59:55–9
Debnath M, Mandal NC, Ray S (2009) The study of cyanobacterial flora from geothermal springs of Bakreswar, West Bengal, India. Algae 24:185–193
Draper N, Cox D (1969) On distributions and their transformations to normality. J R Stat Soc Ser B 31:472–476
Gouveia L, Marques AE, da Silva TL, Reis A (2009) Neochloris oleabundans UTEX #1185: a suitable renewable lipid source for biofuel production. J Ind Microbiol Biotechnol 36:821–6
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–86
Hu Q, Sommerfeld M, Jarvis E et al (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–39
Illman A, Scragg A, Shales S (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 27:631–635
Ionescu D, Hindiyeh M, Malkawi H, Oren A (2010) Biogeography of thermophilic cyanobacteria: insights from the Zerka Ma’in hot springs (Jordan). FEMS Microbiol Ecol 72:103–113
Jolliffe I (2002) Principal component analysis, 2nd edn. Springer, New York
Karatay SE, Dönmez G (2011) Microbial oil production from thermophile cyanobacteria for biodiesel production. Appl Energy 88:3632–3635
Kenyon CN (1972) Fatty acid composition of unicellular strains of blue-green algae. J Bacteriol 109:827–34
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070
Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ Sci 2:759–766
Knothe G (2013) Production and properties of biodiesel from algal oils. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 207–221
Kützing FT (1843) Phycologia generalis oder Anatomie, Physiologie und Systemkunde der Tange. Mit 80 farbig gedruckten Tafeln, gezeichnet und gravirt vom Verfasser. pp. [part 1]: [i]-xxxii, [1]-142, [part 2:] 143–458, 1, err.], pls 1–80. Leipzig: F.A. Brockhaus
Li Y, Han D, Hu G et al (2010) Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerol. Metab Eng 12:387–91
Li X, Hu H, Zhang Y (2011) Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour Technol 102:3098–102
Liang X, Dong W, Miao X, Dai C (2006) Production technology and influencing factors of microorganism grease. Food Res Dev 27:46–47
Liu X, Duan S, Li A et al (2008) Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J Appl Phycol 21:239–246
Lv J-M, Cheng L-H, Xu X-H et al (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101:6797–804
Medina I, Aubourg S, Gallardo JM, Pérez-Martin R (1992) Comparison of six methylation methods for analysis of the fatty acid composition of albacore lipid. Int J Food Sci Technol 27:597–601
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 5:600–608
Mühling M, Belay A, Whitton BA (2005) Variation in fatty acid composition of Arthrospira (Spirulina) strains. J Appl Phycol 17:137–146
Murata N, Wada H (1995) Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J 308:1–8
Naz S, Masud U, Shameel M (2004) Taxonomic study of Chroocophyceae (Cyanophyta) from northern areas of Pakistan. Pak J Bot 36:247–281
Parmar A, Singh NK, Pandey A et al (2011) Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour Technol 102:10163–72
Peltier MR, Wilcox CJ, Sharp DC (1998) Technical note: application of the Box-Cox data transformation to animal science experiments. J Anim Sci 76:847–849
Piorreck M, Baasch K-H, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 23:207–216
Pruvost J, Van Vooren G, Cogne G, Legrand J (2009) Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour Technol 100:5988–95
Renaud SM, Thinh L-V, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214
Rencher AC (2002) Methods of multivariate analysis, 2nd edn. John Wiley & Sons, New York
Rezanka T, Dor I, Prell A, Dembitsky VM (2003) Fatty acid composition of six freshwater wild cyanobacterial species. Folia Microbiol (Praha) 48:71–5
Richmond A (1986) Cell response to environmental factors. In: Richmond A (ed) Handbook of microalgal mass cultures. CRC, Boca Raton, pp 69–101
Rippka R, Deruelles J, Waterbury JB et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Rodolfi L, Chini Zittelli G, Bassi N et al (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–12
Sato N, Murata N (1981) Studies on the temperature shift-induced desaturation of fatty acids in monogalactosyl diacylglycerol in the blue-green alga (cyanobacterium), Anabaena variabilis. Plant Cell Physiol 22:1043–1050
Selvan BK, Revathi M, Piriya PS et al (2013) Biodiesel production from marine cyanobacteria cultured in plate and tubular photobioreactors. Indian J Exp Biol 51:262–8
Seyfabadi J, Ramezanpour Z, Khoeyi ZA (2011) Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J Appl Phycol 23:721–726
Sharma NK, Tiwari SP, Tripathi K, Rai AK (2011) Sustainability and cyanobacteria (blue-green algae): facts and challenges. J Appl Phycol 23:1059–1081
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) Look Back at the U.S. Department of Energy’s Aquatic Species Program: biodiesel from algae; close-out report. doi:10.2172/15003040
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bact Rev 35:171–205
Stansell GR, Gray VM, Sym SD (2011) Microalgal fatty acid composition: implications for biodiesel quality. J Appl Phycol 24:791–801
Tedesco MA, Duerr EO (1989) Light, temperature and nitrogen starvation effects on the total lipid and fatty acid content and composition of Spirulina platensis UTEX 1928. J Appl Phycol 1:201–209
Thompson PA, Guo M, Harrison PJ, Whyte JNC (1992) Effects of variation in temperature. II. On the fatty acid composition of eight species of marine phytoplankton. J Phycol 28:488–497
Vargas MA, Rodríguez H, Moreno J, Olivares H, Del Campo JA, Rivas J, Guerrero MG (1998) Biochemical composition and fatty acid content of filamentous nitrogen-fixing cyanobacteria. J Phycol 34:812–817
Vasudevan PT, Briggs M (2008) Biodiesel production—current state of the art and challenges. J Ind Microbiol Biotechnol 35:421–30
Vladimirova MG, Semenenko VE (1962) Intensive culture of unicellular algae. Acad Sci USSR 44–46. (In Russian)
Walsh K, Jones GJ, Hugh Dunstan R (1997) Effect of irradiance on fatty acid, carotenoid, total protein composition and growth of Microcystis aeruginosa. Phytochemistry 44:817–824
Ward DM, Castenholz RW (2002) Cyanobacteria in geothermal habitats. In: Whitton BA, Potts M (eds) Ecol. cyanobacteria. Springer, Netherlands, pp 37–59
Whitelam G, Codd G (1986) Damaging effects of light on microorganisms. In: Herbert R, Codd G (eds) Microbes in extreme environments. Academic, London, pp 129–169
Acknowledgments
The authors would like to express their gratitude to Prof. Glenda El Gamal, faculty at Masdar Institute of Science and Technology (Abu Dhabi, UAE) for her critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zili, F., Mezhoud, N., Trabelsi, L. et al. Fatty acid composition of the thermophilic Gloeocapsa gelatinosa under different combinations of temperature, light intensity, and NaNO3 concentration. J Appl Phycol 27, 97–107 (2015). https://doi.org/10.1007/s10811-014-0296-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0296-4


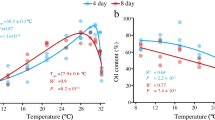






 G1—monounsaturated 14:1, 16:1n7, 18:1n9c, and short-chain fatty acids.
G1—monounsaturated 14:1, 16:1n7, 18:1n9c, and short-chain fatty acids.  G2—saturated 16:0, 18:0, and long-chain fatty acids under different combinations of temperature (T), light intensity (L), and NaNO3 concentration (N)
G2—saturated 16:0, 18:0, and long-chain fatty acids under different combinations of temperature (T), light intensity (L), and NaNO3 concentration (N)