Abstract
Value-added abalone Haliotis discus hannai containing bioactive phlorotannins is produced by simply changing the feed to phlorotannin-rich brown seaweed Ecklonia cava 2 weeks prior to harvesting. We assessed the accumulation of phlorotannins by feeding with the seaweed after 4 days of starvation. Reverse-phase high-performance liquid chromatography afforded isolation of the major phlorotannins, which were identified by mass spectrometry and 1H-nuclear magnetic resonance to be 7-phloroeckol and eckol. Throughout the E. cava feeding period of 20 days, 7-phloroeckolol accumulated in the flesh (foot muscle tissue), up to 0.85 ± 0.21 mg g−1 dry weight of tissue after 12 days. Eckol reached 0.31 ± 0.08 mg g−1 dry tissue after 14 days. Feeding Laminaria japonica as a control, we detected no phlorotannins in the abalone muscle tissue. Abalone seaweed consumption and growth rate were similar when fed with E. cava or L. japonica for 20 days. Reduction in phlorotannins to half-maximal accumulation took 1.0 and 2.7 days for 7-phloroeckol and eckol, respectively, after replacement of the feed with L. japonica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abalone is an herbivorous marine gastropod belonging to the genus Haliotis. They are found in most temperate oceans, living on near-shore rocky substrates, reefs, and crevices (Leighton 2000). Juveniles feed on benthic microflora; larger adults feed on larger marine algae, such as Laminaria and Undaria spp. The attractiveness of an alga as a food to abalone may be due to its texture (ease of ingestion) or nutritional value, coupled with the presence of chemical feeding stimulants (Harada et al. 1996) or repellants (Winter and Estes 1992). Abalone has long been consumed and is a highly valued seafood worldwide. It is produced in many countries, and the most important species with regard to aquaculture in Asia is Haliotis discus hannai (FAO 2009). Annual production levels in Korea in 2010 were estimated to be 6,228 and 208 tons by aquaculture and natural harvest, respectively (Korean Fisheries Association 2011). A high degree of management of production processes is necessary to minimize risk in such highly capital-intensive operations, as well as to guarantee a regular supply of product of uniform size and quality. Moreover, production of higher-quality abalone could render it a value-added product. One simple technique for improving quality is feeding a valuable seaweed containing biologically active substances, thus transferring these compounds to the abalone. A value-added abalone containing bioactive phlorotannins is proposed to be produced by simply changing the feed to phlorotannin-rich brown seaweed Ecklonia cava. The E. cava contains marine polyphenols known as phlorotannins (Li et al. 2009), which are found only in brown seaweeds, synthesized via an acetate–malonate pathway by polymerization of phloroglucinol (Ragan and Glombitza, 1986). Phlorotannins exhibit antioxidant (Kang et al. 2004), anti-inflammatory (Kang et al. 2012), antidiabetic (Okada et al. 2004), and antihypertensive (Jung et al. 2006) properties, and inhibit melanogenesis (Heo et al. 2009), metalloproteinases (Kim et al. 2006), and reverse transcriptase (Ahn et al. 2004). Eckol and 7-phloroeckol, the major phlorotannins in E. cava, have shown potential for prevention of Alzheimer's disease through inhibition of BACE1 (Hyun et al. 2010). E. cava components are also used in treatments for hemorrhoids and gastroenteritis, as well as insecticides, as recorded in the Oriental medical textbook Donguibogam published in 1613 (Donguibogam Committee 1999). However, the seaweed has bitter and tannin tastes, and thus people prefer not to eat it directly. It is known that polyphenolic compounds from brown algae also deter grazing by abalone (Winter and Estes 1992). Thus, we assessed the accumulation of phlorotannins by feeding with the bitter-tasting seaweed after 4 days of starvation. After feeding with the seaweed, amounts of 7-phloroeckol and eckol in edible flesh (foot muscle tissue) of abalone, relative growth rates, phlorotannin distribution in abalone tissues, diminishing phlorotannin levels, and enzymatic degradation pattern of phlorotannins were determined.
Materials and methods
Seaweed material
The brown seaweed Ecklonia. cava Kjellman was collected from the coast of Gijang (35°12′49″ N, 129°13′28″ E), Busan, Korea in 2010 and 2011. A voucher specimen was deposited in the author's laboratory (Y.K. Hong). Seaweed thalli were dried completely for 1 week at room temperature and then stored at 4 °C until feeding. Commercial dry thalli of Laminaria japonica, commonly fed in aquaculture farms, were used as a reference feed.
Abalone
The aquacultured abalone Haliotis discus hannai, with an initial mean wet weight of 52.8 ± 6.6 g and mean shell length of 7.2 ± 1.0 cm, were purchased from the fish market. They were kept in an aquarium tank (200 L) with a semi-closed circulating and filtering system, and acclimatized for 7 days with feeding on L. japonica. Flow-through seawater (3 L min−1) was supplied to the tank, and adjusted to 20 ± 1 °C. A photoperiod of 12 h light and 12 h dark was maintained. Fecal matter was removed from the filter daily, and seawater was renewed at the rate of 30 % 1 h before feeding.
Feeding trials
Prior to feeding trials, abalone was starved for 4 days. The 13 abalone were maintained in each plastic container (10 cm long, 8 cm wide, 5 cm high) with slits on all sides, allowing water flow and preventing egress of seaweed thalli. Abalone was fed at a rate of 0.8 g seaweed per one abalone at 17:00 daily for the 20-day feeding trial. At least six abalone were taken out randomly from each container for phlorotannin analysis. To quantify the seaweed consumed, the thalli remaining after daily feeding were collected, dried at room temperature, and weighed. Thalli under identical conditions but in the absence of abalone were compared as a control for reasons other than abalone grazing. Abalone seaweed consumption is expressed as: amount of reference thalli − amount of remaining thalli after grazing. Abalone relative growth rate (%) was calculated as: [(final weight − initial weight) / initial weight] × 100.
Quantification of phlorotannins from E. cava
Phlorotannins were quantified from seaweed powder according to Chowdhury et al. (2011). Briefly, the E. cava powder (10 g) was shaken in a mixture of methanol (40 mL) and chloroform (80 mL) and then partitioned by adding deionized water (30 mL). The upper layer was collected and extracted again with ethyl ether (30 mL). This crude phlorotannin residue was dissolved in methanol (1 mg mL−1) and quantified by reverse-phase high-performance liquid chromatography (RP-HPLC).
Quantification of phlorotannins from abalone
To quantify abalone 7-phloroeckol and eckol levels, tissues detached from the shell were cleaned thoroughly with distilled water to remove contaminants and other mucilage, chopped into small pieces, and ground in paste for 5 min using a hand-held blender. The procedure was conducted on ice to inhibit enzymatic degradation of phlorotannins. Paste of the tissue was stored at −20 °C until use. Crude phlorotannins were extracted from the tissue paste according to the method of Chowdhury et al. (2011) with some modifications. Abalone paste (2.5 g) was immersed in methanol (10 mL) and shaken (180 rpm) for 3 h at room temperature. Chloroform (40 mL) was added, and the mixture was homogenized by shaking for 5 min, and then filtered through defat-cotton. After filtration, the mixture was partitioned into upper and lower layers by adding deionized water (7.5 mL) with shaking (5 min) and centrifugation (5 min, 4,000×g). The upper water layer (nonlipid fraction) was collected and extracted twice with ethyl ether (7.5 mL). The ethyl ether fraction was combined and then dried completely with a nitrogen generator (G-4510E; Domnick Hunter Ltd., Dukesway, England). The crude phlorotannins were dissolved in 300 μL of methanol; 200 μL was injected into the HPLC. To isolate each compound, a C18 column (250 × 10.0 mm; Altech Associates Inc., USA) was used. The HPLC system included a Waters 486 Tunable Absorbance Detector (Waters Associate Inc., USA). HPLC elution was performed at a flow rate of 1.0 mL min−1 using a linear gradient of 30 to 100 % methanol for 40 min. The UV detector was set at 290 nm. All compounds were isolated on the basis of retention time. Phlorotannin content was assessed by measuring the dimensions of HPLC peaks and comparing with a standard curve for each purified compound. Calibration plots of peak height (y, in cm) versus pure compound (x, in mg) in the methanol showed a straight line. The regression equation for 7-phloroeckol was y = 38.8x − 0.82 and the equation for eckol was y = 16.6x − 0.14. The correlation coefficients (r 2) were 0.950 and 0.928, respectively.
Identification of phlorotannins
Isolated compounds were dissolved in methanol and reinjected into the HPLC using the above isolation conditions to ensure complete purification. The purified compounds were analyzed by 1H nuclear magnetic resonance (NMR) spectroscopy using a JNM-ECP 400 NMR spectrometer (JEOL, Japan) with methanol-d (CD3OD). The GC-MS spectra were analyzed using a Shimadzu GC-MS-QP5050A. The structure of each compound was verified by comparison with previously published spectral data (Kim et al. 2009).
Enzymatic degradation of phlorotannins
For the preparation of crude enzyme from abalone muscle, abalone were fed with E. cava or L. japonica, as a control, for 20 days. The muscle (2.5 g) was ground in 3 mL of distilled water and centrifuged (3,000×g, 15 min); the supernatant containing the enzyme was collected. Crude enzyme (400 μL) was mixed with 300 μL of each pure phlorotannin compound, and reacted at 30 °C for 4 h. Then, 200 μL of each reaction was removed periodically and injected into the RP-HPLC to determine the remaining amount of each phlorotannin. Degradation rates (mg mL−1 h−1) were calculated by measuring the slope of phlorotannin level according to reaction time.
Statistical analysis
All data are presented as means ± SE of at least four independent replicates. Statistical comparisons of the means were performed by analysis of variance (ANOVA), followed by Duncan's multiple test using the SPSS software (ver. 10.1). Mean values indicated by different letters are statistically significantly different (P < 0.05).
Results
The brown seaweed E. cava feeding material contains several phlorotannin compounds. MS and 1 H-NMR data of major compounds revealed that some matched with known compounds and confirmed their chemical structures. Separation of the solvent extract from E. cava detected five major peaks—eckol, dieckol, unidentified P34 compound, unidentified P37 compound, and phlorofucofuroeckol-A—by RP-HPLC at retention times of 28, 32, 34, 37, and 39 min, respectively. Water extract from boiling for 5 min showed five major peaks—unidentified P13 compound, 7-phloroeckol, eckol, dieckol, and phlorofucofuroeckol-A—by RP-HPLC at retention times of 13, 20, 28, 32, and 39 min, respectively. The yields of crude phlorotannin extracted using solvent or hot water were approximately 3 and 347 mg g−1 dry tissue, respectively. After solvent extraction, the yields from E. cava of 7-phloroeckol and eckol were 0 and 3 mg g−1 dry seaweed, respectively. After hot-water extraction, the yields from E. cava of 7-phloroeckol and eckol were 29 and 0.6 mg g−1 dry seaweed, respectively.
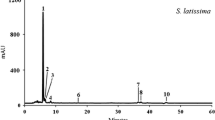
After feeding E. cava to abalone for 20 days, fresh muscle tissue was chopped and ground, and the tissue paste (containing 92 % moisture) was subjected to phlorotannin extraction. By RP-HPLC, using the same conditions, the solvent extract showed three major peaks—unidentified P13 compound, 7-phloroeckol, and eckol—at retention times of 13, 20, and 29 min, respectively (Fig. 1a). The water extract of abalone showed two peaks—P13 and unidentified P18 compound—at retention times of 13 and 18 min, respectively. When comparing the isolation patterns of solvent and water extracts, the former clearly contained 7-phlorotannin and eckol. Both compounds are water-soluble and were likely partitioned into the water layer because the tissue paste itself had a high water content. Thus, phlorotannins were extracted from abalone using a solvent process. After feeding L. japonica to abalone as a control, no 7-phlorotannin or eckol were detected in the muscle tissue, although P13 was present at ∼0.2 mg g−1 dry weight of tissue (Fig. 1b).
Representative HPLC profiles of phlorotannins extracted from the muscle tissue of abalone fed with E. cava (a) and L. japonica (b) for 20 days. Muscle paste was subjected to organic solvent extraction, and separated using a 30 to 100 % methanol gradient on a C18 column by RP-HPLC. Peaks 1, 2, and 3 at 290 nm represent P13, 7-phloroeckol, and eckol, respectively
After feeding E. cava, abalone showed phlorotannin accumulation in the muscle tissue. To quantify accumulation of the major phlorotannin compounds in abalone muscle tissue, RP-HPLC was conducted using a C18 column. Two major phlorotannins (approximate retention times, 20 and 29 min) were detected in the solvent extract of muscle paste, and their levels were calculated based on the HPLC peak dimensions and standard curves. 7-Phloroeckol accumulated during the feeding period to a maximum of 0.85 ± 0.21 mg g−1 dry weight of abalone tissue after 12 days. Control L. japonica-fed abalone showed no accumulation of this compound (Fig. 2a). Eckol accumulated to a maximum of 0.31 ± 0.08 mg g−1 dry weight of tissue after 14 days. L. japonica-fed abalone showed no accumulation of this compound (Fig. 2b). Individual abalone consumed 2.09 ± 0.20 g of E. cava and 2.36 ± 0.22 g of L. japonica during the 20-day feeding trial. Daily consumption of feed was similar; 13 and 15 % of the E. cava and L. japonica provided, respectively. During the 20-day trial, the relative growth rates of abalone fed with E. cava and L. japonica were similar (Fig. 3). After the abalone is adapted to the feeds after 4 days of starvation, the relative growth rates reached ∼0.7–0.8 % within 14 days. Thus, E. cava had no effect on feed preference or growth rate, compared with L. japonica. After feeding E. cava (0.8 g day−1) for 20 days, all abalone were sacrificed, and part of the foot muscle, heart, gonads, and gut were chopped and ground to paste on ice. Table 1 shows the amounts of phlorotannins accumulated in each tissue type. The edible foot muscle contained the highest 7-phloroeckol and eckol (0.83 ± 0.03 and 0.30 ± 0.04 mg g−1 dry tissue) levels.
Accumulation of 7-phloroeckol (a) and eckol (b) in abalone muscle tissue after feeding E. cava. Black circles, E. cava feeding; white circles, L. japonica feeding (control). Abalone was fed with 0.8 g of seaweed daily. Compounds were quantified by RP-HPLC and expressed as amounts per 1 g of dry foot muscle tissue (n ≥ 6)
Relative growth rates of abalone after feeding with E. cava and L. japonica. Black circles, E. cava feeding; white circles, L. japonica feeding (control). Abalone was fed with 0.8 g of seaweed daily. Relative growth rates (%) were calculated as: [(final weight − initial weight) / initial weight] × 100
To determine the decline pattern of phlorotannin levels in muscle tissue, abalone were first fed with E. cava for 20 days to accumulate phlorotannins, and then their food was replaced with L. japonica for the following 7 days. Levels of both phlorotannins decreased quickly after stopping E. cava feeding. Reduction to half-maximal accumulation of 7-phloroeckol and eckol values took 1.0 and 2.7 days with L. japonica feeding (Fig. 4). Abalone lost 7-phloroeckol and eckol from muscle tissue at a mean rate of −0.37 and −0.07 mg g−1 dry tissue per day, respectively. When abalone are starved after 20 days of phlorotannin accumulation, the pattern of decline in the level of each compound was similar to that in L. japonica feeding (data not shown). To confirm the enzymatic degradation of phlorotannins in abalone tissue, aliquots from the muscle of abalone fed for 20 days with E. cava or L. japonica were used as enzyme sources. Both of the tissues, fed with E. cava and L. japonica, had enzymes that decomposed 7-phloroeckol and eckol (Table 2). L. japonica contained no phlorotannins, but tissue of abalone fed with it also demonstrated the degradation of eckol, at −0.051 mg mL−1 h−1. Thus, it seems that the phlorotannin-decomposing enzymes are produced constitutively by the abalone. Abalone fed with E. cava, exposed to phlorotannins, showed a lower degradation rate, –0.035 mg mL−1 h−1, against eckol than those fed with L. japonica. It is unclear whether the phlorotannin may inhibit or suppress some enzyme(s) related to its decomposition.
Diminishing phlorotannin levels in abalone muscle tissue. Abalone was fed with E. cava for 20 days, and then replaced by L. japonica for measurement of remained 7-phloroeckol (Black circles) and eckol (white circles). 0.8 g of seaweeds per each abalone was fed everyday. Each phlorotannin amount is expressed as mean ± SE (n ≥ 6) against dry weight of tissue
Discussion
Abalone are a valuable human food source in many areas of the world where the species is abundant. The foot muscle of abalone is consumed raw or cooked in a variety of dishes, and the shell is used as a decorative item and as a source of mother of pearl. To produce a value-added abalone with flesh containing biologically active substances, we changed the feed to the brown seaweed E. cava for a short period before harvest. The seaweed species E. cava contains high levels of diverse phlorotannins, which have diverse biological activities, including antioxidative properties (Kang et al. 2004). Usually, polyphenolic compounds from brown algae are considered to deter grazing by and growth of abalone (Winter and Estes 1992). Abalone prefer to eat phenolic-poor rather than phenolic-rich seaweeds. Humans consume E. cava as a foodstuff after removal of bitter-tasting substances by blanching (Kim and Lee 2004). Thus, we starved the abalone for 4 days to enhance the grazing rate and then exposed them to the phenolic-rich E. cava as the sole food source. After adaptation, abalone consumed the seaweed readily. Significant and similar weight gain (P < 0.05) occurred after feeding with phlorotannin-rich E. cava and phlorotannin-poor L. japonica (Fig. 3), suggesting that the high level of phlorotannins may not affect weight gain, at least during the short 20-day feeding period used here. Kubanek et al. (2004) reported significant increases in the survival and growth of amphipods upon addition of purified phlorotannins to their feeds. Some herbivores with basic or surfactant-rich digestive systems readily consume phlorotannin-rich seaweeds (Targett and Arnold 1998). Deterrent effects on herbivores vary geographically, even among herbivores with similar digestive systems (Steinberg et al. 1995). Herbivore digestive efficiency or willingness to feed on seaweeds may or may not be related to the phlorotannin concentration in seaweeds (Denton and Chapman 1991; Targett et al. 1995). Some phlorotannins depress herbivore assimilation while others do not (Boettcher and Targett 1993). Accumulation of diet-derived compounds by many herbivores has been reported. Abalone previously fed with Ulva lactuca accumulated more dimethylsulfoniopropionate than did wild-collected abalone or those that received manufactured feeds (Smit et al. 2007). Caribbean sacoglossan Costasiella ocellifera fed with the green seaweed Avrainvillea longicaulis accumulated the metabolite avrainvilleol in tissue (Hay et al. 1990). Sacoglossans Cyerce nigricans and Elysia sp. from the Great Barrier Reef, Australia, fed with the green seaweed Chlorodesmis fastigiata, accumulated the diterpenoid chlorodesmin (Hay et al. 1989). The sea hare Aplysia dactylomela grazing on several types of seaweed accumulated mycosporine-like amino acids in the body tissues and spawn (Carefoot et al. 2000). The sea hare Aplysia parvula, grazing on the red seaweed Delisea pulchra, accumulated halogenated furanone secondary metabolites from the seaweed (de Nys et al. 1996). The accumulated furanones were lost at a mean rate of −0.92 mg g−1 dry weight per day from A. parvula when fed with Ulva sp. (Rogers et al. 2000). Abalone lost phlorotannins at a mean rate of −0.07 to −0.37 mg g−1 dry tissue per day, depending on the chemical nature of the phlorotannin. Abalone may possess enzymes capable of degrading phlorotannins present in muscle. Tissue enzymes in abalone fed with E. cava showed a lower phlorotannin degradation rate than those fed with L. japonica. This suggests that these degradative enzymes are expressed constitutively. When fed with L. japonica, abalone lost phlorotannins faster, ∼1.5-fold in the case of eckol. The slower rate of phlorotannin degradation in E. cava-fed abalone may be due to substrate inhibition of the enzyme reaction or suppression by accumulated phlorotannins of production of detoxification enzymes. Uptake and retention of metabolites also depend on their chemical characteristics. Common dietary polyphenols undergo extensive degradation in the small intestine and liver, facilitating their elimination from the body (van Dorsten et al. 2010). E. cava phlorotannins may be degraded to simpler phenolic compounds. Although such ring-fission metabolites tend to have lower biological activities (Scalbert and Williamson 2000), the understanding of the bioavailability and role of metabolites of dietary polyphenols in animals and humans in vivo is limited. NMR- and GC-MS-based metabolomics methods may provide valuable information on polyphenol degradation and its potential impact on endogenous metabolism. In conclusion, our data indicate that maximum phlorotannin accumulation in abalone flesh occurs by feeding with the brown seaweed E. cava for 2 weeks. Moreover, our findings suggest the possibility of producing value-added abalone containing high level of phlorotannins by simply changing the feed used. Continuous feeding with phlorotannin-rich seaweed is necessary to maintain phlorotannin levels in abalone flesh.
References
Ahn MJ, Yoon KD, Min SY, Lee JS, Kim JH, Kim TG, Kim SH, Kim SH, Kim NG, Hun H, Kim J (2004) Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol Pharm Bull 27:544–547
Boettcher AA, Targett NM (1993) Role of polyphenolic molecular size in reduction of assimilation efficiency in Xiphister mucosus. Ecology 74:891–903
Carefoot TH, Karentz D, Pennings SC, Young CL (2000) Distribution of mycosporine-like amino acids in the sea hare Aplysia dactylomela: effect of diet on amounts and types sequestered over time in tissues and spawn. Comp Biochem Phys C 126:91–104
Chowdhury MTH, Bangoura I, Kang JY, Park NG, Ahn DH, Hong YK (2011) Distribution of phlorotannins in the brown alga Ecklonia cava and comparison of pretreatments for extraction. Fish Aquat Sci 14:198–204
de Nys R, Steinberg PD, Rogers CN, Charlton TS, Duncan MW (1996) Quantitative variation of secondary metabolites in the sea hare Aplysia parvula and its host plant Delisea pulchra. Mar Ecol Prog Ser 130:135–146
Denton A, Chapman ARO (1991) Feeding preferences of gammarid amphipods among four species of Fucus. Mar Biol 109:503–506
Donguibogam Committee (1999) Translated Donguibogam. Bubinmunwha, Seoul
FAO (2009) The state of world fisheries and aquaculture 2008. Food and Agriculture Organization of the United Nations, Rome
Harada K, Miyasaki T, Kawashima S, Shiota H (1996) Studies on the feeding attractants for fishes and shellfishes. XXVI. Probable feeding attractants in allspice Pimenta officinalis for black abalone Haliotis discus. Aquaculture 140:99–108
Hay ME, Pawlk JR, Duffy JE, Fenical W (1989) Seaweed–herbivore–predator interactions: host plant specialization reduces predation on small herbivores. Oecologia 81:418–427
Hay ME, Duffy JE, Fenical W (1990) Host-plant specialization decreases predation on a marine amphipod: an herbivore in plant's clothing. Ecology 71:733–743
Heo SJ, Ko SC, Cha SH, Kang DH, Park HS, Choi YU, Kim D, Jung WK, Jeon YJ (2009) Effects of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol in Vitro 23:1123–1130
Hyun RG, Choi JS, Na DH (2010) Quantitative determination of major phlorotannins in Ecklonia stolonifera. Arch Pharm Res 33:539–544
Jung HA, Hyun SK, Kim HR, Choi JS (2006) Angiostensins-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fish Sci 72:1292–1299
Kang HS, Chung HY, Kim JY, Son BW, Jung HA, Choi JS (2004) Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch Pharm Res 27:194–198
Kang JY, Choi JS, Park NG, Ahn DH, Hong YK (2012) In vivo antipyretic, analgesic, and anti-inflammatory activities of the brown Alga Ecklonia cava extracts in mice. Fish Aquat Sci 15:73–76
Kim JA, Lee JM (2004) The changes of biologically functional compounds and antioxidant activities in Ecklonia cava with blanching times. Korea J Food Cult 19:369–377
Kim MM, Ta QV, Mendis E, Rajapakse N, Jung WK, Byun HG, Jeon YJ, Kim SK (2006) Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci 79:1436–1443
Kim AR, Shin TS, Lee MS, Park JY, Park KE, Yoon NY, Kim JS, Choi JS, Jang BC, Byun DS, Park NK, Kim HR (2009) Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J Agric Food Chem 57:3484–3489
Korea Fisheries Association (2011) Korean Fisheries Yearbook. Samshin, Seoul
Kubanek J, Lester SE, Fenical W, Hay ME (2004) Ambiguous role of phlorotannins as chemical defenses in the brown alga Fucus vesiculosus. Mar Ecol Prog Ser 277:79–93
Leighton DL (2000) The biology and culture of the California abalone. Dorrance Publishing Co, Pittsburgh
Li Y, Qian ZJ, Ryu B, Lee SH, Kim MM, Kim SK (2009) Chemical components and its antioxidant properties in vitro: an edible marine brown alga Ecklonia cava. Bioorg Med Chem 17:1963–1973
Okada Y, Ishimaru A, Suzuki R, Okuyama TA (2004) New phloroglucinol derivative from the brown alga Eisenia bicyclis: potential for the effective treatment of diabetic complications. J Nat Prod 67:103–105
Ragan MA, Glombitza K (1986) Phlorotannins, brown algal polyphenols. Prog Phycol Res 4:177−241
Rogers CN, de Nys R, Charlton TS, Steinberg PD (2000) Dynamics of algal secondary metabolites in two species of sea hare. J Chem Ecol 26:721–744
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073S–2085S
Smit AJ, Robertson-Andersson DV, Peall S, Bolton JJ (2007) Dimethylsulfoniopropionate (DMSP) accumulation in abalone Haliotis midae (Mollusca: Prosobranchia) after consumption of various diets, and consequences for aquaculture. Aquaculture 269:377–389
Steinberg PD, Estes JA, Winter FC (1995) Evolutionary consequences of food chain length in kelp forest communities. Proc Natl Acad Sci U S A 92:8145–8148
Targett NM, Arnold TM (1998) Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J Phycol 34:195–205
Targett NM, Boettcher AA, Targett TE, Vrolijk NH (1995) Tropical marine herbivore assimilation of phenolic-rich plants. Oecologia 103:170–179
van Dorsten FA, Grün CH, van Velzen EJJ, Jacobs DM, Draijer R, van Duynhoven JPM (2010) The metabolic fate of red wine and grape juice polyphenols in humans assessed by metabolomics. Mol Nutr Food Res 54:1–12
Winter FC, Estes JA (1992) Experimental evidence for the effects of polyphenolic compounds from Dictyoneurum californicum Ruprecht (Phaeophyta: Laminariales) on feeding rate and growth in the red abalone Haliotus rufescens Swainson. J Exp Mar Biol Ecol 155:263–277
Acknowledgments
This work was supported by the Ministry of Marine Affairs and Fisheries R&D Project “Aquaculture 10 Species” in Korea. We thank National Institute for International Education Development of Korea for graduate scholarship (IB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bangoura, I., Chowdhury, M.T.H., Kang, JY. et al. Accumulation of phlorotannins in the abalone Haliotis discus hannai after feeding the brown seaweed Ecklonia cava . J Appl Phycol 26, 967–972 (2014). https://doi.org/10.1007/s10811-013-0104-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-0104-6