Abstract
Temperature is a common and effective environmental driver employed for promoting lipid accumulation in microalgae, while the thermal reaction norms associated with total lipid, fatty acid, photosynthesis and growth are little known. Here, we investigated the thermal responses of growth rate, photosynthetic parameters, lipid accumulation, and fatty acid composition at a wide range of temperatures (9, 13, 17, 21, 25, 28, 30, 31, 32, and 32.3 ℃) in a terrestrial high-oil-production microalga Vischeria sp. WL1 on days 4 and 8. We identified the thermal reaction norm of growth rate for Vischeria sp. WL1 with the optimal temperature of 30.5 ± 0.3 and 27.9 ± 0.6 ℃ at day 4 and 8, respectively. The percentage of oil content increased with decreasing cultivation temperature on both days 4 and 8. We found that several photosynthetic parameters (Chl a content, Fv/Fm, PIabs, φE0, and ψ0) showed similar thermal reaction norms as that of the growth rate. While, the thermal responses of fatty acids exhibit remarkable diversity, and only linoleic (C18:2), linolenic (C18:3), and arachidonic (C20:4) show comparable thermal reaction norms as that of the growth rate. Our study reveals the consistent/intricate physiological and lipidic adaptations to temperature fluctuations, providing insights for optimizing oil yield and targeted fatty acid production via temperature manipulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae have emerged as promising candidates for the sustainable production of edible oils or biodiesel because of their numerous advantages like high photosynthetic efficiency (Li et al. 2008), fast reproduction, high oil production rate and low land requirement (Dismukes et al. 2008; Xue et al. 2018; Deshmukh et al. 2019). Among various biofuel options, microalgae-based fuels are considered as one of the most promising pathways due to their ability to produce lipids in the form of triacylglycerols (TAGs) for synthesis of biodiesel through ester exchange reactions (Chisti 2007). This approach has demonstrated its potential to meet the global fuel demand while simultaneously reducing emitted atmosphere CO2. Additionally, microalgae contain valuable compounds (such as such as proteins, exopolysaccharides, phenolic compounds, and omega fatty acids) that can be utilized for various purposes such as nutraceuticals and in infant formulations (Barkia et al. 2019). However, the high cost of biodiesel production through microalgae remains the main bottleneck and thus the identification of high lipid-producing microalgal strains is of paramount importance. Biological soil crusts (BSCs), encompassing approximately 70% of arid and semiarid regions, are considered natural bioresource repositories that may offer promising microalgae strains capable of producing valuable metabolites (Chamizo et al. 2016; Weber et al. 2018). Nevertheless, the exploration of exceptional microalgal strains from BSCs for oil production remains limited.
Strategies, such as nitrogen-starvation, phosphate-starvation, and high salinity conditions, have been demonstrated to be effective triggers capable of promoting lipid accumulation in microalgae, but occur with inhibition of their growth and photosynthesis (Courchesne et al. 2009). In contrast, temperature is a very sensitive factor affecting microalgal growth and microalgal cell metabolic activity (Gillooly et al. 2001), but less affecting light absorption, excitation energy transfer, and primary photochemistry in photosynthesis (Raven and Geider 1988). The reported findings indicate that there are robust correlations between temperature and the growth rate in various microalgae species, displaying a characteristic thermal reaction norm (a function relating environmental temperature to the phenotype (Angilletta Jr et al. 2003)) with optimal temperatures (Thomas and Litchman 2015; Listmann et al. 2016; Kremer et al. 2018; O'Donnell et al. 2018). Thermal reaction norms have also been observed in studies concerning photosynthetic parameters like Fv/Fm and rETR (Barton et al. 2020; Lepori‐Bui et al. 2022). Furthermore, it is noteworthy that temperature exerts significant but intricate influences on lipid production in microalgae (Table S1). Studies have found that lowered (sub-optimal) temperatures tend to increase the lipid content in Chlorella sorokiniana LS-2, Scenedesmus sp, Isochrysis galbana TK1, and Porosira glacialis (Zhu et al. 1997; Li et al. 2011; Wang et al. 2016; Svenning et al. 2019), but also could decrease the lipid content in Ochromonas danica and Nannochloropsis salina, Nannochloropsis oculate, Chlorella vulgaris, and Scenedesmus sp. Z-4 (Converti et al. 2009; Fakhry and Maghraby 2015; Ma et al. 2017). Other studies have reported that both higher or lower temperatures could increase the lipid content in Chlorella minutissima and Monoraphidium dybowskii Y2 (Cao et al. 2014; He et al. 2018). It is well known that lower temperatures can trigger an increase in the ratio of unsaturated to saturated fatty acids in microalgae, conferring membrane fluidity and permeability (Renaud et al. 2002; Hoffmann et al. 2010; Boelen et al. 2013; O'Donnell et al. 2019) and protecting the photosynthetic systems in thylakoid membrane glycolipids (Renaud et al. 2002; Hu 2013; Boelen et al. 2013). However, this correlation varies largely for specific fatty acid among microalgae. Some studies have found the negative linear relationships between increasing temperature and the 16:0 fatty acid in Cryptomonas sp., Rhodomonas sp. and N. paleacea (Renaud et al. 2002), 14:0 fatty acid in Isochrysis sp. and Rhodomonas sp. (Renaud et al. 1995), C18:3n3 fatty acid in Nannochloropsis sp. FIKU036 (Chaisutyakorn et al. 2017), 16:1n-7 fatty acid in Prymnesiophyte NT19, 18:1n-9 fatty acid in Cryptomonas sp. and Rhodomonas sp., and 20:5 fatty acid in Navicula UMACC 231 (Teoh et al. 2004). Interestingly, a positive correlation between increasing temperature and C16:0, C18:0 and C18:1n9 fatty acids also has been reported in Chaetoceros sp. FIKU035, Tetraselmis suecica FIKU302 and Nannochloropsis sp. FIKU036 (Chaisutyakorn et al. 2017). These contradictory findings may be due to variations in species, incomplete temperature ranges, or insufficient resolution of temperature explored in these studies. Nevertheless, the thermal reaction norms of lipid and fatty acids in high-lipid-production algae, and optimal temperatures for lipid and fatty acids, as well as their correlations with growth and photosynthesis, remain poorly understood due to limited research (Table S1).
To assess the above gaps, we conducted an experiment using a high oil-producing microalga Vischeria sp. WL1 strain (with ~50% and ~60% oil yield under nitrogen and NaCl treatment, respectively) (She et al. 2022). We then quantified the growth rates, photosynthetic pigments and parameters, total lipid content and fatty acid compositions of this strain across a temperature gradient spanning (9-32.3 °C). This study provides us a comprehensive understanding of the thermal response of the overall fatty acid profile in Vischeria sp. WL1 and provides a theoretical basis for improving oil and target fatty acid production in productive life.
Materials and methods
Strain and culture conditions
Vischeria sp. WL1 was isolated from a large biological soil crust (BSCs) in 2019 from the eastern side of Helan Mountain in Yinchuan, Ningxia Hui Autonomous Region, China (She et al. 2022). The cultures were statically maintained in 250 mL glass flasks with 150 mL BG-11 medium (Stanier et al. 1971). The static cultivation was achieved by placing cultures in a stationary liquid medium for cultivation, with cultures gently shaken three times daily, and their positions in incubator switched randomly to reduce cell sedimentation and ensure consistent incident light in different bottles. Continuous illumination of 100 µmol photons m−2 s−1 was provided by a light incubator (GDN-600D, Ningbo Yanghui instrument Co., Ltd., China) with LED tri-color light. Vischeria sp. WL1 was initially cultured at 25°C before starting the experiment. The mid-log phase cultures were then transferred to each experimental temperature for pre-culturing for 8 days. These cultures were subsequently used as inocula for the formal experiment. Based on the preliminary experiments, temperatures of 9, 13, 17, 21, 25, 28, 30, 31, 32, and 32.3 ℃ were selected in the range of upper limit and lower limit. Three replicates were performed in each temperature regime. Cultures at different temperatures were sampled at a cell concentration of 3-4 × 105 cells mL–1 on day 4 and a final cell concentration of ~5 × 105 cells mL–1 on day 8 with sampling volume of 50 mL in a batch. Preliminary experimental work identified the ideal cell density to initiate the experiment and for sampling and also to identify correlation between cell density and growth stage. The cultures at both day 4 and day 8 were all in the mid-log phase.
Measurement of cell density, specific growth rates and thermal performance curves
Cell density under was quantified by cell counting using a hemocytometer. The specific growth rate (μ day-1) was calculated as:
where Nt1 is the cell density at day 8, Nt0 is the inoculation density, and Δt is the time passed in days. Thermal performance curves for growth, photosynthetic parameters, fatty content and fatty acids were fitted by Eq. (2) (O'Donnell et al. 2018) and the optimal growth temperature (Topt) was obtained.
where Topt is the optimum temperature, T is temperature, d1 is the pre-exponential constant for death, b2 and d2 are the exponential rates of increase in birth and death terms, and d0 is the temperature-independent death rate.
Measurement of photosynthetic pigments concentration
A 2 mL sample was centrifuged (8,000×g, 5 min) to remove the supernatant,and was then extracted using 2 mL 95% ethanol and ground using a Tissue Lyser (Wonbio-L, China) for 10 min at 60 Hz. The extract was then centrifuged at 8,000×g for 5 min. The OD664.1, OD648.6 and OD470 values of the supernatant were measured and concentrations of chlorophyll a and carotenoids were calculated as follows (Lichtenthaler 1987):
Photochemical traits
To assess the photochemical response of the cells under different temperatures, 2 mL samples were pre-incubated in the dark for 20 min (Yuan et al. 2023). Photochemical parameters were then measured using a portable PAM fluorometer (FluorPen FP 110, Czech Republic). Parameters of the maximum quantum yield of PSII photochemistry (Fv/Fm), performance index on absorption basis (PIabs), maximum values of quantum yield for electron transport (φE0), maximum values of efficiency that a trapped exciton can move an electron into the electron transport chain beyond QA- (ψ0), the change of chlorophyll fluorescence value of the OJIP curve at about 300 μs (Wk), the effective antenna size per reaction center (ABS/RC), the dissipated energy flux per reaction center (DIo/RC), the trapped energy flux per reaction center (TRo/RC), and the electron transport per active reaction centers (ETo/RC) were then measured according to the manufacturer’s instructions. All measurements were made at the corresponding incubation temperature .
Dry weight and total oil content
A 50 mL sample from each flask was harvested by centrifugation (8,000×g, 3 min), and the precipitate was then frozen overnight at −20 ℃, followed by freeze drying until a constant weight was achieved. The dried pellet was then weighed. Lipid was extracted using the method of Xu et al. (2020) with slight modification. Briefly, the above algal powder was placed in a 2-mL centrifuge tube. 2 mL chloroform-methanol (2:1, v/v) was then added followed by grinding using a Tissue Lyser (Wonbio-L, China) for 10 min at 60 Hz. Then, samples were centrifuged (10 min, 8,000×g) to collect the lipid-containing phase which was dried using a nitrogen blower and weighed. The oil content (%) of each culture was calculated as follows:
where w1 is the total oil weight, w2 is the dry weight.
Fatty acid composition analysis
To perform methyl esterification, 10 mg oil was mixed with 4 mL iso-octane in a 10 mL centrifuge tube. Then 200 μL 2 M KOH-CH3OH was added to the mixture for reaction for 20 min, followed by adding about 1 g NaHSO4 for neutralization. The neutralized solution was mixed with 1 mL distilled water for organic phase separation. The organic phase was then collected and filtered using a 0.45 μm filter. The fatty acid composition was determined using a GC2010-Pro gas chromatograph (Shimadzu, Japan) equipped with an FBX-10 column (100 m × 0.25 mm × 0.20 μm) (ANPEL Laboratory Technologies (Shanghai) Inc., China). The temperature of the flame ionization detector (FID) and the injector temperature (with a split ratio of 50:1) were set to 280 °C and 250 °C, respectively. Nitrogen was used as the carrier gas at a flow rate of 25 mL min−1. The column temperature was programmed as previously described (Zhong et al. 2007), with a final 40 min isothermal period at 230 °C. The fatty acids were qualitatively identified according to the chromatographic peaks of a 37-component Fatty Acid Methyl Ester (FAME) mixture (ANPEL Laboratory Technologies (Shanghai) Inc. China). The relative contents (%) of the individual fatty acids were calculated using the area normalization method (Satchithanandam et al. 2002). The absolute content of eicosapentaenoic acid (EPA) was determined by an internal standard and calculated by the following standard curve:
where y is the peak area and x is the EPA concentration (mg mL-1).
Statistical analysis
Data are presented as means ± standard deviation of three replicates and tested for statistical significance using one-way analysis of variance or t-test. Values were considered significantly different when the probability (P) was < 0.05. Tests were performed using the SPSS 19.0 (SPSS Inc., USA).
Results
The thermal response of growth rate and intracellular oil content
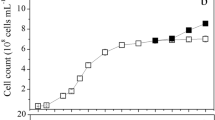
The thermal response of the growth rate of Vischeria sp. WL1 under different cultivation temperatures is shown in Fig. 1a. The growth rate gradually increased with the increase of the cultivation temperature from 9 to ~31 ℃ on day 4 and from 9 to ~28 ℃ on day 8, but decreased sharply when the temperatures exceeds ~31 ℃ on day 4 and exceeds ~28 ℃ on day 8. The growth temperature of Vischeria sp. WL1 ranged from 9 to 32.3 °C and the optimum growth temperatures (Topt) were 30.5 ±0.3 ℃ and 27.9 ± 0.6 ℃ on day 4 and 8, respectively. The optimal specific growth rate was ~0.17 day-1 on day 4 and ~0.16 day-1 on day 8, which are about four times the lowest specific growth rate (~0.04 day-1) at 9 ℃.
The oil content of Vischeria sp. WL1 was strongly depended on temperature like the growth rate but with some different patterns (Fig. 1b). In general, although the oil content showed a noticeable increase trend as the temperature decreases on both days 4 and 8, no significant difference between day 4 and day 8 under each temperature was observed (P > 0.05, Dataset S1). However, with the decrease of temperature, the fitted thermal performance curves and values of oil content observed on day 4 show a quicker increase when temperatures are equal and higher than 25 ℃ (oil content of 52.0 %), but show a slower increase when temperature was lower than 25 ℃, which contrast with the stepped change of the fitted curve that observed on the day 8, with the maximum oil content (64.6 %) reached at 9 °C (Fig. 1b, Table S2).
The thermal response of photosynthetic pigments
Different photosynthetic pigments show different thermal responses (Fig. 2). The thermal response of chlorophyll a concentration complies well with the thermal reaction norm (Fig. 2a) and show a very similar Topt on day 4 (30.3 ± 0.8 ℃) with that of the Topt for growth rate on day 4 (30.5 ± 0.3 ℃), and also shows similar Topt on day 8 (28.2 ± 1.2 ℃) with that of the Topt for growth rate on day 8 (27.9 ± 0.6 ℃) (Table S3). In contrast, the carotenoid concentration and the ratio of carotenoid/Chl a show a bow-shaped curve in response to increasing temperatures with the lowest values at mid temperatures close to 20 ℃ but with their highest values at both lower and higher temperatures than 20 ℃ (Fig. 2b and c). Moreover, compared with that on day 4, the thermal response curves of Chl a concentration, carotenoid concentration and carotenoid/Chl a ratio are much higher on day 8 (P < 0.05, Dataset S1).
The thermal response of photosynthetic pigments on day 4 (pale blue symbols) and day 8 (red symbols). (a) Chlorophyll a concentration (b) Carotenoid concentration (c) Carotenoid/Chlorophyll a ratio. The pale blue and red text in each panel represent the R2 and P of the fitted curves on day 4 and day 8, respectively. Topt, optimal temperature
The thermal response of photosynthetic parameters
Several of the photosynthetic parameters, such as Fv/Fm, PIabs, φE0, and ψ0, can be fitted well with the thermal reaction norm, which shows that optimal temperatures for these parameters were close to or lower than the optimal temperature for population growth rate (Fig. 3a-d, Table S3). We found similar optimal temperatures of Fv/Fm on day 4 (25.7 ± 0.7 °C) and day 8 (25.8 ± 0.9 °C), but higher optimal temperatures of PIabs and φE0 on day 4 (28.4 ± 0.3 ℃, 27.7 ± 0.6 ℃) than on day 8 (24.3 ± 0.7 ℃, 24.1 ± 0.9 ℃), and lower optimal temperatures of ψ0 on day 4 (25.1 ± 0.0 ℃) and day 8 (27.0 ± 0.5 ℃) (Table S3). There were significant differences between the optimum growth temperature and the Topt of Fv/Fm, PIabs, φE0 and ψ0 on day 4, and the Topt of Fv/Fm, PIabs and φE0 on day 8 (Table S3). As shown in Fig 3e, f and Fig. S1a, the Wk value, effective antenna size per reaction center (ABS/RC) and the dissipated energy flux per reaction center (DIo/RC) have obvious bow-shaped curves in response to increasing temperatures at day 4 and day 8, and the values of Wk were higher on day 8 than that on day 4. The thermal response of the trapped energy flux per reaction center (TRo/RC), the electron transport per active reaction centers (ETo/RC) showed a flat trend at both days 4 and 8, but only few values on day 4 were higher than those on day 8 (Fig. S1, Dataset S1).
The thermal response of photosynthetic parameters on day 4 (pale bule symbols) and day 8 (red symbols). (a) Fv/Fm. (b) PIabs. (c) φE0. (d) ψ0. (e) ABS/RC. (f) DIo/RC. The pale blue and red text in each panel represent the R2 and P of the fitted curves on day 4 and day 8, respectively. Fv/Fm, the maximum quantum yield of PSII photochemistry; PIabs, performance index on absorption basis; φE0, maximum values of quantum yield for electron transport; ψ0, maximum values of efficiency that a trapped exciton can move an electron into the electron transport chain beyond QA-; ABS/RC, the effective antenna size per reaction center; DIo/RC, the dissipated energy flux per reaction center; Topt, optimal temperature
The diverse thermal response of the fatty acids
The percentage of saturated fatty acid (SFA) of Vischeria sp. WL1 can be fitted well with the thermal reaction norm on day 4 (R2 = 0.61, P = 6.5 × 10-5; Topt = 31.0 ± 5.3 ℃), but not on day 8 (R2 = 0.11, P = 0.58, Topt = 22. 7 ± 5.8 ℃) (Fig. 4a). The percentage of unsaturated fatty acid (UFA) showed a decreasing trend on day 4 and a flat trend on day 8 in response to increasing temperatures (Fig. 4b). A general decrease of the percentage of monounsaturated fatty acids (MUFA) was observed on both days 4 and 8 as the temperature increased (Fig. 4c). This decrease was more rapid when temperature increases from 9 ℃ to 21 ℃ on day 4, while it increased after reaching 25 ℃. It then reached a similar level between day 4 and day 8 at 32 ℃. Interestingly, the percentage of polyunsaturated fatty acid (PUFA) can be fitted well with the thermal reaction norms with the optimal temperature of 23.8 ± 5.5 ℃ and 29.5 ± 0.9 ℃ on day 4 and 8, respectively (Fig. 4d).
The thermal response of (a) SFA. (b) UFA. (c) MUFA. (d) PUFA as percentages of total fatty acid on day 4 (pale blue symbols) and day 8 (red symbols). The pale blue and red text in each panel represent the R2 and P of the fitted curves on day 4 and day 8, respectively. SFA, saturated fatty acid; UFA, unsaturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; TFA, total fatty acid; Topt, optimal temperature
Vischeria sp. WL1 has a fatty acid composition of myristic (C14:0), palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1), linoleic (C18:2), linolenic (C18:3), arachidonic (C20:4) and EPA (Fig. 5).
The thermal response of fatty acid composition as percentage of total fatty acids. The fatty acid composition on (a) day 4 and (b) day 8. (c) the thermal response of fatty acids on day 4 (pale blue symbols) and day 8 (red symbols). Numbers in the upper middle indicate the fatty acid classes shown in each panel. The pale blue and red text in each panel represent the R2 and P of the fitted curves on day 4 and day 8, respectively. TFA, total fatty acid; Topt, optimal temperature
There were obvious changes in the relative content of different fatty acids in response to temperature variations and across different sampling days (Fig. 5a and b).
Among different fatty acids, C16:1 and C16:0 are the most abundant total fatty acids, accounting for ~50-70% and ~5-20% of the total fatty acids, respectively. Remarkably, the thermal responses of PUFAs (C18:2, C18:3) can be fitted well with thermal reaction norms, with optimal temperatures of C18:2 and C18:3 at 31.7 ± 1.2 ℃ and 27.6 ± 1.0 ℃ on day 4, and 31.4 ± 0.1 ℃ and 28.6 ± 0.7 ℃ on day 8, respectively (Fig. 5c). The optimal temperature of C20:4 also fitted well with the thermal reaction norm on day 8 (R2 = 0.57, P = 2.2 × 10-4; Topt = 32.0 ± 4.4 ℃) but not on day 4 (R2 = 0.25, P = 0.11; Topt = 23.8 ± 4.1 ℃). Interestingly, we found that Topt of C18:2 and C18:3 were not significantly different with the optimum growth temperature on both day 4 and day 8 (Table S4). Moreover, temperature and sampling day are both factors affecting the MUFAs. The C16:1 content decreased with increasing temperature on both day 4 and day 8, similar to the trajectories of total lipid content shown in Fig. 1b. However, C18:1 showed bow-shaped curves in response to increasing temperatures on both day 4 and day 8, and with lower values on day 8 than that on day 4 at temperatures below 9 ℃ and higher than 30 ℃. For the SFAs C14:0, C16:0 and C18:0, although their content can be fitted by the typical thermal reaction norm with a P value ≤ 0.016, only C18:0 on day 4 had an optimal temperature of 26.6 ± 5.3 ℃. In general, within the increase of temperature, all SFAs have an increasing trend on day 4, but for C14:0 and C16:0 there was little change and C18:0 showed a decreasing trend on day 8 (Fig. 5c).
For EPA, a general trend was found with both the relative (%TFA) and absolute content (mg mL-1) of EPA decreasing with increasing temperatures on day 4 but increasing with increasing temperatures on day 8 (Fig. 6). The maximum level of relative EPA was 16 % TFA, and the absolute EPA content of 0.07 and 0.08 mg mL-1 was observed on day 4 and day 8, respectively.
Discussion
Considerable effort has been made to investigate the effect of temperature on photosynthesis, oil content and fatty acid composition in microalgae (Sushchik et al. 2003; Ivanova et al. 2015; Svenning et al., 2019), with the aim of understanding the mechanisms underlying the accumulation of cellular oil and to formulate effective strategies for improving oil production in microalgae (Tan and Lee 2016). Here, we investigated the thermal reaction norms of the growth, photosynthesis, oil content and fatty acid compositions of the high-production microalga Vischeria sp. WL1 over a wide temperature range and establishing the thermal traits of these parameters. We found that: 1. Several photosynthetic parameters (Chl a content, Fv/Fm, PIabs, φE0, and ψ0) fitted well by the thermal reaction norm, with close or slightly lower optimal temperatures than the growth rate; 2. The total lipid content generally increased with decreasing cultivation temperature on both days 4 and 8; 3. The thermal responses of fatty acids exhibit remarkable diversity with obvious distinct patterns.
Thermal reaction norms of growth rate, including its evaluated thermal traits such as the optimal temperature, are commonly used to assess thermal tolerance and evolution (Angilletta et al. 2003; Thomas et al. 2012). Here, we revealed that photochemical traits, including photosynthetic pigments (Chl a) and photosynthetic parameters (Fv/Fm, PIabs, φE0, and ψ0), also fitted well with the thermal reaction norm, showing very similar shapes to that of the growth rate (Fig. 1 and 2). Although biochemical reactions are known to be temperature-dependent (Gillooly et al. 2001), light absorption, excitation energy transfer, and primary photochemistry in photosynthesis are temperature-independent (Raven & Geider 1988). However, the optimal temperatures for some of these photochemical traits were very colse to that of the growth rate (Table S3), which is parallel with a previous study where gross photosynthesis rates exhibit comparable thermal reaction norms to the growth rate in the marine diatom Thalassiosira pseudonana (Schaum et al. 2018). These results suggest that these photosynthetic parameters may serve as a predictor for optimal growth (Ranglova et al. 2019) for thermal responses in Vischeria sp. WL1, attributed to the strong coupling between photosynthesis and growth during temperature treatment as most enzymes in cells are temperature-dependent (Toseland et al. 2013). Among these photosynthetic parameters, only the optimal temperatures of Chl a on both day 4 and day 8 are similar with those of the growth rate, relative to the optimal temperatures of Fv/Fm on both day 4 and day 8 are much lower than those of the growth rate (Table S3). Although Fv/Fm is one of the most commonly used parameters to reflect the photosynthesis efficiency, the thermal responses of Fv/Fm seem less sensitive than PIabs, φE0, and ψ0. This suggests that Fv/Fm might not be the best indicator s to represent the growth physiology of Vischeria sp. WL1 during thermal tolerance but Chl a did.
Although temperature is commonly used as an important strategy for lipid accumulation in microalgae, the observations regarding its effect vary among different studies and microalgal species. Lower temperature, higher temperature, or both lower and higher temperature have been found to induce lipid accumulation (Converti et al. 2009; Cao et al. 2014; Wang et al. 2016; Ma et al. 2017; He et al. 2018; Svenning et al. 2019). Here, after evaluating the lipid content of Vischeria sp. WL1 at a wide range of temperatures (9-32.3 ℃), we found that the total lipid content generally increased with decreasing temperature on both days 4 and 8 but exhibited different patterns (Fig. 1b). These results suggest that cellular total lipid is not always linearly accumulated under lower temperature in Vischeria sp. WL1, differing from previous reports (Deng et al. 2020). Furthermore, in comparison to an oil yield of approximately 50% and 60% under nitrogen and NaCl treatment, respectively, for this strain (She et al. 2022), low temperature induced a significantly higher oil yield reaching up to approximately 70% at 9 ℃. This suggests that low temperature could be an effective strategy for oil production in this strain. One possible reason for the high oil yield may be attributed to evolved adaptive strategies to habitats with large diurnal temperature ranges.
The plasticity of fatty acid profiles under different temperatures has been described in a wide variety of microalgal taxa (Mortensen et al. 1988; Thompson et al. 1992a; Helamieh et al., 2021; Krimech et al., 2022), while thermal reaction norms of fatty acids have been rarely analysed. Here, we found an increasing trend of UFA but a decreasing trens of SFA as the temperature decreased at day 4 (Fig. 6a and b), suggesting the maintenance of membrane fluidity at lower temperatures by altering the saturation state of the fatty acids in the membrane (Barati et al., 2019). However, this trend is not applicable on day 8, where there was only a very slight alteration of both the UFA and SFA across the temperature spectrum, suggesting sampling cell density/culture volume are important factors affecting the thermal response of fatty acid unsaturation. Studies have found that the response trajectory of MUFA across temperature is variable with often nonsignificant differences (Thompson et al. 1992b; Renaud et al. 2002; Pasquet et al. 2014), but that PUFA increases with increasing temperature (Thompson et al. 1992b). Intriguingly in this study, with the increase of temperature, the total MUFA (on both day 4 and day 8) and UFA (on day 4) generally show a decreasing trend, while the total PUFA fitted well with the thermal reaction norms at both day 4 and day 8. Moreover, we further observed a high diversity of the thermal responses of fatty acids in Vischeria sp. WL1 with only C18:2 and C18:3 exhibiting typical thermal reaction norms and the evaluated optimal temperatures (Fig. 5c). Notably, there were no significant differences in optimal temperatures for growth rate and for C18:2 and C18:3 (Table S4), indicating the coupling of these PUFAs with growth. It is worth noting that the thermal response of EPA content differs from the thermal response of other PUFAs (Fig. 5).
The EPA content was negatively correlated with increasing temperature on day 4, while it showed a positive correlation with increasing temperature on day 8. This highlights the complex effect of sampling cell density/culture volume on the thermal response of EPA in Vischeria sp. WL1.
In conclusion, our results reveal intriguing parallels of thermal reaction norms and optimal temperatures between several photosynthetic parameters, PUFAs and the growth rate. The most outstanding finding is that the thermal response of fatty acids of Vischeria sp. WL1 exhibits remarkable diversity, characterized by distinct patterns of change. The comprehensive understanding of thermal responses and patterns observed in this study underscores the complexity of Vischeria sp. WL1’s physiological and lipidic adaptations to temperature. By understanding how temperature affect the lipid and fatty acids of microalgae, targeted temperature strategies to optimize these processes can be developed. This could have significant implications for industries such as biofuels and food production, where targeted fatty acid synthesis and high oil yields are desirable outcomes. Overall, this research provides practical insights for enhancing oil production and selective fatty acid synthesis through temperature manipulation. Further investigations are needed to elucidate the underlying mechanisms driving these distinct responses and their ecological implications.
Data availability
All data supporting the findings of this study are included in the main article and its supplementary file.
References
Angilletta MJ, Wilson RS, Navas CA, James RS (2003) Tradeoffs and the evolution of thermal reaction norms. Trends Ecol Evol 18:234–240
Barati B, Gan SY, Lim PE, Beardall J, Phang SM (2019) Green algal molecular responses to temperature stress. Acta Physiol Plantarum 41:26
Barkia I, Saari N, Manning SR (2019) Microalgae for high-value products towards human health and nutrition. Mar Drugs 17:304
Barton S, Jenkins J, Buckling A, Schaum CE, Smirnoff N, Raven JA, Yvon-Durocher G, Ezenwa V (2020) Evolutionary temperature compensation of carbon fixation in marine phytoplankton. Ecol Lett 23:722–733
Boelen P, Dijk R, Damsté JSS, Rijpstra WIC, Buma AG (2013) On the potential application of polar and temperate marine microalgae for EPA and DHA production. AMB Express 3:26
Cao J, Yuan H, Li B, Yang J (2014) Significance evaluation of the effects of environmental factors on the lipid accumulation of Chlorella minutissima UTEX 2341 under low-nutrition heterotrophic condition. Bioresour Technol 152:177–184
Chaisutyakorn P, Praiboon J, Kaewsurelikhit C (2017) The effect of temperature on growth and lipid and fatty acid composition on marine micaroalgae used for biodiesel production. J Appl Phycol 30:37–45
Chamizo S, Belnap J, Eldridge D, J., Cantón Y, Malam Issa O, (2016) The role of biocrusts in arid land hydrology. In: Weber B, Büdel B, Belnap J (eds) Biological Soil Crusts: An Organizing Principle in Drylands. Springer, Cham, pp 321–346
Chisti Y (2007) Biodiesel from microalgae. Biotech Adv 25:294–306
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Courchesne NM, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotech 141:31–41
Deng W, Sun J, Chang ZG, Gou NN, Wu WY, Luo XL, Zhou JS, Yu HB, Ji H (2020) Energy response and fatty acid metabolism in Onychostoma macrolepis exposed to low-temperature stress. J Thermal Biol 94:102725
Deshmukh S, Kumar R, Bala K (2019) Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process Technol 191:232–247
Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC (2008) Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotech 19:235–240
Fakhry EM, Maghraby DME (2015) Lipid accumulation in response to nitrogen limitation and variation of temperature in Nannochloropsis salina. Bot Stud 56:2–8
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
He Q, Yang H, Hu C (2018) Effects of temperature and its combination with high light intensity on lipid production of Monoraphidium dybowskii Y2 from semi-arid desert areas. Bioresour Technol 265:407–414
Helamieh M, Gebhardt A, Reich M, Kuhn F, Kerner M, Kummerer K (2021) Growth and fatty acid composition of Acutodesmus obliquus under different light spectra and temperatures. Lipids 56:485–498
Hoffmann M, Marxen K, Schulz R, Vanselow KH (2010) TFA and EPA productivities of Nannochloropsis salina influenced by temperature and nitrate stimuli in turbidostatic controlled experiments. Mar Drugs 8:2526–2545
Hu Q (2013) Environmental effects on cell composition. In: Richmond A, Hu Q (eds) Handbook of Microalgal Culture. Applied Phycology and Biotechnology. Wiley-Blackwell, Oxford, pp 114–122
Ivanova JG, Kabaivanova LV, Petkov GD (2015) Temperature and irradiance effects on Rhodella reticulata growth and biochemical characteristics. Russ J Plant Physiol 62:647–652
Kremer CT, Fey SB, Arellano AA, Vasseur DA (2018) Gradual plasticity alters population dynamics in variable environments: thermal acclimation in the green alga Chlamydomonas reinhartdii. Proc R Soc B 285:20171942
Krimech A, Helamieh M, Wulf M, Krohn I, Riebesell U, Cherifi O, Mandi L, Kerner M (2022) Differences in adaptation to light and temperature extremes of Chlorella sorokiniana strains isolated from a wastewater lagoon. Bioresour Technol 350:126931
Lepori-Bui M, Paight C, Eberhard E, Mertz CM, Moeller HV (2022) Evidence for evolutionary adaptation of mixotrophic nanoflagellates to warmer temperatures. Global Change Biol 28:7094–7107
Li X, Hu HY, Zhang YP (2011) Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresource Technol 102:3098–3102
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae. Biotechnol Progr 24:815–820
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Listmann L, LeRoch M, Schlüter L, Thomas MK, Reusch TBH (2016) Swift thermal reaction norm evolution in a key marine phytoplankton species. Evol Appl 9:1156–1164
Ma C, Wen H, Xing D, Pei X, Zhu J, Ren N, Liu B (2017) Molasses wastewater treatment and lipid production at low temperature conditions by a microalgal mutant Scenedesmus sp. Z-4. Biotechnol Biofuels 10:111
Mortensen SH, Borsheim KY, Rainuzzo JR, Knutsen G (1988) Fatty acid and elemental composition of the marine diatom Chaetoceros gracilis Schütt. Effects of silicate deprivation, temperature and light intensity. J Exp Mar Biol and Ecol 122:173–185
O’Donnell DR, Du ZY, Litchman E (2019) Experimental evolution of phytoplankton fatty acid thermal reaction norms. Evol Appl 12:1201–1211
O’Donnell DR, Hamman CR, Johnson EC, Kremer CT, Klausmeier CA, Litchman E (2018) Rapid thermal adaptation in a marine diatom reveals constraints and trade-offs. Glob Chang Biol 24:4554–4565
Pasquet V, Ulmann L, Mimouni V, Guihéneuf F, Jacquette B, Morant-Manceau A, Tremblin G (2014) Fatty acids profile and temperature in the cultured marine diatom Odontella aurita. J Appl Phycol 26:2265–2271
Ranglova K, Lakatos GE, Manoel JAC, Grivalsky T, Masojidek J (2019) Rapid screening test to estimate temperature optima for microalgae growth using photosynthesis activity measurements. Folia Microbiol 64:615–625
Raven JA, Geider RJ (1988) Temperature and algal growth. New Phytol 110:441–461
Renaud SM, Thinh L, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211:195–214
Renaud SM, Zhou HC, Parry DL, Thinh L, Woo KC (1995) Effect of temperature on the growth, total lipid content and fatty acid composition of recently isolated tropical microalgae Isochrysis sp., Nitzschia closterium, Nizschia paleacea, and commercial species Isochrysis sp. (clone T.ISO). J Appl Phycol 7:595–602
Satchithanandam S, Fritsche J, Rader JI (2002) Gas chromatographic analysis of infant formulas for total fatty acids, including trans fatty acids. J AOAC Int 85:86–94
Schaum CE, Buckling A, Smirnoff N, Studholme DJ, Yvon-Durocher G (2018) Environmental fluctuations accelerate molecular evolution of thermal tolerance in a marine diatom. Nat Commun 9:1719
She Y, Gao X, Jing X, Wang J, Dong Y, Cui J, Xue H, Li Z, Zhu D (2022) Effects of nitrogen source and NaCl stress on oil production in Vischeria sp. WL1 (Eustigmatophyceae) isolated from dryland biological soil crusts in China. J Appl Phycol 34:1281–1291
Stanier RY, Kunisawa R, Mandel M, Cohen-bazire G (1971) Purification and properties of unicellular blue-green algae (Order Chroococcales). Bact Rev 35:171–205
Sushchik NN, Kalacheva GS, Zhila NO (2003) A temperature dependence of the intra- and extracellular fatty-acid composition of green algae and cyanobacterium. Russ J Plant Physiol 50:374–380
Svenning JB, Dalheim L, Eilertsen HC, Vasskog T (2019) Temperature dependent growth rate, lipid content and fatty acid composition of the marine cold-water diatom Porosira glacialis. Algal Res 37:11–16
Tan KWM, Lee YK (2016) The dilemma for lipid productivity in green microalgae: importance of substrate provision in improving oil yield without sacrificing growth. Biotechnol Biofuels 9:255
Teoh M-L, Chu W-L, Marchant H, Phang S-M (2004) Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J Appl Phycol 16:421–430
Thomas MK, Kremer CT, Klausmeier CA, Litchman E (2012) A global pattern of thermal adaptation in marine phytoplankton. Science 338:1085–1088
Thomas MK, Litchman E (2015) Effects of temperature and nitrogen availability on the growth of invasive and native cyanobacteria. Hydrobiologia 763:357–369
Thompson PA, Guo MX, Harrison PJ (1992) Effects of variation in temperature. I. On the biochemical composition of eight species of marine phytoplankton. J Phycol 28:481–488
Thompson PA, Guo MX, Harrison PJ, Whyte JNC (1992) Effects of variation in temperature. II. On the fatty acid composition of eight species of marine phytoplankton. J Phycol 28:488–497
Toseland A, Daines SJ, Clark JR, Kirkham A, Strauss J, Uhlig C, Lenton TM, Valentin K, Pearson GA, Moulton V, Mock T (2013) The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat Climate Change 3:979–984
Wang Y, He B, Sun Z, Chen Y-F (2016) Chemically enhanced lipid production from microalgae under low sub-optimal temperature. Algal Res 16:20–27
Weber B, Tamm A, Maier S, Rodríguez-Caballero E (2018) Biological soil crusts of the Succulent Karoo: a review. Afr J Range Forage Sci 35:335–350
Xu J, Li T, Li CL, Zhu SN, Wang ZM, Zeng EY (2020) Lipid accumulation and eicosapentaenoic acid distribution in response to nitrogen limitation in microalga Eustigmatos vischeri JHsu-01 (Eustigmatophyceae). Algal Res 48:101910
Xue Z, Wan F, Yu W, Liu J, Zhang Z, Kou X (2018) Edible oil production from microalgae: A review. Eur J Lipid Sci Technol 120:1700428
Yuan X, Gao X, Zheng T, Wang J, Dong Y, Xue H (2023) Carbon nanomaterial-treated cell cultures of Nostoc flagelliforme produce exopolysaccharides with ameliorative physio-chemical properties. Int J Biol Macromol 227:726–735
Zhong HY, Bedgood DR, Bishop AG, Prenzler PD, Robards K (2007) Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chem 100:1544–1551
Zhu CJ, Lee YK, Chao TM (1997) Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. J Appl Phycol 9:451–457
Funding
This research was supported by the Key Research and Development Program of Shaanxi Province (No. 2022NY-194) and the Shaanxi Province Qin Chuangyuan “Scientist+Engineer” Team Construction Project (No. 2023KXJ-206).
Author information
Authors and Affiliations
Contributions
ZL and XG designed research. DW, XW, XY, XG performed the experiments. DW and ZL wrote the manuscript. All authors contributed to the corrections of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Gao, X., Wang, X. et al. Diverse thermal responses of the growth, photosynthesis, lipid and fatty acids in the terrestrial oil-producing microalga Vischeria sp. WL1. J Appl Phycol 36, 29–39 (2024). https://doi.org/10.1007/s10811-023-03152-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03152-3