Abstract
Proliferations of green, brown and red algae appear in shallow sandy bays in North Brittany (France), and they represent a real economic constraint for the affected communities. In addition to the nuisance for residents and tourist activity, the communities must carry out systematic collection. The collected algae are spread on agricultural land spreading or composted, but these solutions reach their limits rapidly, bringing little added value to the collected algae. Seaweeds are potentially excellent sources of bioactive metabolites that could represent useful leads in the development of new functional ingredients in pharmaceutical and cosmetic industries. The aim of this study was to propose the use of an enzyme-assisted extraction as a tool to improve the extraction efficiency of antiviral compounds from three invasive French seaweeds. We selected the red Solieria chordalis, the green Ulva sp. and the brown Sargassum muticum as models for these experiments. In comparison with water extraction at 50 °C for the same time of treatment, enzymatic hydrolysis increased the yields. The data suggest the potential of enzymatic hydrolysis for producing active fractions in the function of the algal biomass, the behaviour of the cell wall, the selectivity and the action of the enzyme. Enzymatic hydrolysis appeared less effective for polyphenol recovery, but was a promising softer technique for recovering proteins, neutral sugars, uronic acids and sulphate groups. The solvent-free process, higher extraction rate and higher yields, coupled to time-saving and lower cost, make this method economical and sustainable. By using a cell viability assay, all hydrolysate fractions tested were shown to be non-toxic to Vero cells. After 3 days of treatment, no microscopically visible alteration of normal cell morphology was observed even at 500 μg mL−1. S. chordalis extracts have an effective antiviral activity with EC50 between 23.0 and 101.1 μg mL−1 at a multiplicity of infection of 0.001 ID50/cells; 100 % and 98 % cellular protection were obtained for 500 μg mL−1 of hydrolysate extracts carbohydrase C3 and blank, respectively. Other extracts from S. chordalis inhibited viral production less effectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine algae from the French Brittany coasts (North West of France) constitute a significant and diverse natural vegetable production. The diversity of macroalgal species occurring in Brittany is due to abiotic factors (substrate, seawater temperature, hydrodynamism, light conditions), which are optimal for the development of macroalgae. As a result, nearly 700 species of algae are currently listed for the Breton littoral (Arzel 2000; Dizerbo and Herpé 2007; Bourgougnon and Stiger-Pouvreau 2011). The use of algae has existed for centuries in Brittany. Since the 1920s, marine macroalgae have been harvested by many companies along the Brittany coast (Arzel 1987, 2000; Arzel and Barbaroux 2003) initially to extract sodium carbonate, iodine and then later the colloids—agars, alginates and carrageenans. Indeed, marine algae had to be processed as soon as possible after their harvesting to preserve all their qualities. Some companies cultivate the algae in the natural environment or out of ranks transforming fresh algae, choosing to settle near the resource. Since the 1980s, cosmetic, food, thalassotherapy and the pharmaceutical fields have been interested in algal resources. The increasing demand worldwide for raw materials for food, cosmetics, bioactive and more recently for chemistry and bioenergy raises the issue of the sustainability of the French and European industries; 50 000 to 60 000 t of wild collected algae are transformed each year in Brittany into colloids and are intended for agri-food or cosmetic industries, or are employed as chemical compounds (Bourgougnon and Stiger-Pouvreau 2011).
Among the flora encountered in Brittany, native or introduced macroalgae can become invasive or proliferative and have profound adverse ecological impacts including the alteration of ecosystem structure, reduction of indigenous biodiversity and economic losses. Excessive growth of some opportunist seaweeds in reaction to medium disturbance is observed with increasing frequency in coastal ecosystems close to agricultural or strongly urbanized and industrialized zones. Proliferations of green, brown and red algae and large piles of decomposing biomass appear along the north shallow sandy bays of Brittany and represent a true economic constraint for the affected communities: Solieria sp. has been observed in the Gulf of Morbihan (France) since 2005 and in the Sarzeau Peninsula (Morbihan, France) where strandings have become more abundant between July and October (Tavennec, personal communication, 2009). During the summer, it is then a question of removing more than 15 000 t year−1 of red algae mainly of S. chordalis (Rhodophyta, Gigartinales). In North Brittany, the opportunistic growth ability of Ulvales genera Ulva and Enteromorpha are associated with proliferation in eutrophicated coastal waters or with contamination of algal closed cultures (Lahaye and Robic 2007). This biomass makes them good candidates for biogas, bio-composites and water recycling in integrated invertebrate and fish aquaculture systems and of urban waters, but most of the generated biomass is of little value today. The collected algae (80–100 000 t year−1) are often incorporated into compost, but are generally dumped although conversion to biogas is possible. The brown macroalga Sargassum muticum (Fucales), a native from Japan, is presently distributed along the Atlantic coasts, from southern Portugal to the southern coast of Norway (Plouguerné et al. 2006). The upgrading of the invasive S. muticum began after eradication failures and progress in its geographic areas. In recent years, algal stranding has appeared more and more precociously and the phenomenon has gained significance. An expanding market for these products is a fact and is facing a new challenge of growing algae on a large scale without harming any further the marine environment.
Seaweeds are potentially excellent sources of highly bioactive metabolites that could represent useful leads in the development of new functional ingredients in the food, pharmaceutical and cosmetic industries (Cardozo et al. 2007; Ioannou and Roussis 2009; Mayer et al. 2007; Stengel et al. 2011; Mohamed et al. 2012). Herpes simplex virus (HSV), a DNA enveloped virus, was a common human pathogen with between 60 and up to 95 % of certain populations infected with Herpes simplex virus type 1 (HSV-1) and between 6 and 50 % infected with Herpes simplex virus type 2 (HSV-2). The frequency of HSV-seropositive males was significantly higher in populations infected with human immunodeficiency virus (HIV). As HIV disease progresses, cutaneous and mucosal complications become more severe and occur in up to 92 % of HIV-infected individuals (Sassi et al. 2008). Acyclovir is the compound of choice for clinical use against HSV-1 and HSV-2, in systemic or topical therapy (Bouhlal et al. 2011). Other acyclovir-related nucleoside analogs, all targeted against viral DNA synthesis, have been more recently licensed for human use. However, these drugs are not always efficacious or well-tolerated, and the emergence of viral resistant variants after prolonged treatment in immuno-compromised AIDS or transplant patients is the main reason for the continuous search for novel antiherpetic agents.
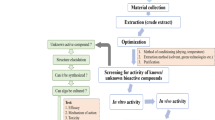
Macroalgal proteins and oligo- and polysaccharides represent potential raw materials for the new generation of health ingredients having both techno- and bio-functional applications. With interest in new renewable sources of chemicals and polymers, this underexploited biomass represents a potential source to be explored. The marine bioprocess industry has evolved and novel technologies have been developed to convert and use biomass or marine food byproducts. The aim of this work is then to propose the use of the two commercial protease and four carbohydrase treatments as a tool to improve the extraction efficiency of bioactive compounds from three invasive French seaweeds: S. chordalis, Ulva sp. and S. muticum. Extraction yield, analysis and recovery of chemical compounds in the water-soluble fractions were analysed and are discussed in relation to the different enzymes used during hydrolysis. The fractions were tested using laboratory in vitro assays against the Herpes simplex virus.
Materials and methods
One kilogram of different specimens of seaweeds was handpicked in Brittany (France). Ulva sp. (Chlorophyta, Ulvales, Ulvaceae) was collected on the beach in Plestin-les-Grèves (48°39′28″N, 3°37′47″W), English Channel, in June 2012. S. chordalis (J. Agardh) C. Agardh (Rhodophyta, Gigartinales, Solieriaceae) was collected in March 2012 from the littoral zone of the Saint Gildas de Rhuys (47°30′0″N, 2°49′60″W). S. muticum (Yendo) Fensholt (Ochrophyta, Fucales, Sargassaceae) was harvested in June 2012 on Grand Dellec, Plouzané (48°22′56″N, 4°37′0″W). To remove adherent seawater, sediment, organic debris, macrofauna and epibiota, they were scraped and rinsed with distilled water. The seaweeds were ground to pieces of about 3 mm with a hammer mill. The ground biomass was stored at −25 °C prior to extraction.
Preparation of enzymatic hydrolysates from seaweeds
Six commercial enzymes were used for the hydrolysis of seaweeds: two types of proteases (endo-peptidase, endo-protease) which will be named P1 and P2 and four types of carbohydrases (cellulase, xylanase, β-glucanase, arabanase) which will be named C1, C2, C3 and C4.
Hydrolysis experiments with Ulva
Extractions were performed in a bioreactor on 500 g of crushed algae (8.3 % dry matter) added to 500 mL of distilled water. Algae were submitted to each enzyme (0.5 %, w/w) for a short period of 5 h at 50 °C.
Hydrolysis experiments with S. chordalis
Extractions were performed in a bioreactor on 100 g of crushed algae (19.9 % dm) added to 200 mL of distilled water. Algae were submitted to each enzyme (0.5 %, w/w) for a short period of 5 h at 50 °C.
Hydrolysis experiments with S. muticum
Two g of dried algal sample was homogenized in 48 mL phosphate buffer and incubated in a batch thermostatic reactor. The enzymatic hydrolysis was performed under conditions (5 % MS, 50 °C, 5 h).
Enzymes were then denatured at 90 °C for 15 min. After hydrolysis, the hydrolysates were centrifuged at 8,000 × g for 15 min at 20 °C to separate undigested residues and solubilized compounds. After centrifugation, the supernatants were filtered and then recovered. The supernatants were lyophilized, weighed and stored at −20 °C until analysed. A blank, water extract (algal powder extracted with distilled water/phosphate buffer for 5 h at 50 °C) served as control. These results correspond to the composition of hydrolysate fractions defined as the dry weight percentage of each chemical compound in hydrolysate obtained with respect to the initial algal sample (Fig. 1).
Composition analysis of hydrolysis extracts
Specimens of the hydrolysed extract samples were used for composition analysis. Ash values were determined gravimetrically after incineration of samples at 550 °C for 16 h, followed by 2 h at 900 °C. Neutral sugars were determined by the phenol sulphuric acid colorimetric method of Dubois et al. (1956). This method is based on the reduction of neutral sugars, and a little part of uronic acids, in 5-hydroxymethylfurfural by phenol, giving a yellow colour. Anhydrous d-glucose was used as standard (0–100 μg mL−1). Fifty μL of phenol 75 % (v/v) in water was added to 1 mL of standard or diluted sample and stirred; 2.5 mL of sulphuric acid was added directly into the sample, stirred, cooled in fresh water and incubated for 10 min at room temperature and 10 min at 30 °C. Titrations were done in triplicate. The OD was measured at 490 nm.
Uronic acids were determined using the meta-hydroxy-di-phenyl (MHDP) method (Blumenkrantz and Asboe-Hansen 1973). Under the action of hot concentrated mineral acids, uronic acids undergo internal dehydration followed by cyclization leading to the formation of derivatives 5-formylfuroic acids. These derivatives react with the MHDP (0.15 % in sodium hydroxide 0.5 %, w/v) to form a pink chromophore absorbing at 525 nm. The colour was enhanced by the presence of tetraborate sodium salt (75 mM in sulphuric acid 96 %, w/v), but remains highly sensitive to interferences of neutral sugars. The addition of sulfamic acid potassium (4 M) salt limited these interferences; 400 μL of sample or standard was added to 40 μL of sulfamic acid and stirred; 2.4 mL of tetraborate was added to the mixture, stirred and incubated for 20 min at 80 °C and then cooled in fresh water for 5 min. After the addition of 80 μL of MHDP, the mixture was incubated for 10 min at room temperature and the OD measured at 525 nm. Samples were done in triplicate.
The sulphated groups content of polysaccharides was determined by the Azure A (Sigma-Aldrich A6270) method (Jaques et al. 1968) which reacts specifically with sulphates linked in polysaccharides. This reaction is based on the formation of a complex between sulphate groups and the 3-amino-7-(dimethylamino) phenothizin-5-ium (Azure A) to give a purple colour. A sulphated dextran (17 %) was used as standard (0–100 μg mL−1). Two hundred μL of standard or samples added to 2 mL of Azure A reagent (10 mg L−1) were used for the reaction. The OD was measured at 535 nm.
The protein content was determined by the bicinchinonic acid colorimetric method (BCA) (Smith et al. 1985) with a Micro BC Assay Kit (cat. UP75860C, Interchim). Bovine serum albumin was used as standard (0–100 μg mL−1). Titrations were done in triplicate. The OD was measured at 540 nm. The total nitrogen content (% N) was determined using the Kjeldahl method (Crooke and Simpson 1971).
The lipid content was determined by a two-time extraction of 500 mg of dried algae with 10 mL of acetone at 60 °C. Samples were pooled and dried under air flow. Lipids were then extracted twice from the residue with 10 mL of hexane. Samples were then pooled, dried and weighted. The measurement was done in duplicate.
The total phenolic content (TPC) of enzymatic extracts from hydrolysate was quantified spectrophotometrically according to the method of Turkmen et al. (2005) with minor modifications. One milliliter of aliquot of extract solution (concentration ranges from 1 to 5 mg mL−1) was mixed with 5 mL Folin Ciocalteu reagent (10 % in distilled water). After 5 min, 4 mL of sodium carbonate (7.5 % in distilled water) was added. The samples were incubated for 2 h at room temperature in the dark. The absorbance was measured at 760 nm. A standard curve with serial gallic acid solutions was used for calibration. Results were expressed as g gallic acid equivalents (GAE) per kg of extract (Wang et al. 2010).
Determination of antiviral activity of hydrolysate fractions
Cells and viruses
African green monkey kidney cells (Vero, ATCC CCL-81) were grown in Eagle's minimum essential medium (MEM, Eurobio) supplemented with 8 % fetal calf serum (FCS, Eurobio) and 1 % of antibiotics PCS (10,000 IU mL−1 penicillin, 25,000 IU mL−1 colimycin, 10 mg mL−1 streptomycin; Sigma-Aldrich). HSV-1 (wild-type strain 17, sensitive to acyclovir) was obtained from Pr. Agut, Laboratoire de Dynamique, épidémiologie et traitement des infections virales de la Pitié Salpétrière (Paris, France).
Cytotoxicity assays based upon cell viability
Using the Vero cell/HSV-1 model, cytotoxicity was evaluated by incubating cellular suspensions (3.5 × 105 cells mL−1) with various dilutions (concentration from 1 to 500 μg mL−1, four wells per concentration) of fractions in 96-well plates (72 h, 37 °C, 5 % CO2) in Eagle's MEM containing 8 % FCS. The cells were examined daily under a phase-contrast microscope to determine the minimum concentration of hydrolysate dry matter that induced alterations in cell morphology, including swelling, shrinkage, granularity and detachment. Cytotoxicity by cell viability was tested using the neutral red dye method. Optical density (OD) was measured at 540 nm. The 50 % cytotoxic concentration (CC50) was defined as the concentration that reduced the OD of treated cells to 50 % of that of untreated cells.
CC50 values were expressed as the percentage of destruction (%D): [(ODc)C − (ODc)MOCK / (ODc)C] × 100, where (ODc)C and (ODc)MOCK are the OD values of the untreated cells and treated cells, respectively (Langlois et al. 1986).
Antiviral assays based upon cell viability
Using the Vero cell/HSV-1 model, 100 μL of cell suspension (3.5 × 105 cells mL−1) in Eagle's MEM containing 8 % FCS was incubated with 50 μL of a dilution of fractions (concentration from 10 to 500 μg mL−1) in 96-well plates (72 h, 37 °C, 5 % CO2). Three replicates were infected using 50 μL of medium and a virus suspension at a multiplicity of infection (MOI) of 0.001 ID50/cells. After incubation, antiviral activity was evaluated by the neutral red dye method. The antiherpetic compound acyclovir [9-(2-hydroxyethoxymethyl) guanine] was used as reference inhibitor. The 50 % effective antiviral concentration (EC50) was expressed as the concentration that achieved 50 % protection of virus-infected cells from virus-induced destruction. The OD was related directly to the percentage of viable cells, which was inversely related to the cytopathic effect (CPE). The linear regression was determined for each assay on the basis of cell controls (0 % CPE) and virus controls (100 % CPE). Data were expressed as a percentage of protection (%P): [((ODt)virus − (ODc)virus) / ((ODc)MOCK − (ODc)virus)] × 100, where (ODt)virus is the OD of the test sample, (ODc)virus is the OD of the virus control and (ODc)MOCK is the OD of the mock-infected control (McLaren et al. 1983; Langlois et al. 1986).
Statistical analysis
Results are expressed as means ± standard deviation (SD) (n = 3). The statistical analysis was carried out on SPSS v20 (IBM, USA) using one-way analysis of variance (ANOVA) followed by Duncan test at 5 % level (p < 0.05) to evaluate differences between samples. For each series of values, the significant differences are labelled by superscript letters.
Results
Proximate composition analyses showed that the dried Ulva sp. sample contained 15.9 ± 1.2 % ash, 23.2 ± 1.4 % neutral sugars, 21.1 ± 0.9 % uronic acids, 20.1 ± 0.9 % sulphated groups, 24.4 ± 0.1 % proteins and 0.6 % total phenol. The dried S. chordalis powder contained 27.4 ± 1.0 % ash, 9.8 ± 1.1 % neutral sugars, 8.0 ± 0.2 % uronic acids, 5.6 ± 0.1 % sulphated groups, 29.3 ± 4.3 % proteins and 2.1 % total phenol. The dried S. muticum sample contained 25.5 ± 1.1 % ash, 39.7 ± 1.3 % neutral sugars, 1.8 ± 0.1 % uronic acids, 8.8 ± 0.6 % sulphated groups, 22.1 ± 0.6 % proteins and 0.9 % total phenol (Table 1). Enzymatic hydrolysis was performed using the two proteases and four carbohydrases on the three seaweeds. Two fractions were generated: an insoluble sludge and soluble aqueous phase. Only the soluble aqueous phase was studied. Yields, total proteins, polyphenols, neutral sugars, uronic acids and the sulphated groups content from the hydrolysates were quantified and compared. For each analysis, the results were presented by the percentage of dry matter yield in hydrolysate. We obtained two data: (1) the best enzyme for improving the extract yield and (2) the capacity of selective enzyme for isolating a type of compound.
Dry matter yields
The dry matter yields are shown in Fig. 2a–c. Important differences can be observed between the algal raw material and the choice of enzymes. For the water extract (blank), 35.0 %, 28.8 % and 11.6 % of dry matter yields were obtained in the soluble phase, respectively, from Sargassum, Ulva and Solieria biomass. After 5 h of hydrolysis, the major percentage of dry matter yield was contained in the supernatant of the two proteases for Ulva sp.—51.0 % and 52.5 % for P1 and P2, respectively. For Ulva biomass (Fig. 2a), proteolysis clearly improved solubilization of dry matter. Similar results but slightly lower were found on Sargassum biomass showing 48.2 % and 46.9 % dry matter yields, respectively, for P1 and P2 (Fig. 2b). C3 carbohydrase also gave good results with the highest yield of 48.4 % of dry matter. The results are very different with Solieria biomass (Fig. 2c). Indeed, the dry matter yield never exceeded 22.1 % (C2 carbohydrase). In comparison with water extraction at 50 °C for the same time of treatment, enzymatic hydrolysis increased the level of yields.
Dry matter yield hydrolysates of Ulva (a), Sargassum (b) and Solieria (c). Values are means ± SD (n = 3). Values with different superscript letters are significantly different (p < 0.05). Protease hydrolysates are indicated with dark grey bars; carbohydrase hydrolysates by light grey bars; water extracts by white bars
Proteins
Protein recoveries from the soluble fraction after hydrolysis by the six enzymes are given in Fig. 3. The percentages of dry matter yield in hydrolysates correspond to the composition of hydrolysate fractions defined as the dry weight percentage of each chemical compound in hydrolysate obtained with respect to the initial algal sample. For Ulva sp. (Fig. 3a), they ranged between 6.27 ± 0.35 % for C2 carbohydrase and 9.92 ± 0.30 % for P1 protease. For Sargassum (Fig. 3b), they ranged between 38.40 ± 1.3 % for C2 carbohydrase and 53.80 ± 2.1 % for C1 carbohydrase. The percentages of dry matter in hydrolysate are lower than the percentage of the blank (58.10 ± 1.7 %). For Solieria (Fig. 3c), they ranged between 11.60 ± 0.20 % for P2 protease and 15.20 ± 0.30 % for P1 protease. Except P2 protease (11.60 ± 0.20 %) and C4 carbohydrase (12.20 ± 0.30 %), the percentages of dry matter in hydrolysate are higher than in the blank (12.70 ± 0.50 %), but they do not exceed 15.20 ± 0.30 % with P1 protease. Carbohydrases present the same recovery levels (13.5 % dry matter in hydrolysate). P1 protease appears as the most effective enzyme for protein recovery and accessibility. Regardless of the raw material or the enzyme used, hydrolysis increased the level of protein in the hydrolysates only for the Solieria biomass. For Sargassum, in spite of yields lower than those obtained by aqueous extraction, the results show good levels of extraction with the enzyme.
Content of proteins in hydrolysates of Ulva (a), Sargassum (b) and Solieria (c). Values are means ± SD (n = 3). Values with different superscript letters are significantly different (p < 0.05). Protease hydrolysates are indicated with dark grey bars; carbohydrase hydrolysates by light grey bars; water extracts by white bars
Total polyphenols
Analysis of the percentage of dry matter yield shows that, compared to the blank extract, the enzymatic hydrolysis entails extraction of polyphenol from the green biomass. The polyphenolic content in hydrolysate samples is higher than in the water extract, particularly for protease hydrolysis. The action of the enzymes is less marked on Solieria and Sargassum biomass.
Considerable variation was observed in total polyphenolic content among different enzymatic hydrolysates and biomass ranging from 0.41 ± 0.02 % (C3) to 0.99 ± 0.17 % (P2) dry matter of total polyphenolic content in hydrolysates for Ulva sp. (Fig. 4a), from 4.40 ± 0.18 % (C3) to 5.40 ± 0.13 % (C2) dry matter of total polyphenolic content in hydrolysates for S. muticum (Fig. 4b) and from 0.60 ± 0.10 % (C3) to 1.00 ± 0.30 % (P1) dry matter of total polyphenolic content in hydrolysates for S. chordalis (Fig. 4c). All the proteases and C1/C4 carbohydrase-treated groups had significantly higher percentage values than the water extract of Ulva biomass. The total polyphenolic contents of the hydrolysates for Solieria and Sargassum biomass extracted by proteases and carbohydrases were generally lower except P1 protease for Solieria. In the case of Sargassum, all hydrolysates had lower total polyphenolic content values than the water extract.
Total polyphenol content in hydrolysates in Ulva (a), Sargassum (b) and Solieria (c). Values are means ± SD (n = 3). Values with different superscript letters are significantly different (p < 0.05). Protease hydrolysates are indicated with dark grey bars; carbohydrase hydrolysates by light grey bars; water extracts by white bars
Neutral sugars content
Except one example with action of C2 carbohydrase on Sargassum biomass, enzymatic hydrolysis appears to be a good method to improve extraction efficiency of soluble neutral sugars (Fig. 5). For each biomass, the percentage of dry matter yield in hydrolysate for neutral sugar is superior to the percentage of dry matter yield of the water extract. Like the protein or polyphenol content, the access to soluble molecules is not the same according to the choice of enzyme and the type of biomass. All the carbohydrase-treated groups had significantly higher percentage values than the water extract (8.99 ± 1.79 %) with values for Ulva biomass ranging from 15.35 ± 3.25 % (C2) to 21.09 ± 3.98 % (C3) dry matter of total sugars in hydrolysate (Fig. 5a). For Sargassum, except C2 (7.76 ± 1.81 %), all the enzymes had significantly higher percentage values than the water extract (8.57 ± 2.21 %) ranging from 11.22 ± 6.41 % (C3) to 36.07 ± 4.37 % (P2) dry matter of total sugar in hydrolysates (Fig. 5b). For Solieria, all the enzymes had significantly higher percentage values than the water extract (4.7 ± 0.3 %) ranging from 36.8 ± 0.5 % (C2) to 5.2 ± 1.2 % (C4) dry matter of total sugar in hydrolysates (Fig. 5c).
Neutral sugars content in hydrolysate of Ulva (a), Sargassum (b) and Solieria (c). Values are means ± SD (n = 3). Values with different superscript letters are significantly different (p < 0.05). Protease hydrolysates are indicated with dark grey bars; carbohydrase hydrolysates by light grey bars; water extracts by white bars
Uronic acids content
Considerable variation was observed in uronic acids content among the different enzymatic hydrolysates (Fig. 6) ranging from 8.91 ± 2.19 % (C2) to 18.57 ± 1.15 % (C3) dry matter of total uronic acid in hydrolysates of Ulva sp. (Fig. 6a) compared with the water extract (21.45 ± 4.19 %); in S. muticum, it ranged from 14.15 ± 0.28 % (P1) to 28.41 ± 1.87 % (C3) dry matter of total uronic acids in hydrolysates (Fig. 6b) compared with the water extract (21.43 ± 1.14 %); and finally in S. chordalis, it ranged from 11.5 ± 4.2 % (C1) to 22.1 ± 4.2 % (C2) dry matter of total uronic acids in hydrolysates (Fig. 6c). For uronic acids, water hydrolysis appears to be more efficient than enzymatic hydrolysis for green biomass.
Uronic acids content in hydrolysate of Ulva (a), Sargassum (b) and Solieria (c). Values are means ± SD (n = 3). Values with different superscript letters are significantly different (p < 0.05). Protease hydrolysates are indicated with dark grey bars; carbohydrase hydrolysates by light grey bars; water extracts by white bars
Sulphated groups
Differences in the presence of sulphated groups were observed between green–brown raw materials and red raw material (Fig. 7). Indeed, for green and brown algae raw materials, the percentage of dry matter yield in hydrolysates is slightly less than the hot aqueous extract (17.24 ± 0.38 %) for the sulphate group content except carbohydrase C1-treated (18.04 ± 0.95 %) Ulva biomass (Fig. 7a, b). For Solieria, the behaviour of the enzymes is very different (Fig. 7c). Except for the C3 carbohydrase-treated material (5.0 ± 0.3 %), the percentage of dry matter yield in hydrolysates is slightly less than the hot aqueous extract (4.7 ± 0.2 %). In comparison with previous results, the percentages do not exceed 5 % dry matter of sulphated groups in hydrolysates.
Sulphate groups in hydrolysate of Ulva (a), Sargassum (b) and Solieria (c). Values are means ± SD (n = 3). Values with different superscript letters are significantly different (p < 0.05). Protease hydrolysates are indicated with dark grey bars; carbohydrase hydrolysates by light grey bars; water extracts by white bars
All the commercial enzymes tested in this study were effective in improving the extraction yield, but to a different extent compared with water extraction. Table 2 summarises the best results in the hydrolysates in comparison with the water-soluble extracts. Except for Sargassum material, proteases are more adapted for the extraction of proteins. In the case of Sargassum, the C1 carbohydrase (multicomplex enzyme) exhibited the highest extraction efficiency of protein content compared to other proteases and carbohydrases tested. For extraction of polyphenolic compounds, enzymatic hydrolysis seems less effective. The percentages of dry matter of polyphenolic compounds do not exceed 5.4 % (for Sargassum material). However, for neutral sugars extraction, enzymatic hydrolysis was effective in enhancing the recovery of neutral sugars. Proteases for Sargassum or carbohydrases for Ulva and Solieria exhibited the greatest percentage of dry matter in hydrolysates. For the uronic acids and sulphated groups contents, carbohydrases exhibited the greatest percentage of dry matter in hydrolysates.
In vitro antiviral activity of hydrolysate fractions
After 3 days of treatment, microscopically visible alteration of normal cell morphology was observed and viability assay showed destruction of the cell layer. No cytotoxic effect of the compounds on the Vero cells was observed in the range of the concentrations assayed for all compounds. No cytotoxicity effect was detected for the hydrolysate fractions from the three seaweeds (Table 3).
For hydrolysate fractions extracted from Ulva sp. and S. muticum, no anti-HSV-1 activity was observed for a MOI of 0.01 ID50/cells. S. chordalis extracts had an effective antiviral activity with EC50 between 23.0 and 101.1 μg mL−1 at a MOI of 0.001 ID50/cells; 100 % and 98 % cellular protection were obtained for 500 μg mL−1 of hydrolysate extracts carbohydrase C3 and blank, respectively. Other extracts from S. chordalis were less effective in inhibiting viral production.
Discussion
Composition of seaweeds
The percentage concentrations of protein, neutral sugars, uronic acids and sulphate contents in the dried seaweed samples were in agreement with previous French studies. French species of Ulva lactuca (sea lettuce) show a lower protein level (10–25 % of dry weight). Analysis of Centre d’Etude et de Valorisation des algues (CEVA http://www.ceva.fr/) showed that French “Ulva lactuca” specimens had the following content of proteins (10–24 % of dry weight), carbohydrates (38–60 % of dry weight in which 45 % of polysaccharides can be detected) and minerals (14–29 %). Ulva pertusa and Ulva intestinalis, from Southern Thailand, contained high levels of protein (14.6–19.5 % dry weight (dw)). Biomass of Ulva has very low added value and ways to use it besides compost and methane production could be by taking advantage of specific properties of their cell wall polysaccharides. The latter are essentially represented by ulvan, which is a complex sulphated polysaccharide drawing increasing interest as a potential source of new functional biopolymer (Ray and Lahaye 1995; Lahaye and Robic 2007). Ulvan represents about 8–29 % of the algae dry weight (Robic et al. 2008). Significant seasonal fluctuations of protein, neutral sugars or uronic acids content in seaweeds have been previously reported in relation with species and geographic areas. The chemical composition and some functional properties of the dried “Ulva lactuca”, collected from Tunisia, were determined by Yaich et al. (2011). They showed that “U. lactuca” alga powder was characterized by a high content of fibers (54.0 %—soluble fibers 20.5 %), soluble sugars (0.64 %), uronic acid (9.9 %), minerals (19.6 %), proteins (8.5 %) and lipids (7.9 % w/w on dry basis). In comparison, samples of cultivated Ulva clathrata had a crude protein content ranging from 20 to 26 % and the main monosaccharides were rhamnose (36–40 %), uronic acids (27–29 %), xylose (10–13 %) and glucose (10–16 %) (Peña-Rodríguez et al. 2011). Murakami et al. (2011) aimed to elucidate the relationship between the chemical composition and the growth of Sargassum horneri (Turner) C. Agardh harvested from the Chikuzen Sea (Japan), as well as the changes in the chemical composition during pre-heating of harvested S. horneri. The mean chemical composition of S. horneri was 87.7 % moisture, 1.0 % protein, 0.1 % lipid, 3.4 % ash and 5.7 % total dietary fiber. With increased demands for renewable biofuels, the development of processes for the production of third generation biofuel from invasive algal biomass information about composition of biomass before and after hydrolysis will be useful (Wang et al. 2011).
For example, Borines et al. (2013) focused on the pretreatment, enzyme saccharification and fermentation of Sargassum spp. for bioethanol production. Proximate analysis of dried Sargassum spp. gave 10.2 % protein, 9.8 % crude fiber, 26.2 % ash and 41.8 % total carbohydrates. After hydrolysis, the amount of carbohydrate in the sample was 47.06 %, which is within the reported range of carbohydrate content of Sargassum species. The ash content of the sample on dry basis, 29.48 %, is lower compared to Sargassum fulvellum (46 %) and Sargassum polycystum (47.08 %), but higher than the one determined in Sargassum vulgare (19.43 %) (Borines et al. 2013). Much less information is available in the literature regarding the chemical composition of S. chordalis. Only the study of Khanzada et al. (2007) gives information on the total protein of Solieria robusta collected in Pakistan with a percentage between 25 and 32 %.
Hydrolysis extraction process
Studies on the extraction of active compounds from natural products have attracted special attention in recent years. For industrial applications, the ideal extraction method should be quantitative, non-destructive and solvent-less, characterized also by higher extraction rate and time-saving with lower energy and cost. The potential use of enzyme treatment as a tool to improve the extraction efficiency of bioactive compounds (peptides, proteins, oligo- and polysaccharides) from seaweeds has been studied for several years and shows promising results (e.g. Samarakoon and Jeon 2012; Wijesinghe and Jeon 2012).
The present study shows that in comparison with the aqueous extraction at 50 °C for the same time (5 h), enzymatic extraction (50 °C) appears as a promising softer technique to recover proteins, neutral sugars, uronic acids and sulphate groups with a higher extraction rate, no solvent requirement and time-saving with lower cost. Access to active compounds will be different according to the preparation of the biomass. Before extraction, the conditioning (fresh, dry, lyophilized, ultrasound sonication, frozen seaweeds) and the preparation (whole or crushed seaweeds) of the biomass are starting steps in the success of enzymatic extraction. Dumay et al. (2013) showed that roughly cut wet Palmaria palmata provides the most interesting results in terms of extract quality and economic cost of phycoerythrin. In our study, the seaweeds were ground to pieces of about 3 mm with a hammer mill to access more easily the soluble substances. Extraction is the second important step in isolating different types of bioactive compounds from plant materials. The degradation of cell wall polysaccharide structures by appropriate enzyme treatment will be the fundamental step in the release of active components.
All the commercial enzymes tested in this study were effective in improving the extraction yield. Yield of water extraction was lower. Protease P1 and P2 gave the highest yield except for Solieria material where the C2 carbohydrase is more effective (Table 2, Fig. 2). In comparison, Wang et al. (2010) after 24 h of enzymatic hydrolysis obtained between 40 and 60 % extraction yield with Neutrase and higher (60 %) with carbohydrase (Viscozyme, Ultraflo and Cellulast) on the rhodophyte P. palmata. There are several factors that directly influence the effect of enzymes, especially the choice of the time or temperature of hydrolysis. In the studies of Heo et al. (2005), Ahn et al. (2004), Athukorala et al. (2006, 2007) and Wijesinghe and Jeon (2012), the enzymatic hydrolytic reactions were performed generally for 12 h to achieve an optimum degree of the hydrolysis. Wijesinghe and Jeon (2012) show optimum conditions for some commonly used enzymes. Depending on the enzymes, they are generally used between 40 and 60 °C and pH 3.8 and 8 for brown or red seaweeds. Wang et al. (2010) proposed enzymatic hydrolysis performed under optimal conditions of the particular carbohydrase and protease for a 24-h data. Data from this work suggest the potential of protease treatment to improve value-added utilization of P. palmata extracts as antioxidants in functional foods and nutraceuticals. In comparison to our study, to perform the enzyme activity at maximum level, temperature was adjusted to their optimal conditions during weather time. pH is free because very slight variations of pH were observed even without control. In the logic of an appropriate industrial application, it is important to avoid adding salts.
Numerous macroalgal species, in particular the red seaweeds, have been shown to contain significant levels of protein and, in some cases, contain higher quantities than some conventional protein-rich foods such as soybean, cereals, eggs and fish (Fleurence et al. 2012) as well as the bioactive potentials of specific lectins, enzymes and protein derivatives such as peptides. Seaweeds traditionally consumed in Asian countries as sea vegetables seem to be a suitable source of proteins for human nutrition. The role of proteins as physiologically active components in the diet is being increasingly acknowledged (Harnedy and FitzGerald 2013; Stengel et al. 2011; Fleurence et al. 2012). Harnedy and FitzGerald (2013) used polysaccharidases as prior treatment of P. palmata cells for protein extraction 30 min at 40 °C.
The primary structure of natural proteins consists of particular amino acid sequences that have the ability to exert physiological benefits in human beings. Macroalgae contain relatively high protein levels (10–47 % dw; Fleurence 1999); however, protein content is dependent on season and on the collection site and is linked to variables including nutrient supply. Levels in red macroalgae commonly reach up to 47 % dw, with green and brown macroalgae typically containing lower levels of 9–26 % dw and 3–15 % dw, respectively (Harnedy and FitzGerald 2013; Stengel et al. 2011).
Depending on the enzymes used, hydrolysates extracted from Ulva sp. biomass showed the following composition: 9.92 ± 0.03 % dw protein, 21.09 ± 3.98 % dw neutral sugars, 18.57 ± 1.15 % dw uronic acids and 18.04 ± 0.5 % dw sulphate groups. In comparison, Fleurence (1999) and Castro et al. (2006) showed that enzymatic transformation of Ulvales biomass could increase both the algal protein availability and digestibility, and ulvan degradation to oligosaccharides could be beneficial for stimulating animal defences against diseases. Ulva rigida ulvan as polymer can stimulate macrophage activities against pathogens and contribute to disease resistance in fish. Ulvan also has the capacity to intercalate into clay with applications in animal feed detoxification (Demais 2006a, b). This characteristic opens the way for the preparation of novel interesting nanocomposites for applications in many different areas.
The algal powder from Solieria sp. had the following composition: 15.2 ± 0.3 % dw protein, 36.8 ± 0.5 % dw neutral sugars, 22.1 ± 0.9 % dw uronic acids and 5.0 ± 0.3 % dw sulphate groups. Sargassum sp. material had the following composition: 53.8 ± 2.1 % dw protein, 36.07 ± 4.37 % dw neutral sugars, 26.41 ± 1.87 % dw uronic acids and 16.12 ± 0.04 % dw sulphate groups. Enzymatic hydrolysis degrades the cell wall and gives access to molecules which are not initially available. The use of enzymatic liquefaction of red seaweeds already described in the literature could be an alternative procedure to improve protein solubilization, especially PBP, in mild conditions (Lahaye and Vigouroux 1992; Fleurence et al. 1995a, b). Studies are being performed to understand the physical conditions needed to improve the stability of PBP especially those extracted from P. palmata. Amano and Noda (1990) and Wijesinghe and Jeon (2012) reported the use of algal cell wall degradation enzymes to facilitate the extraction of proteins from the red alga Porphyra (Pyropia) yezoensis. Further, they demonstrated the use of an enzymatic mixture, including digestive enzymes to improve protein accessibility. In addition, the technique was tested on several algae including U. pertusa, Laminaria (Saccharina) japonica and Kallymenia perforata, and significant differences were obtained in protein composition for all the treated seaweeds (Amano and Noda 1992). Fleurence et al. (1995a, b) reported that the simultaneous application of carrageenase and cellulase activities on the red alga Chondrus crispus led to a tenfold increase in extraction efficiency of the proteins. Further, they demonstrated that the combined use of agarase and cellulase on another red alga Gracilaria verrucosa led to a threefold increase in protein yield. Cian et al. (2012) showed that several hydrolysates obtained using trypsin, alcalase and a combination of both sequentially added from a first cold water protein extract derived from Porphyra columbina were enriched in peptides.
Seaweeds appear to be good sources of active polysaccharides having great chemical, physico-chemical and rheological diversities (Lahaye 1991). Unfortunately, seaweeds contain high viscosity parietal polysaccharides which are an anti-nutritional factor limiting the digestibility of protein fractions, and extraction of protein from most seaweeds is difficult due to the presence of large amounts of polysaccharides and phenolic compounds. The main plant proteins used in animal nutrition are soybean proteins which have different problems such as digestibility, non-adapted enzymatic system for the degradation of these proteins or environmental and ecological problem like the requirement for large quantities of fresh water and the question of genetically modified soybeans (Fleurence et al. 2012). The use of seaweeds in the diet of terrestrial or marine farm animals has already been described (Hansen et al. 2003; Fleurence et al. 2012). Numerous studies have reported the beneficial effects of the use of seaweed meal in the diet of fish or mollusks such as abalone. The positive effect of Ulva meal or wet feed including alginates on the immune status of fish such as sea bream or Atlantic salmon has been well known for 20 years (Fleurence et al. 2012). The insoluble fibers are found in small proportions in the form of cellulosic fractions. The soluble fibers or phycocolloids are better represented as follows: in the red seaweeds, 51–56 % by agars, carrageenans and xylans; in the green seaweeds, 51–56 % by ulvans, rhamnans and arabinogalactans; and in the brown seaweeds, 67–87 % by laminarans (β-glucans), alginates and fucans. Generally, these polysaccharides have been extracted using water or polar organic solvents. However, since the cell wall consists of complex polymers, it is not easy to extract active polysaccharides using a solvent extraction process. These polysaccharides are little or non-degraded by digestive enzymes in the human intestine. Nevertheless, the polysaccharides are degraded by some types of carbohydrases derived from microorganisms. Those enzymes can convert water-insoluble seaweeds into water-soluble materials. The production of different bioactive polysaccharides and oligosaccharides with enzymatic digestion is required in order to increase the extraction efficiency of more functional ingredients from seaweeds (Wijesinghe and Jeon 2012). Enzymatic hydrolysis can be employed as an alternative method to improve the extraction efficiency of bioactive polysaccharides from seaweeds. Polysaccharides from red and brown algae, particularly the laminarin, as well as the carrageenan and the alginate, have been extensively studied due to their elicitor properties on plants natural defences, and only a few studies were carried out on green algae (Lahaye and Robic 2007).
Phenolic compounds are known to be the dominant secondary metabolites in temperate brown seaweeds and may amount up to 30 % dw (Plouguerné et al. 2006). Some phenolic compounds, such as phlorotannins in brown seaweeds, also exhibit primary functions, e.g. in growth and the development of the cell wall in Fucales. Commonly characterized as stress compounds, phenolics are involved in the chemical protective mechanisms against biotic factors (Stengel et al. 2011). Enzymatic extraction of seaweeds for the purpose of obtaining natural antioxidant substances would provide several potential advantages: water solubility, simple and large-scale production process of antioxidant extracts from seaweeds. Although vitamin C is water-soluble (but heat-sensitive and easily denatured), natural antioxidants like carotenoids, phenolic compounds and vitamin E presented some disadvantages because they are water-insoluble. Heo et al. (2005) have shown potential antioxidative activities of enzymatic extracts from seven species of brown seaweeds Ecklonia cava, Ishige okamurae, Sargassum fullvelum, S. horneri, Sargassum coreanum, Sargassum thunbergii and Scytosiphon lomentaria. Ultraflo carbohydrase and alcalase protease extracts of S. horneri were dose-dependent and thermally stable. They exhibited strong hydrogen peroxide scavenging activities. The effect of various protease and carbohydrase treatments on the extraction of polyphenols and other antioxidant ingredients from the red alga P. palmata was investigated by Wang et al. (2010). Proteases were more effective than carbohydrases and Umamizyme peptidase extract exhibited the greatest scavenging activity against DPPH and peroxyl radicals. The use of different in vitro antioxidant tests verified the complexity of the antioxidant effects of the seaweed hydrolysates. Crude polyphenols contributed greatly to the peroxyl radical scavenging properties of Umamizyme extract, whereas crude polysaccharides appeared to be more potent metal chelators in experiments. Treatment of P. palmata by Umamizyme improves versatile application of extracts as natural antioxidants and functional food ingredients (Wang et al. 2010). In our study, the relatively lower total polyphenol of hot water, protease and carbohydrase extracts could be partly due to the formation of protein polyphenol complexes during the extraction. When the algal cell wall is disrupted, the intracellular constituents including proteins are released from the cells, which are prone to complex with polyphenols, leading to aggregation and ultimate precipitation (Siriwardhana et al. 2008). Many studies have investigated the mechanisms of polyphenol–protein interactions. Potentially, polyphenols may interact with proteins via hydrogen bonding, p-bonding, hydrophobic interactions, ionic and covalent linkage. Although much less information is available in the literature regarding the complexation of algal polyphenols with proteins, strong interactions between phlorotannins (the largest group of polyphenols present in brown algae) and proteins were reported by Stern et al. (1996). Another explanation for the decreased total polyphenol in carbohydrase extracts could be due to the release of oligosaccharides and simple sugars during the degradation of cell wall polysaccharides, resulting in lower overall levels of polyphenols in the extracts (Siriwardhana et al. 2008).
Evaluation of antiherpetic activities
Screening assays of the antiviral activity of many extracts have led to the identification of a number of polysaccharides (Bergé et al. 1999; Harden et al. 2009; Bouhlal et al. 2010, 2011), the diterpenes (El Gamal 2010) or glycolipides (Vo et al. 2011) having potent inhibitory effects against Herpes simplex virus (HSV) type 1. However, no screening assays of antiviral activity have been carried out for enzymic extracts. Only fractions extracted from S. chordalis present antiviral activities. Fractions obtained by hot water extraction and carbohydrase (C3) hydrolysis with S. chordalis biomass have an effective antiviral activity with EC50 23.0 and 29.3 μg mL−1 for a MOI of 0.001 ID50/cells, respectively, without any cytotoxicity effect. The activity corresponds to fractions rich in sulfates groups (4.7 ± 0.2 % and 5.0 ± 0.3 %, respectively). The importance of red macroalgae as a source of novel anti-HSV agents has been recognized and reported by many researchers (Vo et al. 2011; Bouhlal et al. 2010, 2011). It was revealed that the aqueous extracts rich in sulfate content (Bourgougnon et al. 1993, 2003; Damonte et al. 2004) were capable of inhibiting the replication of HSV-1 at an EC50 range of 2.5–80 μg mL−1 without cytotoxic effect. It has been known that marine red macroalgae contain significant quantities of sulphated polysaccharides such as xylomannan, galactans and carrageenans that exhibited potent antiherpetic activity at the EC50 range of 0.3–10.0 μg mL−1. We can suppose that the hydrolysates are rich in sulphated oligosaccharides or polysaccharides. Bondu et al. (2010) showed that carrageenan extracted from S. chordalis proved to be devoid of direct cytotoxicity on Daudi (human Burkitt's lymphoma), Jurkat (human leukaemic T-cell lymphoblast) and K562 (human chronic myelogenous leukaemia) cell lines and showed great immunostimulating properties (enhancement of neutrophil phagocytosis, cytotoxicity by natural killer cells, antibody-dependent cell cytotoxicity and stimulation of lymphocyte proliferation). However, no antiviral activity was ever detected in an extract of S. chordalis.
In conclusion, the present study shows that in comparison with the aqueous extraction realized in 50 °C for the same time (5 h), enzymatic extraction (50 °C) appears as a promising softer technique to recover some compounds and is solvent-less, has higher extraction rate and is time-saving with lower cost. After 5 h of enzymatic hydrolysis, the behaviour and the selectivity of enzymes on the biomass are different with the type of raw material and the choice of enzyme. All the commercial enzymes tested in this study were effective in improving the extraction yield and the quality of bioactive compounds, but to a different extent compared with water extraction. But enzymatic hydrolysis seems to be less effective for polyphenol extraction and analysis in our choice of the conditions of hydrolysis. Cheap and food grade enzymes could be explored in the future to extract new commercial compounds from algal biomass. Enzymatic extraction tool of bioactive components appears as a useful approach for recovery of industrially important metabolites from invasive seaweeds. Some fractions obtained from S. chordalis present good antiherpetic activities. Further research is underway to determine the structure and nature of the active fractions to establish structure–activity relationships. At the same time, research on potential antioxidant activities to find new useful functional food ingredients and antibacterial activities to find new antifouling compounds is in progress to value the obtained extracts.
References
Ahn CB, Jeon YJ, Kang DS, Shin TS, Jung BM (2004) Free radical scavenging activity of enzymatic extracts from a brown seaweed Scytosiphon lomentaria by electron spin resonance spectrometry. Food Res Int 37:253–258
Amano H, Noda H (1990) Proteins of protoplasts from red alga Porphyra yezoensis. Nippon Suisan Gakkaishi 56:1859–1864
Amano H, Noda H (1992) Proteins of protoplasts from several seaweeds. Nippon Suisan Gakkaishi 58:291–299
Arzel P (1987) Les Goémoniers. Le Chasse-Marée, Editions de l’estran. Douarnez, France, 305 pp
Arzel P (2000) Sur la route des Algues, les goémoniers. Patrimoine maritime de Bretagne. Ed. Uhel Izel, 65 pp
Arzel P, Barbaroux O (2003) Les Algues, produits, saveurs et santé de la mer. Ed. Libris, 104 pp
Athukorala Y, Jung WK, Vasanthan T, Jeon YJ (2006) An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr Polym 66:184–191
Athukorala Y, Lee KW, Kim SK, Jeon YJ (2007) Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour Technol 98:1711–1716
Bergé JP, Bourgougnon N, Alban S, Pojer F, Chermann JC, Billaudel S, Robert JM, Durand P, Franz G (1999) Antiviral and anticoagulant activities of a water soluble compound extracted from the marine diatom Haslea ostrearia. Planta Med 65:604–609
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Bondu S, Deslandes E, Fabre MS, Berthou C, Guangli Y (2010) Carrageenan from Solieria chordalis (Gigartinales): structural analysis and immunological activities of the low molecular weight fractions. Carbohydr Polymers 81:448–460
Borines MG, de Leon RL, Cuello JL (2013) Bioethanol production from the macroalgae Sargassum spp. Bioresour Technol 138:22–29
Bouhlal R, Riadi H, Bourgougnon N (2010) Antiviral activities of Morocco seaweeds extracts. Afr J Biotechnol 9:7968–7975
Bouhlal R, Haslin C, Chermann JC, Colliec-Jouault S, Sinquin C, Simon G, Cerantola S, Riadi H, Bourgougnon N (2011) Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar Drugs 7:1187–1209
Bourgougnon N (2003) Anti-HIV compounds from red seaweeds. In: Fingerman M, Nagabhushanam R (eds) Biomaterials and bioprocessing, vol 9, Recent advances in marine biotechnology. Science, Enfield, pp 16–206
Bourgougnon N, Stiger-Pouvreau V (2011) Chemodiversity and bioactivity within red and brown marine macroalgae along French coasts, Metropole and overseas departments and territories. In: Kim S-K (ed) Handbook of marine macroalgae: Biotechnology and applied phycology. Wiley, Chichester, pp 58–105
Bourgougnon N, Lahaye M, Chermann JC, Kornprobst JM (1993) Composition and antiviral activities of sulfated polysaccharide from Schizymenia dubyi (Rhodophyta, Gigartinales). Bioorg Med Chem Lett 3:1141–1146
Cardozo KHM, Guaratini T, Barros MP (2007) Metabolites from algae with economical impact Comp. Biochem Physiol C 146:60–78
Castro R, Piazzon MC, Zarra I, Leiro J, Noya M, Lamas J (2006) Stimulation of turbot phagocytes by Ulva rigida C. Agardh polysaccharides. Aquaculture 254:9–20
Cian RE, Martínez-Augustin O, Drago SR (2012) Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res Internat 49:364–372
Crooke WM, Simpson WE (1971) Determination of ammonium in Kjeldahl digests of crops by an automated procedure. J Agric Food Chem 27:1256–1262
Damonte EB, Matulewicz MC, Cerezo AS (2004) Sulfated seaweed polysaccharides as antiviral agents. Curr Med Chem 11:2399–2419
Demais H, Brendle J, Jaber M, Laza Anca L (2006a) Exfoliating an intercalated clay, useful in e.g. animal/human feeds, plastification, surface coatings and in nanocomposite, comprises preparing intercalated clay from clay and intercalating compound and lyophilizing in presence of water. French Patent FR2882997
Demais H, Brendle J, Le Deit H, Laza Anca L, Lurton L, Brault D (2006b) Interspersed clay. PCT Patent Application WO2006030075
Dizerbo AH, Herpé E (2007) Liste et répartition des algues marines des côtes françaises de la Manche et de l'Atlantique Iles Normandes incluses Éditions Anaximandre Landernau, 315 pp
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dumay J, Clément N, Morançais M, Fleurence J (2013) Optimization of hydrolysis conditions of Palmaria palmata to enhance R-phycoerythrin extraction. Bioresour Technol 131:21–27
El Gamal AA (2010) Biological importance of marine algae. Saudi Pharm J 18:1–25
Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10:25–28
Fleurence J, Massiani L, Guyader O, Mabeau S (1995a) Use of enzymatic cell wall degradation for improvement of protein extraction from Chondrus crispus, Gracilaria verrucosa and Palmaria palmata. J Appl Phycol 7:393–395
Fleurence J, Le Cœur C, Mabeau S, Maurice M, Landrein A (1995b) Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J Appl Phycol 7:577–582
Fleurence J, Morançais M, Dumay J, Decottignies P, Turpin V, Munier M, Garcia-Bueno N, Jaouen P (2012) What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci Technol 27:57–61
Hansen HR, Hector BL, Feldmann J (2003) A qualitative and quantitative evaluation of the seaweed diet of North Ronaldsay sheep. Anim Feed Sci Technol 105:21–28
Harden EA, Hartline C, Falshaw R, Carnachan SM, Kern ER, Prichard MN (2009) Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir Res 83:282–289
Harnedy PA, FitzGerald RJ (2013) Extraction of protein from the macroalga Palmaria palmata. Food Sci Technol 51:375–382
Heo SJ, Park EJ, Lee KW, Jeon YJ (2005) Anti oxidant activities of enzymatic extracts from brown seaweeds. Bioresour Technol 96:1613–1623
Ioannou E, Roussis V (2009) Natural products from seaweeds. Springer, Berlin, pp 51–81
Jaques LB, Ballieux RE, Dietrich CP, Kavanagh LW (1968) A microelectrophoresis method for heparin. Can J Physiol Pharmacol 46:351–360
Khanzada AK, Kazi WSTG, Kabir S, Soofia S (2007) Antifungal activity, elemental analysis and determination of total protein of seaweed, Solieria robusta (Greville) Kylin from the coast of Karachi. Pak J Bot 39:931–937
Lahaye M (1991) Marine algae as sources of fibres: determination of soluble and insoluble dietary fibre contents in some “sea vegetables”. J Sci Food Agric 54:587–594
Lahaye M, Robic A (2007) Structure and functional properties of ulvans, a polysaccharide from green seaweeds. Biomacromolecules 8:1765–1774
Lahaye M, Vigouroux J (1992) Liquefaction of dulse (Palmaria palmata (L.) Kuntze) by a commercial enzyme preparation and purified endo-β-1,4-d-xylanase. J Appl Phycol 4:329–337
Langlois M, Allard JP, Nugier F, Aymard M (1986) A rapid and automated colorimetric assay for evaluating in the sensitivity of Herpes simplex strains to antiviral drugs. J Biol Stand 14:201–211
Mayer AMS, Rodríguez AD, Berlinck RGS, Hamann MT (2007) Marine pharmacology in 2003–4: marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp Biochem Physiol C 145:553–581
McLaren C, Ellis MN, Hunter GA (1983) A colorimetric assay or the measurement of the sensitivity of Herpes simplex viruses to antiviral agents. Antivir Res 3:223–234
Mohamed S, Hashim SN, Rahman HA (2012) Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci Technol 23:83–96
Murakami K, Yamaguchi Y, Noda K, Fujii T, Shinohara N, Ushirokawa T, Sugawa-Katayama Y, Katayama M (2011) Seasonal variation in the chemical composition of a marine brown alga, Sargassum horneri (Turner) C. Agardh. J Food Compos Anal 24:231–236
Peña-Rodríguez A, Mawhinney TP, Ricque-Marie D, Cruz-Suárez LE (2011) Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chem 129:491–498
Plouguerné E, Le Lann K, Connan S, Jechoux G, Deslandes E, Stiger-Pouvreau V (2006) Spatial and seasonal variations in density, maturity, length and phenolic content of the invasive brown macroalga Sargassum muticum along the coast of Western Brittany (France). Aquat Bot 85:337–344
Ray B, Lahaye M (1995) Cell-wall polysaccharide from the marine green alga Ulva “rigida” (Ulvales, Chlorophyta)—extraction and chemical composition. Carbohydr Res 274:251–261
Robic A, Sassi JF, Lahaye M (2008) Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr Polym 74:344–352
Samarakoon K, Jeon YJ (2012) Bio-functionalities of proteins derived from marine algae—a review. Food Res Int 48:948–960
Sassi AB, Harzallah-Skhiri F, Bourgougnon N, Aouni M (2008) Antiviral activity of some Tunisian medicinal plants against Herpes simplex virus type 1. Nat Prod Res 22:53–65
Siriwardhana N, Kim KN, Lee KW, Kim SH, Ha JH, Song CB (2008) Optimisation of hydrophilic antioxidant extraction from Hizikia fusiformis by integrating treatments of enzymes, heat and pH control. Int J Food Sci Technol 43:587–596
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Stengel DB, Connan S, Popper ZA (2011) Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotech Adv 29:483–501
Stern JL, Hagerman AE, Steinberg PD, Mason PK (1996) Phlorotannin–protein interactions. J Chem Ecol 22:1877–1899
Tavennec M (2009) La gestion d’une problématique algale dans un secteur hautement touristique, la presqu’île de Rhuys. Masters Thesis, Université de Nantes, 107 pp
Turkmen N, Sari F, Velioglu YS (2005) The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem 93:713–718
Vo TS, Ngo DH, Ta QV, Kim SK (2011) Marine organisms as a therapeutic source against herpes simplex virus infection. Eur J Pharm Sci 44:11–20
Wang T, Jónsdóttir R, Kristinsson HG, Hreggvidsson GO, Jónsson JÓ, Thorkelsson G, Ólafsdóttir G (2010) Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. Food Sci Technol 43:1387–1393
Wang X, Liu X, Wang G (2011) Two stage hydrolysis of invasive algal feedstock for ethanol fermentation. J Integr Plant Biol 53:246–253
Wijesinghe WAJP, Jeon Y-J (2012) Enzyme-assistant extraction (EAE) of bioactive components: a useful approach for recovery of industrially important metabolites from seaweeds: a review. Fitoterapia 83:6–12
Yaich H, Garna H, Besbes S, Paquot M, Blecker C, Attia H (2011) Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem 128:895–901
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hardouin, K., Burlot, AS., Umami, A. et al. Biochemical and antiviral activities of enzymatic hydrolysates from different invasive French seaweeds. J Appl Phycol 26, 1029–1042 (2014). https://doi.org/10.1007/s10811-013-0201-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0201-6