Abstract
Gracilaria gracilis and Nannochloropsis oceanica, single or blended, were tested in European seabass (Dicentrarchus labrax) diets. A control (CTRL) diet was compared with experimental diets including either 8% G. gracilis (GRA8), 8% N. oceanica (NAN8), or a blend of 4% of each alga (NAN4GRA4). After 106 days of feeding, growth, nutrient utilization, antioxidant defense, immunological status, and end-product quality were evaluated. All fish exhibited similar feed intake (1.4–1.5%), body weight, growth, and feed conversion ratio (1.6). Dietary inclusion N. oceanica did not affect digestible N intake and gain. Fish fed GRA8 had the lowest digestible N and energy intake (P < 0.05), and simultaneously the highest nitrogen retention efficiency and energy retention efficiency, resulting in a N and energy gain similar to all other treatments. All fish had well-preserved intestinal morphology; feeding NAN8 resulted in a significant increase in neutral goblet cells compared with GRA8. Fish fed the algal diets had significantly lower (P < 0.05) hepatosomatic index (1.7–1.8 vs 2.1) and plasma triglyceride levels than CTRL, but whole body composition remained similar among treatments. The liver total antioxidant capacity of fish fed NAN8 was significantly higher than that of fish fed GRA8 but did not differ significantly from the CTRL group. NAN4GRA4 resulted in lower values of total glutathione, glutathione peroxidase, and alternative complement. N. oceanica decreased fillet springiness; however, with NAN4GRA4, the muscle fillet became less resilient. G. gracilis and N. oceanica biomass, either used single (8%) or blended (4% each), can be valuable natural ingredients for partial replacement of fish meal in European seabass diets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the use of both seaweeds and microalgae has attracted increasing interest from the feed, food, and pharmaceutical industries for the development of novel products, as well as sources of high-value-added compounds. Global trends point towards increasing demand for natural sustainable sources of nutrients and biologically active substances able to simultaneously promote fish health and function as a vehicle of valuable compounds for consumers.
Seaweeds can be natural sources not only of nutrients for aquafeeds, but also of bioactive compounds like pigments and water-soluble and liposoluble vitamins, as well as minerals (Valente et al. 2006; Holdt and Kraan 2011; Bellou et al. 2014; Silva et al. 2015; Sarker et al. 2016). Several studies have demonstrated the benefits of including seaweeds in fish diets in terms of growth, nutrient utilization, stress resistance, immune system, and fillet quality (Mohamed et al. 2012; Araújo et al. 2016; Magnoni et al. 2017; Peixoto et al. 2019). Among seaweeds, red algae (Rhodophyta) such as Gracilaria are rich in polysaccharides being used for the production of valuable bioactive compounds (Torres et al. 2019). Besides, their protein content can increase up to 30–50%, DM basis, if cultivated under integrated multitrophic aquaculture systems (IMTA) (Abreu et al. 2011), making them more suitable ingredients for aquafeeds. According to Valente et al. (2006), Gracilaria bursa-pastoris can replace up to 10% fish-protein hydrolysate in seabass diets, without affecting growth, nutrient utilization or body composition, but the inclusion of Gracilaria cornea should be limited to 5% of the diet. In rainbow trout (Oncorhynchus mykiss), 10% Gracilaria vermiculophylla impaired growth and feed efficiency (Araújo et al. 2016), suggesting that responses are species dependent and also vary with dietary inclusion levels. Gracilaria spp. are rich in antioxidants such as carotenoids (Schubert et al. 2006) that may prevent flesh oxidation, increase shelf life, and affect flesh color (Husni et al. 2013; Araújo et al. 2016). In zebrafish (Danio rerio), up to 1% Gracilaria gracilis increased the expression of catalase intestinal mucosa and had a positive effect on fish immune system without compromising growth or feed efficiency (Hoseinifar et al. 2018). The inclusion of 5% IMTA-cultivated Gracilaria sp. also enhanced the innate immune response of rainbow trout (Araújo et al. 2016).
Microalgae have also attracted interest as important nutrient sources for aquafeeds (Kiron et al. 2012; Tulli et al. 2012; Qiao et al. 2014; Tibaldi et al. 2015; Valente et al. 2019). They have high protein content (up to 70%) and balanced amino acid profile and are also being rich lipid sources including long-chain n-3 PUFAS (Becker 2007; Hemaiswarya et al. 2011). Microalgae are also good sources of bioactive compounds, such pigments, vitamins, and minerals (Tibbetts 2018) with potential to improve stress response, immune status, antioxidant system, and nutritional value of fish species (Natrah et al. 2007; Sheikhzadeh et al. 2012; Vizcaíno et al. 2018; Messina et al. 2019). Nannochloropsis spp. are among the most interesting marine microalgae due to their small size and high protein content, suitable amino acid profile, and particularly high lipid content (up to 40% of DM), normally rich in EPA and DHA (Becker 2007; Bellou et al. 2014; Molino et al. 2018; Ashour et al. 2019). Dietary supplementation with up to 10% Nannochloropsis sp. meal had no negative effects on fish growth and muscular amino acid profile in turbot (Scophthalmus maximus) (Qiao et al. 2019) or Atlantic salmon (Salmo salar) (Sørensen et al. 2017). Moreover, Nannochloropsis sp. defatted meal could replace up to 15% fish meal in European seabass (Valente et al. 2019) and up to 10% crude protein from fishmeal and soy concentrate in red drum (Sciaenops ocellatus) diets (Patterson and Gatlin 2013) without affecting growth, feed utilization, protein, and energy retention of the fish. The use of the eustigmatophyte Nannochloropsis sp. has also been associated with diminishing lipid peroxidation and enhanced antioxidant capacity in turbot (Qiao et al. 2019) and Atlantic salmon (Sørensen et al. 2017).
Most studies in fish evaluated single algae species, but the supplementation of diets with a mix of seaweeds (Gracilaria sp., Ulva sp., and Fucus sp.) improved European seabass immune and antioxidant responses, without compromising growth (Peixoto et al. 2016a). Likewise, a substitution of 15% fishmeal by a mix of the microalgae Tisochrysis lutea and Tetraselmis suecica enhanced non-specific immune system response and induced greater villi height and upregulation of gene coding for certain brush border enzymes (Messina et al. 2019). The effects of concomitantly using microalgae and seaweed species in aquafeeds have never been explored before and may potentiate synergetic effects among distinct species, opening new opportunities for algae producers and the feed industry. Thus, the present work aimed to investigate for the first time the utilization of G. gracilis and Nannochloropsis oceanica, single or blended, in diets for European seabass. After 106 days of feeding, growth, nutrient utilization, antioxidant defense, immunological status, and end-product quality were evaluated.
Materials and methods
The present study was performed by accredited scientists in compliance with the guidelines of the European Union (directive 2010/63/EU) and Portuguese law (Decreto-Lei no. 113/2013, de 7 de Agosto) on the protection of animals used for scientific purposes. All animal procedures were subject to an ethical review process carried out by the CIIMAR animal welfare body (ORBEA-CIIMAR) and further approved by our national competent authority.
Algae and experimental diets
Commercial IMTA-cultivated Gracilaria gracilis (ALGAplus, Ílhavo, Portugal) and Nannochloropsis oceanica (Allmicroalgae, Pataias, Portugal) were dried by convection in a pilot-scale tray dryer (Armfield UOP8, England) with an air flow of 0.6 m s−1 maintained at 50 °C until the constant weight of the sample, and by pilot-scale spray dryer (Niro Atomizer 2394, Denmark) with a vanned wheel rotating at high speed and a concurrent drying chamber (0.8 m in diameter and 0.6 m in height), respectively. The proximate composition, amino acid, and mineral content of each alga are presented in Table 1.
Four isonitrogenous (53% dry matter, DM) isolipidic (17% DM) diets were formulated and extruded by SPAROS Lda. (Olhão, Portugal), based on the nutritional requirements of European seabass (NRC 2011). A commercial-based diet was used as control (CTRL) and compared with three experimental diets with the inclusion of either 8% G. gracilis (GRA8), 8% N. oceanica (NAN8), or a blend of 4% of each alga (NAN4GRA4). Algae were included in diets at the expense of fish meal and wheat meal. Diets were manufactured with a pilot-scale twin-screw extruder (CLEXTRAL BC45, France) to a pellet size of 2 mm, and oil was added after the extrusion process by means of vacuum coating (Dinnissen Pegasus vacuum mixer, PG-10VCLAB). All batches of extruded feeds were dried in a convection oven (OP 750-UF, LTE Scientifics, UK) and stored at 4 °C until use. Formulation and proximate composition of the experimental diets are presented in Table 2.
The apparent digestibility coefficients (ADCs) of the experimental diets were determined by the indirect method, after the incorporation of 1% chromium oxide (Cr2O3, Merck KGaA, Germany), as an inert marker, to each experimental diet. Each extruded diet was ground and mixed with the marker before being dry pelleted through a 3.2-mm die at 50 °C using a laboratory pellet press (CPM, C-300 model, USA).
Growth trial
European seabass from a commercial fish farm (SONRIONANSA S.L., Cantabria, Spain) were transported to CIIMAR (Matosinhos, Portugal). In order to adapt to the experimental conditions, fish were kept in quarantine for 2 weeks and fed with a commercial diet (AQUASOJA—49% crude protein, 20% crude fat). After acclimation, fish were fasted for 24 h, anesthetized with 75 mg L−1 of MS222 (Sigma-Aldrich, USA) and individually weighed (g) and measured (total length, cm). Twelve homogeneous groups (average body weight of 29.7 ± 0.02 g, total length of 13.7 ± 0.08 cm, density of 11.3 kg m−3) of nineteen fish were distributed by 50-L fiberglass tanks within a saltwater recirculation system. Fish were adapted to the new conditions (water temperature of 21 °C, salinity of 35‰, flow rate at 4 L min−1 and 12 h light/12 h dark photoperiod regime) for 2 days, and then each diet was randomly assigned to triplicate tanks and fed until apparent satiation, three times a day, by automatic feeders. The amount of feed supplied to each tank was daily adjusted based on the presence or absence of uneaten feed in the tank (Borges et al. 2009). Nitrogenous compounds and pH were monitored during the trial and kept at levels recommended for marine species. The trial lasted 106 days, and fish were bulk weighed monthly to monitor feed consumption and weight gain.
Before the growth trial began, ten fish from the initial fish stock were collected after a 24-h fasting period, sacrificed by anesthetic overdose (150 mg L−1 of MS222), and kept at − 20 °C, until initial whole body composition was analyzed. At the end of the growth trial, and after a 5-h fasting period, four fish per tank were anesthetized with 75 mg L−1 of MS222, individually weighed (g) and measured (total length, cm), and sacrificed with a sharp blow on the head. The intestinal tract of those fish was divided into pyloric caeca (PC), anterior intestine (AI; section below the tract with PC until the increase in diameter indicating the start of the posterior intestine), and posterior intestine (PI; the terminal part of the intestine with larger diameter until the anus), and samples were kept at − 80 °C until the determination of brush border membrane (BBM) enzyme activity. After a 24-h fasting period, all fish were anesthetized with 75 mg L−1 of MS222, before individually weighed (g) and measured (total length, cm). Blood was collected from the caudal vein of four fish per tank with heparinized syringes, centrifuged (5000×g for 10 min at 4 °C), and the resulting plasma was stored at − 80 °C for the analysis of innate immune parameters and determination of postprandial metabolite levels. Fish were then sacrificed by a sharp blow on the head. Fish intestine and liver weighs were registered to determine hepatosomatic and viscerosomatic indexes. The liver from each fish was immediately frozen in liquid nitrogen and then kept at − 80 °C until the determination of oxidative stress parameters. Left dorsal muscle was collected and immediately frozen in liquid nitrogen and then kept at − 80 °C until the determination of its nutritional value (DM; crude protein and crude fat). The right dorsal muscle was also collected for the instrumental texture determination. For intestinal histomorphological evaluation, approximately 0.5 cm of the anterior (after the pyloric caeca) and posterior (before the rectum sphincter) intestine was collected, washed, and fixed in 10% neutral-buffered formalin for 24 h. Five fish per tank, sacrificed by anesthetic overdose (150 mg L−1 of MS222), were collected for the whole body composition analysis and frozen at − 20 °C until analysis.
Digestibility trial
After the growth trial, the remaining fish from each diet (12–18 fish) were individually distributed by tanks similar to those used for the growth trial, but equipped with individual feces sedimentation columns, in a system specially designed for in vivo digestibility studies (Guelph system) according to Cho and Slinger (1979). Fish were subjected to the same rearing conditions used during the growth trial. The experimental diets supplemented with 1% Cr2O3 were fed to the fish until apparent satiation, twice a day (10:00 and 17:00) for 10 days to adapt to each new diet before the feces collection began. During the digestibility trial, about 30 min after feeding, each tank was carefully cleaned to assure that no uneaten feed remained in the tanks or in the sedimentation column. Daily collection of the feces was performed twice a day (9:00 and 16:00), before feeding, for 1 month. Feces were centrifuged (7200 rpm, 5 min, 4 °C) to eliminate excess water, frozen at − 20 °C and freeze-dried prior to the analysis.
Proximate analysis of algae, feeds, whole body, muscle, and feces
Fish collected from each tank for whole body composition were ground and pooled, and moisture was determined (105 °C for 24 h). Afterwards, fish were freeze-dried, ground, and homogenized before being analyzed. Algae and experimental diets were also homogenized before analysis. All chemical analyses followed AOAC (2006) methods and were performed in duplicate. Samples were analyzed for DM (105 °C for 24 h); ash, by combustion in a muffle furnace (500 °C for 5 h); crude protein (N × 6.25), using a Leco nitrogen analyzer (Model FP-528, Leco Corporation, USA); lipid content, by petroleum ether extraction using a Soxtherm Multistat/SX PC (Germany) and after a pre-hydrolysis with hydrochloric acid for algae and diets; gross energy, by an adiabatic bomb calorimeter (Werke C2000, IKA, Germany); and phosphorous content by digestion at 230 °C in a Kjeldatherm block digestion unit followed by digestion at 60 °C in a water bath and absorbance determination at 820 nm (adapted from AFNOR 1992). Chromic oxide content in diets and feces was determined according to Bolin et al. (1952). Algae and feed crude fiber content was analyzed according to the intermediate filtration method (ISO 6865:2000) and neutral detergent fiber (NDF) according to ISO 16472:2006 (Robertson and Van Soest 1981; Van Soest and Robertson 1985); carbohydrates of the algae were calculated by deducting the sum of ash, CP, and total lipids from DM. The mineral content of the algae was determined according to USEPA (1995). Aliquots (0.3 g) of dry algae biomass were introduced in Teflon microwave vessels, and 9 mL of concentrated HNO3 and 1.0 mL aqua regia were added. Samples were processed in a microwave digester (CEM Mars Xpress Matthews, USA) at 175 °C and elevated frequency of 2450 MHz. The temperature was kept at 170–180 °C for 10 min. After cooling, digested solutions were filtered through a PTFE filter of 0.2-μm size, transferred into 20-mL volumetric flasks, and stored at 5 °C for determination by inductively coupled plasma optical emission spectroscopy (ICP-OES). A Varian Vista Pro axial instrument (Varian Inc., USA) equipped with a cross-flow nebulizer and auto-sampler was used. The calibration was performed using an ICP-standard 23-element solution in 5% HNO3 (Merck solution IV) using yttrium (Y) as an internal standard. The calibration curve and two blanks were run during each set of analyses, to check the purity of the chemicals. The method detection limit (MDL) was calculated as 3SD/Ss (where SD is the standard deviation of 10 replicate blanks and S is the slope of the calibration curve) for each element. For the amino acid profile, algae samples were subjected to acid hydrolysis (6 M HCl) in an oven for 18 h at 110 °C. The hydrolysis was performed using an amount of the samples corresponding to 5–10 mg protein per mL HCl. After hydrolysis, the samples were cooled to RT °C, and 100 μL was diluted with 1.5 mL 1 M NaCO3 and filtered through a 0.2-μm syringe filter (Q-max PTFE, Denmark) before derivatization using the EZ:FaastTM Amino Acid Analysis kit from Phenomenex (USA). The samples (50 μL) were then analyzed by LC-(APCI)-MS (Agilent 1100, Agilent Technology) according to the procedure described by Sabeena Farvin et al. (2010).
Intestine histomorphological evaluation
After fixation, intestine samples were preserved in ethanol 70%. Samples were further processed according to standard histological procedures and embedded in paraffin. Two fish per tank were then selected for histologic analyses (6 fish per diet). Cross intestinal sections (3 μm) were obtained in a semi-automated rotary microtome (Leica RM 2245) and stained with specific Alcian blue/PAS (pH 2.5). Micrographs of each individual section were examined under a light microscope (Olympus BX51, Germany) with a camera (Olympus DP50). An imaging software (cell^B software, Olympus cellSens Dimension Desktop) was used to measure the following parameters in two sections of each sample, according to previous studies (Galeano et al. 2015; Batista et al. 2016; Ramos et al. 2017): cross-sectional area (mm2); villus length and width (μm); outer longitudinal and inner circular layers of muscularis externa (μm); and goblet cells (no. GC per fold) divided into neutral and acid cells (magenta and blue cells, respectively). The muscularis externa thickness was measured in eight points of each transverse section analyzed, and the mean of the measurements was calculated. The fold’s length and width were measured in the eight highest folds, with the width being measured at the base of the fold and length measured from the fold tip to the bottom, following the curves of the fold. Goblet cells (mucus-producing cells) were counted on the previous selected highest folds; the average number of total goblet cells per fold was determined as well as the number of each GC type.
Brush Border Membrane (BBM) enzyme activities
The gut-stored sections (pyloric caeca, anterior and posterior intestine) were thawed, and whenever needed, the remaining content was gently squeezed out. Gut tissue was diluted 1:10 in iced saline buffer and crushed in a tissue-lyser disruption system (Tissue Lyser II, Qiagen, Germany) at 30 Hz for 1 min. Samples were centrifuged at 13.500×g for 10 min at 4 °C, and the supernatant was used to measure the BBM enzyme activities (Messina et al. 2019). The hydrolysis of maltose and sucrose, by the BBM enzyme maltase and the complex sucrase-isomaltase (SI), was determined according to Harpaz and Uni (1999). Alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (γ-GT) activity was determined using commercial kits (Paramedical, Pontecagnano Faiano, SA, Italy) as indicated by the manufacturer. One unit (U) of enzyme activity corresponded to the amount of enzyme that transforms or hydrolyzes 1 μmole of substrate mL−1 min−1. The specific enzymatic activity was calculated as U of enzyme activity per g of tissue.
Metabolic parameters
The glucose (Glu, mg dL−1), cholesterol (Chol, mg dL−1), triglycerides (Trig, mg dL−1), total proteins (TP, g dL−1), and albumin (Alb, g dL−1) were determined in plasma by an automated analyzer system for blood biochemistry (Roche Cobas Mira, Biosys, Milan, Italy) and commercially available kits (Biochemical Enterprise, Italy), following the manufacturer’s protocols.
Oxidative stress analysis
Liver samples were homogenized using phosphate buffer (0.1 M, pH 7.4) in a proportion of 1:10 (w:v). Protein content was determined according to Bradford (1976) and used to standardize antioxidant enzyme activities. The following methods were, without exception, measured in triplicate using a microplate reader. Concentration of total antioxidant was determined by using the total antioxidant capacity assay kit (Sigma MAK187) and measuring the formation of Trolox equivalents. Total glutathione (TG) was evaluated at 412 nm by the formation of 5-thio-2-nitrobenzoic acid (TNB), as detailed in Baker et al. (1990). Protein content in the post-mitochondrial supernatant (PMS) was precipitated with trichloroacetic acid (TCA; 12%) for 1 h and centrifuged at 12,000×g for 5 min at 4 °C. TG was determined (in deproteinated PMS) adopting the enzymatic recycling method using GR excess, whereby the sulfhydryl group of GSH reacts with DTNB (5,5′-dithiobis-2-nitrobenzoic acid, Ellman’s reagent) producing a yellow-colored TNB. Formation of TNB was monitored by spectrophotometry at 415 nm, for 7 min, and results were expressed as nmol TNB conjugated formed per min per mg of protein. Glutathione peroxidase (GPx) was evaluated based on NADPH oxidation at 340 nm (Mohandas et al. 1984) through an indirect method based on the oxidation of glutathione (GSH) to oxidized glutathione (GSSG) catalyzed by GPx. The reaction was performed at 25 °C and pH 8.0, using H2O2 and including sodium azide (NaN3) as a catalase inhibitor. The enzyme activity was calculated as nmol NADPH oxidized min−1 mg−1 protein. Glutathione s-transferase (GST) was determined as described by Habig et al. (1974). Total activity (cytosolic and microsomal) was determined by measuring the conjugation of CDNB with reduced glutathione (GSH). The change in absorbance was recorded at 340 nm and 25 °C for 5 min. Enzyme activity was calculated as mmol CDNB conjugate formed per min per mg of protein. Catalase (CAT) activity was measured according to Claiborne (1985), but using hydrogen peroxide (H2O2) 30% as substrate. Changes in absorbance were recorded at 240 nm at 25 °C. CAT activity was calculated in terms of mmol H2O2 consumed per min per mg of protein. Lipid peroxidation (LPO) was determined according to Bird and Draper (1984) by quantifying the presence of thiobarbituric acid reactive substances (TBARS) that induces the formation of malondialdehyde (MDA), quantified colorimetrically following its controlled reaction with TBA. The absorbance of each aliquot was measured at 535 nm and LPO was expressed as nmol of TBARS formed per gram of fresh tissue.
Innate immune parameters analysis
Plasma lysozyme, peroxidase, and alternative complement (ACH50) pathway activities were measured in triplicate on a microplate spectrophotometer (BioTek Synergy HT, USA). Lysozyme activity (EU min−1 mL−1 plasma) was determined using a turbidimetric assay based on the method described by Ellis (1990) and adapted to microtiter as described by Hutchinson and Manning (1996). One lysozyme enzyme unit (EU) was defined as the amount of lysozyme that caused a decrease in 1 OD absorbance per minute. Total peroxidase activity (EU mL−1 plasma) was measured following the procedure described by Quade and Roth (1997) and Costas et al. (2011) and was determined by defining that one unit of peroxidase produces an absorbance change of 1 OD. Alternative complement pathway (ACH50) was based on the lysis of rabbit red blood cells (2.8 × 108 cells mL−1; Probiológica, Belas, Portugal) as target cells in the presence of ethylene glycol tetraacetic acid (EGTA; Sigma) and Mg2+ (MgCl2·6H2O; VWR) as described by Sunyer and Tort (1995). ACH50 units were defined as the concentration of plasma giving 50% lysis of cells.
Instrumental texture and color analysis of fish skin and muscle
Skin and muscle color were measured using a CR-400 chromameter (Konica Minolta) with an aperture of 8 mm, at standard illuminant D65 using the CIE 1976 (L*, brightness; a*, redness; b*, yellowness). Initially, the apparatus was calibrated with a white plate reference standard (Minolta Co, Ltd., Osaka, Japan). Color parameters were measured by applying the colorimeter onto the raw skin and flesh of 12 fish from each diet. Measurements were made above the lateral line and in three points of each fillet, and mean values were considered for each fish. After flashing, L*, a*, and b* reflected light values were recorded. From a* and b* values, the hue angle (h* = tan−1 b*/a*) and the chroma (C* = (a*2 + b*2)1/2) were calculated according to Choubert et al. (1997).
Fillet texture was analyzed immediately post-mortem using a TA.XT plus texture analyzer, fitted with a 5-kg load cell and a 2.0-mm-diameter probe, and controlled by Exponent v6 software (Stable Micro Systems, UK). Texture profile analyses were obtained by double compression (constant speed and penetration depth of 1 mm s−1 and 4.0 mm, respectively) on the maximum thickness part of each raw fillet. Penetration depth was selected according to the maximum distance that did not induce fibers breaking, and the following measurements were determined: hardness (N), chewiness (J), adhesiveness (J), springiness, cohesiveness, and resilience.
Calculations
ADCs of the experimental diets were calculated according to Maynard et al. (1979): DM ADC (%) = 100 × (1 – (dietary Cr2O3 level/feces Cr2O3 level)) and nutrient ADC (%) = 100 × (1 – (dietary Cr2O3 level/feces Cr2O3 level) × (feces nutrient or energy level/dietary nutrient or energy)); condition factor = (final body weight/(final body length)3) × 100; daily growth index = 100 × ((final body weight)1/3 – (initial body weight)1/3)/days; average body weight (ABW) = (final body weight + initial body weight)/2; voluntary feed intake (%) = 100 × dry feed intake/ABW/day; feed conversion ratio = dry feed intake/weight gain; hepatosomatic index = 100 × liver weight/body weight; viscerosomatic index = 100 × weight of viscera/body weight; digestible nitrogen (N) or energy (E) intake = (dry feed consumption × N (%) or E (kJ g−1) in the diet × ADC N or E)/ABW/days); N or E gain = (final carcass N or E content – initial carcass N or E content)/ABW/days; N or E retention efficiency (% digestible N or E) = (N or E gain/digestible N or E intake) ×100.
Statistical analysis
Data were tested for normality and homogeneity of variances by Shapiro-Wilk and Levene’s tests, respectively, and transformed whenever required before being submitted to a one-way ANOVA with the statistical program IBM SPSS STATISTICS, 25.0 package (USA). When this test showed significance, individual means were compared using HSD Tukey’s test. In all cases, the minimum level of significance was set at P < 0.05. The principal component analysis (PCA) was applied to assess the consensus among the variables that differed significantly among dietary treatments using XL-STAT 2019 system software (Addinsoft, USA).
Results
The IMTA-produced G. gracilis and the N. oceanica biomass had high protein contents (> 30%) (Table 1), but the lipid content of G. gracilis was lower (< 1%) than that of N. oceanica (9.8% DM). Although N. oceanica seems to be a better source of essential amino acids (20%), G. gracilis appears an excellent source of minerals, especially K (53.3 g kg−1 DM). The neutral detergent fiber content was similar between the two algae, but G. gracilis had both higher crude fiber and carbohydrate contents.
The apparent digestibility coefficient (ADCs) of DM of fish fed NAN8 did not differ significantly either from the CTRL or from diet NAN4GRA4 (Table 3). Fish fed GRA8 diet displayed the lowest DM ADC value (16%) that differed significantly from all other treatments (P < 0.05). Protein and energy ADC values of NAN8 did not differ from the CTRL and were significantly higher (P < 0.05) than those observed in GRA8. The algae blend (NAN4GRA4) displayed a similar protein ADC value to the CTRL diet. Lipid and phosphorus digestibility was not affected by dietary algae inclusion.
All diets were well accepted by European seabass, resulting in similar voluntary feed intake (1.4–1.5%), final body weight, growth, and feed conversion ratio (1.6) among dietary treatments (Table 3). Moreover, survival rate was high (> 90%) and similar among groups. Fish fed GRA8 had a significantly higher condition factor (1.14) than fed CTRL diet (1.11; P < 0.05). The dietary inclusion of algae resulted in a significant decrease (1.7–1.8; P < 0.05) of the hepatosomatic index (HSI) compared with fish fed the control diet (2.1). Final whole body composition remained unaffected by the dietary treatments. Digestible N intake and N gain of fish fed N. oceanica diets did not differ from the CTRL group, resulting in similar nitrogen retention efficiency (NRE, %DN). Fish fed GRA8 had the lowest digestible N and digestible energy intake values (P < 0.05), but highest NRE and ERE, resulting in a N and energy gain similar to all other treatments.
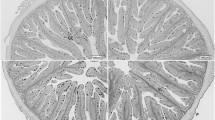
All fish had a very well-preserved intestinal morphology, without major differences among the experimental diets (Fig. 1 and Table 4). The only exception was the number of neutral GC per fold that was significantly higher in fish fed NAN8 than that in fed GRA8 (P < 0.05), both in the anterior (17 vs 8 cells per fold) and posterior (22 vs 1 cell per fold) intestinal sections. In the anterior intestine, the dietary inclusion of the algae blend (NAN4GRA4) resulted in an increased number of neutral GC in relation to both the control and GRA8, but that could not be observed in the posterior intestine.
Histological sections (Alcian blue/PAS staining, pH = 2.5) of the intestine of European seabass showing. a Different parameters measured. CSA, Cross-section area; VL, villus length; muscularis externa (OLL and ICL, outer longitudinal and inner circular layer, respectively; VW, Villus width; goblet cell (AGC and NGC, acid and neutral goblet cells respectively). b Fish fed GRA8 diet. c Fish fed NAN8 diet. Note the lower number of NGC in fish fed GRA8 diet compared with fish fed NAN8 diet. See Table 4 for more details
As shown in Table 5, the activities of the BBM enzymes maltase, SI, and γ-GT were affected by dietary treatments. Diet NAN8 resulted in the lowest activity of the disaccharases, maltase, and sucrose-isomaltase, compared with fish fed NAN8GRA8 diet (P < 0.05) in the pyloric caeca. The highest activity of γ-GT was found in the posterior intestine, with the highest figures registered in fish fed NAN8 diet (12.99 μmol min−1 g−1, P < 0.05). The activity of the alkaline phosphatase was not affected by dietary treatments in the anterior and posterior intestinal tract (P > 0.05) but significantly decreased in the pyloric caeca of fish fed NAN8 diet (P < 0.001). The dietary inclusion of Gracilaria (diets GRA8 and NAN4GRA4) resulted in enhanced IAP activity, with fish fed NAN4GRA4 displaying the highest and significant activity (88.0 μmol min−1 g−1, P < 0.05).
Feeding fish for 106 days with the algae-rich diets resulted in a general increase in circulating glucose level (Table 6), reaching statistical significance in fish fed diet GRA8 (149.6 mg dL−1) relative to the control (120.9 mg dL−1; P < 0.05). Triglyceride levels decreased significantly in all fish fed the algae-based diets. Total cholesterol, protein, and albumin levels in plasma were not significantly affected by the experimental diets.
Considering the oxidative stress parameters (Table 7), all diets presented similar TAC content compared with the CTRL; however, fish from NAN8 groups showed the highest TAC content in the liver (19 μL Trolox equivalents g−1 w/w), being significantly different from GRA8 (16 μL Trolox equivalents g−1,w/w; P < 0.05). The NAN4GRA4 diet leads to a significant decrease (P < 0.05) of the TG (0.9 nanomol min−1 mg−1 protein) and GPx (1.6) activities in the liver, compared with the CTRL groups (2.2 and 5.8 nmol min−1 mg-1 protein, respectively). No differences were found for GST, GR, CAT, and LPO between dietary treatments.
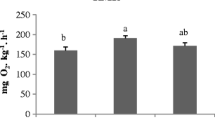
The humoral non-specific immune parameters (Fig. 2) did not show any differences between treatments concerning the peroxidase and lysozyme activities. However, fish fed the blend NAN4GRA4 diet had lower alternative complement (ACH50) pathway activity (132 Units mL−1) than fish fed CTRL diet (239 Units mL−1).
Effects of the experimental diets on humoral non-specific immune parameters of European seabass after 106 days of feeding. Lysozyme and peroxidase expressed in EU mL−1 and alternative complement pathway hemolytic activity (ACH50) in Units mL−1. Values are means ± standard error: n = 12. For each parameter, different superscript letters indicate significant differences between diets (P < 0.05)
No significant differences were observed in seabass skin color among dietary treatments by the end of the growth trial (Table 8). However, the fillet of fish fed the NAN8 diet had a significantly higher hue angle (h*, 130) than those fed the GRA8 diet (h*, 123; P < 0.05). Fillets of fish fed the GRA8 diet had higher redness (a*, − 2.6) compared with fish from all other treatments (a*, − 3.2). Cohesiveness of the fillet was higher in fish fed NAN8 and GRA8 (0.3) than in those fed the CTRL diet (0.2). The dietary inclusion of N. oceanica (NAN8 and NAN4GRA4) significantly decreased the fillet springiness, while the inclusion of the algal blend (NAN4GRA4) decreased fillet resilience in relation to the CTRL. The fillet content of protein and lipids did not change among dietary treatments, but fish fed the algae-rich diets had significantly higher moisture content (> 76%) compared with the control (75%).
The PCA is often applied for data reduction to identify a small number of factors that explain most of the variance observed in a much larger number of manifest variables. This statistical methodology attempts to identify underlying variables or factors that explain the pattern of correlations within a set of observed variables. Figure 3 shows the two first dimensions of the PCA consensus, which explains 82.5% of the variability of the experimental data. The representation shows that although diets are projected into distinct quadrants, algae-rich diets tended to be separated from the CTRL. Fish fed the CTRL and NAN8 diets are separated from diets containing G. gracilis (GRA8 and NAN4GRA4) along F1 with 50.1% of the variance. Fish fed diets with N. oceanica (NAN8 and NA4GRA4) are discerned in relation to the CTRL and GRA8 along F2 with 32.4% of the variance.
Principal component analysis biplot of the mean scores and loadings for all the variables with significant difference (P < 0.05) between European seabass fed the experimental diets (CTRL, GRA8, NAN8, and NA4GRA4). ADC, apparent digestibility coefficient; AI, anterior intestine; ERE, energy retention efficiency; GPx, glutathione peroxidase; ɤGT, ɤ-glutamyltransferase; HSI, hepatosomatic index; IAP, intestinal alkaline phosphatase; K, final condition factor; NGC, neutral goblet cells; NRE, nitrogen retention efficiency; PC, pyloric caeca; PI, posterior intestine; SI, sucrose-isomaltase; TAC, total antioxidant capacity; TG, total glutathione
Discussion
The sustainable growth of aquaculture largely depends on the use of new nutrient sources to partially replace fish meal, without compromising fish growth, health, and nutritional value of end products (Gatlin et al. 2007). The present study clearly shows that both G. gracilis and N. oceanica biomass, used either single or blended, can be valuable natural ingredients to partially replace FM in diets for European seabass without affecting fish growth. Both algae have high protein and mineral content, despite their low lipid level. Seaweeds such as Gracilaria concentrate minerals from seawater, reaching a mineral content 10–20 times higher than terrestrial plants (Moreda-Piñeiro et al. 2012). Among the macro minerals present in the G. gracilis biomass used in our study, K was the most abundant element, as corroborated by other studies (Reka et al. 2017; Radha 2018). The microalga N. oceanica also has been shown to be a good source of minerals, namely Na, K, Mg, Ca, and P; when compared with G. gracilis, this microalga also had a higher content of essential amino acids, confirming previous observations (Archibeque et al. 2009). Nevertheless, besides the nutritional value of each alga, their nutrient bioavailability is of major importance for nutritional studies. In the present study, the dietary inclusion of 8% N. oceanica did not affect nutrient digestibility, or nitrogen and energy retention efficiencies, but the diet with 8% G. gracilis had the lowest protein and energy ADC values. In European seabass, previous studies have reported that the inclusion of G. bursa-pastoris at 5–10% (Valente et al. 2006) resulted in protein ADC values similar to the control, but defatted Nannochloropsis sp. biomass up to 15% (Valente et al. 2019) impaired energy digestibility. The same defatted Nannochloropsis sp. included at 10% in diets for Atlantic salmon significantly reduced protein and energy ADCs (Sørensen et al. 2017). Moreover, a recent study in Atlantic salmon showed that the incorporation of 10% pre-extruded N. oceanica in plant-based commercial-like feeds did not affect protein digestibility but reduced the lipid digestibility (Gong et al. 2020). The significant reduction of energy ADC observed in diets containing G. gracilis (GRA8 and NAN4GRA4) might be explained by the presence of indigestible fibers in the seaweed cell wall, which may interfere with the digestion (Angell et al. 2016). Seaweeds are multi-cellular organisms with cell walls composed of complex polysaccharides. A recent study by Zheng et al. (2020) showed that red seaweeds are rich in sulfated polysaccharides of the carrageenan, agar, and agarose types which resist enzymatic degradation in the stomach and small intestine. The presence of such cell wall polysaccharides may limit the access of the digestive enzymes to algal proteins (e.g., phycobiliproteins) and can partially have contributed to the observed reduction of protein and energy ADC values in fish fed Gracilaria. Although seaweeds may have even higher levels of total dietary fiber than terrestrial plants, it is mostly soluble and, in the case of Gracilaria, comprises agar (Rajapakse and Kim 2011). This soluble fiber forms a viscous mass in the gut with binding properties that may trap digestive enzymes and some other nutrients, slowing down the digestibility and impairing nutrient absorption in the intestine. Although fish fed GRA8 had the lowest digestible N and digestible energy intake values, they also displayed the highest NRE and ERE, resulting in similar nutrient gain, final body size and whole body composition to all other treatments. According to Valente et al. (2006), macroalgae such as Gracilaria sp. have great potential as alternative ingredients in diets for European seabass juveniles at dietary inclusion levels up to 10%. In rainbow trout, the dietary inclusion of 10–12% G. vermiculophylla meal or G. pygmaea impaired growth (Araújo et al. 2016; Sotoudeh and Mardani 2018), but in herbivorous fish such as Nile Tilapia (Oreochromis niloticus) inclusion levels could go up to 20% (Stadtlander et al. 2013; Younis et al. 2018). Few studies have evaluated the inclusion of whole Nannochloropsis sp. in diets for European seabass, but defatted Nannochloropsis sp. biomass was successfully used up to 15% without affecting feed intake, fish growth, or whole body composition (Valente et al. 2019). Likewise, a study with turbot (S. maximus) carried out by Qiao et al. (2019) showed that Nannochloropsis sp. meal could be successfully used as a feed ingredient, contributing up to 10% of the diet without negative effects on fish growth performance. In Atlantic salmon, incorporation of 10% pre-extruded N. oceanica in plant-based commercial-like feeds did not affect the growth, feed utilization, or body proximate composition (Gong et al. 2020). However, reduced feed intake was observed in Atlantic cod Gadus morhua fed with 14 and 28% of a Nannochloropsis sp. and Isochrysis sp. blend (Walker and Berlinsky 2011).
Reduced growth reported in many fish species fed algae-rich diets was often associated with decreased intestinal absorption area due to a reduction in villi length or width (Silva et al. 2015; Araújo et al. 2016; Moutinho et al. 2018). Although intestinal morphology was not affected by the experimental diets used in the present study, the inclusion of N. oceanica (NAN8 and NAN4GRA4) resulted in a significant increase of neutral GC in the anterior intestine compared with fish fed GRA8. Goblet cells reside throughout the length of the small and large intestine and are responsible for the production and maintenance of the protective mucus blanket by synthesizing and secreting high-molecular-weight glycoproteins known as mucins (Specian and Oliver 1991). The increased number of total GC observed from the anterior to posterior intestine also indicates increased mucus production and lubrication for nutrient passway. Moreover, the mucin-containing mucus layer coating the gastrointestinal epithelium is the front line of innate host defense (Kim and Khan 2013). Mucins are likely to be the first molecules that invading pathogens interact with at the cell surface and thus can limit binding to other glycoproteins and neutralize the pathogen (Kim and Khan 2013). Changes in goblet cell functions and in the chemical composition of intestinal mucus are detected in response to a broad range of luminal insults, including alterations of microbiota (Deplancke and Gaskins 2001). It has been suggested that acidic mucins protect against bacterial translocation (Deplancke and Gaskins 2001), whereas neutral mucins have been related to digestion and absorption processes (Grau et al. 1992). The higher number of neutral GC associated with a higher protein and energy ADC values in fish fed N. oceanica (NAN8 and NAN4GRA4) compared with fish fed GRA8 suggests that N. oceanica inclusion is positively influencing the digestion and absorption processes in the anterior intestine, mitigating the negative effect of G. gracilis in the blended diet (NAN4GRA4). However, this warrants further studies for clarification. In Atlantic salmon, increased cell proliferation (PCNA) was observed in the distal intestine of fish fed 10% N. oceanica but without causing any histomorphological changes (Gong et al. 2020).
The present study has investigated the effect of dietary algae inclusion on the activity of a panel of BBM enzymes 5 h after the last meal, that is, during the digestive process, when the presence of the substrates could trigger their activity. The presence of N. oceanica in the diet has significantly affected the activity of both the disaccharidases and the intestinal alkaline phosphatase. In particular, diet NAN8 decreased the activity of maltase and sucrase-isomaltase in the pyloric caeca and anterior intestine and such effect could be due to the lower carbohydrate content of N. oceanica and consequently of diet NAN8, compared with other diets. The alkaline phosphatase (IAP) is expressed in mature enterocytes and is generally considered a marker of intestinal cell differentiation (Hinnebusch et al. 2004). The activity of IAP in the pyloric caeca was enhanced in fish fed seaweed-containing diets while the diet including N. oceanica resulted in a significant decrease, indicating that the dietary substitution of fishmeal with algae can modulate IAP gut activity. The IAP decrease observed in the anterior intestine of fish fed NAN8, although not statistically significant, is consistent with the increase of the neutral mucins in the same tract of the gut, thus describing a likely positive effect of N. oceanica on gut physiology. A different role of IAP has been proposed in zebrafish by Bates et al. (2007) where the alkaline phosphatase of the intestinal brush border has been shown to act as a detoxifier agent when dephosphorylates the lipopolysaccharides produced by the luminal microflora. Anyway, the interaction between algae and microflora is poorly investigated and was not considered in the present study.
The γ-GT enzyme participates in the final steps of the dietary protein digestion in the microvilli surface. Its activity was not significantly affected by dietary treatments, and the highest level of activity was registered in the posterior intestine as already reported by Tibaldi et al. (2006) and Messina et al. (2019). These results are consistent with total protein concentration in plasma, observed 24 h postprandial, suggesting that dietary algae inclusion may have limited effects on protein metabolism of European seabass.
At the end of the feeding trial, the physiological status of fish was assessed through the analysis of certain blood biochemical parameters which influence energy metabolism and are considered important diagnostic parameters (Coz-Rakovac et al. 2005). The algae-rich diets resulted in a general increase in circulating glucose level, reaching statistical significance in fish fed diet GRA8 relative to the control diet. Also, Belal et al. (2012) observed that Nile Tilapia fingerlings fed with diets containing 1% of Spirulina exhibited higher glucose values in fish serum. However, Vizcaíno et al. (2016) found an inverse relationship of glucose concentration when increasing dietary macroalgae (G. cornea and Ulva rigida) levels in S. aurata diets. Algae inclusion had no effect on blood cholesterol and protein levels which is in accordance with a previous study in S. aurata fed 5% of seaweed biomass (Guerreiro et al. 2019). However, triglyceride levels were significantly decreased in fish fed the algae-based diets and could probably account for the reduced HSI observed in those fish. In red sea bream (Pagrus major) fed Spirulina sp., reduced total lipids both in serum and liver were associated with elevated activity of carnitine palmitoyltransferase, which is related to fatty acid β-oxidation, activating lipid mobilization (Nakagawa et al. 2000). In addition, 1% Chlorella extract could improve lipolysis in Plecoglossus altivelis by a synergistic effect with vitamin C (Mustafa et al. 1997). In humans, DHA supplementation from algal oil reduced serum triglycerides and increased HDL and LDL cholesterol (Bernstein et al. 2012). It seems that algae may modulate lipid metabolism although in the present study fish whole body composition and muscle fat remained unchanged. Moreover, lipid peroxidation (LPO) also remained unaffected by the dietary inclusion of algae. Further studies are warranted to better clarify the effect of algae biomass on fish intermediary lipid metabolism.
Marine algae are rich sources of bioactive compounds able to modulate oxidative stress (Lee et al. 2013) which occurs when the production of excessive reactive oxygen species (ROS) is not balanced by an endogenous antioxidant defense system or exogenous dietary antioxidants that maintain these oxidizing compounds at acceptable levels (Biller and Takahashi 2018). Exogenous antioxidants provided by microalgae uptake can prevent oxidative stress by activating a wide range of enzymes, as previously observed in fish fed Chlorella vulgaris (Rahimnejad et al. 2017), Spirulina platensis (Teimouri et al. 2019), and N. oceanica (Sørensen et al. 2017). In the present study, the inclusion of algal biomass in fish diets had limited effects on oxidative stress, confirming previous studies using seaweeds (Guerreiro et al. 2019). All diets induced similar total antioxidant capacity (TAC) in the seabass liver, when compared with the CTRL. However, fish fed NAN8 had a higher TAC than fish fed GRA8, suggesting that N. oceanica might have higher levels of exogenous compounds with antioxidant capacity than G. gracilis. In Atlantic salmon, the dietary inclusion of defatted Nannochloropsis sp. had no effect on TAC (Kiron et al. 2016), but in rainbow trout, the TAC increased concomitantly with increasing levels of S. platensis (Teimouri et al. 2019). This effect was attributed to a possible role of carotenoids with recognized antioxidant potential in S. platensis. In fact, both the chemical composition, mainly the carotenoid type and content, and the inclusion level of each alga will certainly determine the effect on each fish species. Antioxidant enzymes such as GPx indicate the scavenging capacity for ROS and the ability to protect cells from oxidative damage, thus preventing lipid peroxidation. The antioxidant function of GPx is largely dependent on its interaction with TG (Lu 2009), reducing H2O2 and lipid peroxide as GSH is oxidized to GSSG. Contrarily to what was observed by Peixoto et al. (2016a), who reported increased GPx activity associated with the inclusion of Gracilaria sp. extracts, seabass fed NAN4GRA4 had a lower activity of GPx and lower bioavailability of TG in the liver. This implies that these fish may be less prepared to cope with eventual biotic stressors. Data from humoral non-specific immune parameters also reinforces that hypothesis, as fish fed NAN4GRA4 diet had a lower ACH50 activity, which further points to an increased vulnerability to biotic stressors. Moreover, our study showed that lipid peroxidation (LPO) did not vary among dietary treatments, indicating that algae inclusion did not have any major effect in terms of the antioxidant status of European seabass. Likewise, Peixoto et al. (2016a) did not find any influence on LPO in the liver of seabass fed diets supplemented with two different Gracilaria sp. extracts, as well as on the activity levels of the antioxidant bioindicators CAT and GR. Different results were observed in turbot where increasing inclusion levels (2.5–10%) of N. oceanica were associated with diminished rates of LPO (Qiao et al. 2019). Moreover, Magnoni et al. (2017) reported a downregulation of genes of antioxidant enzymes in gilthead sea bream (S. aurata) fed heat-treated Gracilaria sp. and subjected to hypoxia challenge, suggesting a protective role against oxidative stress. Further studies, perhaps involving a pro-oxidant challenge, are necessary in order to fully assess the potential of algae in fish oxidative stress.
The ability of fish to cope with stress events will definitely be a priority for aquaculture in the coming years, and the dietary inclusion of dietary sources rich in biologically active substances seems a promising strategy to improve fish robustness. In this study, some humoral innate immune parameters were evaluated in seabass plasma after being fed the experimental diets. Despite the fact that humoral peroxidase and lysozyme activities were not affected by dietary treatments, fish fed NAN4GRA4 had a lower alternative complement pathway (ACH50) than fish fed the CTRL diet. The complement system, made up of a large number of distinct plasma proteins that react with one another, plays an essential role in alerting the host of the presence of potential pathogens, as well as in their clearing (Boshra et al. 2006). It seems that the algae blend may be contributing towards the depletion of plasma serum hemolytic activity, perhaps due to the inhibition of the protein cascade that composes the alternative pathway of the complement system. This lack of certain complement proteins may lead to an increased susceptibility to infections, as well as the development of autoimmune diseases in fish. A similar trend was observed in seabass fed with 7.5% Gracilaria sp. that resulted in a significant reduction of ACH50 (Peixoto et al. 2016b). Opposite results were reported in rainbow trout, where the dietary inclusion of 5% Gracilaria sp. increased both ACH50 and peroxidase activity (Araújo et al. 2016). A dose-dependent stimulation of ACH50 was also observed with the inclusion of defatted Nannochloropsis sp. up to 10%, in spite of results not differing significantly from the control (Valente et al. 2019).
Besides their nutritional properties, algae are also good sources of pigments that may affect the appearance of fish, namely in terms of skin and muscle color. Such attributes are known to influence market value, flavor perception, and acceptability of fish food products (Spence et al. 2010). Although fish used in the present study were not commercial sized, the instrumental color of skin and muscle textural properties were evaluated to predict any possible effect of the algal biomass that could compromise these parameters at later stages. The dietary inclusion of N. oceanica or G. vermiculophylla did not influence the skin color of seabass. However, Peixoto et al. (2018) reported a significantly lighter (L*) skin in fish fed diets with Gracilaria sp. extracts compared with control groups. This same author did not find any effect of the Gracilaria sp. on the fillet color, but in our study fish fed GRA8 had higher redness (a*) compared with fish from all other treatments and lower hue angle (h*) than fish fed NAN8. Increased a* was also previously reported in raw and cooked fillets of rainbow trout fed G. vermiculophylla, which were perceived by a sensory panel as having increased color intensity (Valente et al. 2015; Araújo et al. 2016). Moreover, in large-sized seabass, increased skin greenish and fillet redness were observed in fish fed Isochrysis sp., but the sensory panel was not able to discriminate among samples (Tibaldi et al. 2015). The extent of such pigmentation patterns on the impact on consumer preferences remains to be clarified.
The texture and structure of fish muscle are important freshness quality attributes that depend on several parameters such as hardness, cohesiveness, springiness, chewiness, resilience, and adhesiveness, as well as the internal cross-linking of connective tissue and the detachment of fibers (Cheng et al. 2014). In our study, muscle cohesiveness and moisture content were higher in fish fed NAN8 and GRA8, compared with CTRL. According to Jonsson et al. (2001) expressible moisture of salmon fillet was positively correlated with textural properties being of major importance for commercial value and consumer acceptance. Water holding capacity and expressible water content significantly affect the tenderness of the fish muscle (Varghese and Mathew 2017). Furthermore, the dietary inclusion of N. oceanica somehow decreased the fillet springiness while the blend of both algae (NAN4GRA4) decreased fillet resilience. This suggests soft flesh traits in the NAN4GRA4 group and a lower elasticity in the NAN8 fillets. Water holding capacity of muscle is greatly influenced by changes in protein structure, and it was demonstrated that the water binding capacity of the proteins shows a noticeable decrease due to the increase in the moisture content of meat (Aaslyng et al. 2003). Collagen is the most significant constituent of the connective tissues, playing a vital role in maintaining fillet integrity and muscle cohesiveness (Aussanasuwannakul et al. 2012). In yellowtail and red sea bream fed with algae (such as Spirulina, brown algae, and Chlorella), high muscle total collagen was associated with a firm raw meat texture (Nakagawa and Montgomery 2007). In the present study, fillet hardness was not affected by the dietary treatments, but a longer trial until fish reach commercial size is required to fully understand the impact of algae on fish textural properties.
According to the PCA plot, all diets are projected into distinct quadrants, but algae-rich diets tended to be separated from the CTRL. Diets containing N. oceanica (NAN8 and NAN4GRA4) were negatively loaded along F2 and projected in opposite quadrants in relation to CTRL and GRA8. NAN8 diet demonstrated a strong correlation with the number of posterior and anterior intestinal neutral GC, and also with the total antioxidant capacity and activity of both γ-GT and SI in the posterior intestine. GRA8 dietary group was positively loaded along F1 and F2 and associated with the condition factor, N and energy retention efficiency, and muscle redness. CTRL dietary group positively loaded along F1 and was associated with the HSI, plasma triglycerides, and activity of both SI and maltase in the anterior intestine. Moreover, PCA analysis also unveiled the effects of the dietary treatments on the nutrient and energy ADC where the NAN8 and CTRL diets were correlated with better digestibility.
In conclusion, the present study clearly shows that both G. gracilis and N. oceanica biomass, used single or blended, can be valuable natural ingredients for partial replacement of FM in diets for European seabass, as they do not affect growth performance, feed efficiency, N gain, or whole body composition. However, triglyceride levels significantly decreased in fish fed the algae-based diets and could probably account for the reduced HSI observed in those fish. The dietary inclusion of N. oceanica did not affect nutrient digestibility, but G. gracilis led to the lowest protein and energy ADC values enlightening the need for some processing prior use. N. oceanica had higher total antioxidant capacity than G. gracilis, despite not differing from the control diet. Moreover, the algae blend (NAN4GRA4 diet) resulted in lower values of TG, GPx, and ACH50, suggesting that these fish may be less prepared to respond in case of possible infection or stress. However, an infection and/or pro-oxidant challenge would be necessary to further asses the implications of these results. Finally, an impact of algal biomass of muscle textural properties was demonstrated: the inclusion of N. oceanica decreased fillet springiness and elasticity, but when G. gracilis was added (NAN4GRA4), the muscle fillet became softer. A longer feeding trial is recommended to ascertain these effects on commercial-sized fish.
References
Aaslyng MD, Bejerholm C, Ertbjerg P, Bertram HC, Andersen HJ (2003) Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual Prefer 14:277–288
Abreu MH, Pereira R, Yarish C, Buschmann AH, Sousa-Pinto I (2011) IMTA with Gracilaria vermiculophylla: productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 312:77–87
AFNOR (1992) Viandes, préparations de viande et produits à base de viande - Détermination de la teneur en phosphore total. NF:V04–V406
Angell AR, Angell SF, de Nys R, Paul NA (2016) Seaweed as a protein source for mono-gastric livestock. Trends Food Sci Technol 54:74–84
AOAC (2006) Official methods of analysis of AOAC International, 18th edn. AOAC International, Maryland
Araújo M, Rema P, Sousa-Pinto I, Cunha LM, Peixoto MJ, Pires MA, Seixas F, Brotas V, Beltrán C, Valente LMP (2016) Dietary inclusion of IMTA-cultivated Gracilaria vermiculophylla in rainbow trout (Oncorhynchus mykiss) diets: effects on growth, intestinal morphology, tissue pigmentation, and immunological response. J Appl Phycol 28:679–689
Archibeque S, Ettinger A, Willson B (2009) Nannochloropsis oculata as a source for animal feed. Acta Agro Hung 57:245–248
Ashour M, Elshobary ME, El-Shenody R, Kamil A-W, Abomohra AE-F (2019) Evaluation of a native oleaginous marine microalga Nannochloropsis oceanica for dual use in biodiesel production and aquaculture feed. Biomass Bioenergy 120:439–447
Aussanasuwannakul A, Slider SD, Salem M, Yao J, Brett Kenney P (2012) Comparison of variable-blade to Allo-Kramer shear method in assessing rainbow trout (Oncorhynchus mykiss) fillet firmness. J Food Sci 77:S335–S341
Baker MA, Cerniglia GJ, Zaman A (1990) Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem 190:360–365
Bates JM, Akerlund J, Mittge E, Guillemin K (2007) Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in Zebrafish in response to the gut microbiota. Cell Host Microbe 6:371–382
Batista S, Medina A, Pires MA, Moriñigo MA, Sansuwan K, Fernandes JMO, Valente LMP, Ozório ROA (2016) Innate immune response, intestinal morphology and microbiota changes in Senegalese sole fed plant protein diets with probiotics or autolyzed yeast. Appl Microbiol Biot
Becker EW (2007) Micro-algae as a source of protein. Biotech Adv 25:207–210
Belal E, Khalafalla M, El-hais AMA (2012) Use of spirulina (Arthrospira fusiformis) for promoting growth of Nile Tilapia fingerlings. Afr J Microbiol Res 6
Bellou S, Baeshen MN, Elazzazy AM, Aggeli D, Sayegh F, Aggelis G (2014) Microalgal lipids biochemistry and biotechnological perspectives. Biotech Adv 32:1476–1493
Bernstein AM, Ding EL, Willett WC, Rimm EB (2012) A meta-analysis shows that docosahexaenoic acid from algal ail reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J Nutr 142:99–104
Biller JD, Takahashi LS (2018) Oxidative stress and fish immune system: phagocytosis and leukocyte respiratory burst activity. An Acad Bras Cienc 90:3403–3414
Bird RP, Draper HH (1984) Comparative studies on different methods of malonaldehyde determination. Meth Enzymol 105: 299–305.
Bolin DW, King RP, Klosterman EW (1952) A simplified method for the determination of chromic oxide (Cr2O3) when used as an index substance. Science 116:634–635
Borges P, Oliveira B, Casal S, Dias J, Conceição L, Valente LMP (2009) Dietary lipid level affects growth performance and nutrient utilisation of Senegalese sole (Solea senegalensis) juveniles. Brit J Nutr 102:1007–1014
Boshra H, Li J, Sunyer JO (2006) Recent advances on the complement system of teleost fish. Fish Shellfish Immun 20:239–262
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cheng J-H, Sun D-W, Han Z, Zeng X-A (2014) Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: a review. Compr Rev Food Sci Food Saf 13:52–61
Cho CY, Slinger SJ (1979) Apparent digestibility measurement in feedstuffs for rainbow trout. In: Halver JE, Tiews K (eds) Finfish nutrition and fish feed technology, vol 2. Heenemann, Berlin, pp 239–247
Choubert G, Blanc JM, Vallée F (1997) Colour measurement, using the CIELCH colour space, of muscle of rainbow trout, Oncorhynchus mykiss (Walbaum), fed astaxanthin: effects of family, ploidy, sex, and location of reading. Aquac Res 28:15–22
Claiborne A (1985) Catalase activity. In: Greenwald R (ed) CRC handbook of methods in oxygen radical research. CRC Press, Boca Raton, pp 283–284
Costas B, Conceição LEC, Aragão C, Martos JA, Ruiz-Jarabo I, Mancera JM, Afonso A (2011) Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 316:68–76
Coz-Rakovac R, Strunjak-Perovic I, Hacmanjek M, Topic Popovic N, Lipej Z, Sostaric B (2005) Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Vet Res Commun 29:677–687
Deplancke B, Gaskins HR (2001) Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 73:1131S–1141S
Ellis AE (1990) Lysozyme assays. In: FT SJS, Anderson DP, Roberson BS, van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publications, Fair Haven, pp 101–103
Galeano J, Lopez-Herrera A, Suescún J (2015) The probiotic Enterococcus faecium modifies the intestinal morphometric parameters in weaning piglets. Rev Fac Nac Agro 69:7803–7811
Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl Å, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza EJ, Stone D, Wilson R, Wurtele E (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579
Gong Y, Sørensen SL, Dahle D, Nadanasabesan N, Dias J, Valente LMP, Sørensen M, Kiron V (2020) Approaches to improve utilization of Nannochloropsis oceanica in plant-based feeds for Atlantic salmon. Aquaculture. https://doi.org/10.1016/j.aquaculture.2020.735122:735122
Grau A, Crespo S, Sarasquete MC, de Canales MLG (1992) The digestive tract of the amberjack Seriola dumerili, Risso: a light and scanning electron microscope study. J Fish Biol 41:287–303
Guerreiro I, Magalhães R, Coutinho F, Couto A, Sousa S, Delerue-Matos C, Domingues VF, Oliva-Teles A, Peres H (2019) Evaluation of the seaweeds Chondrus crispus and Ulva lactuca as functional ingredients in gilthead seabream (Sparus aurata). J Appl Phycol 31:2115–2124
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Harpaz S, Uni Z (1999) Activity of intestinal mucosal brush border membrane enzymes in relation to the feeding habits of three aquaculture fish species. Comp Biochem Physiol A 124:155-160
Hemaiswarya S, Raja R, Kumar R, Ganesan V, Anbazhagan C (2011) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27:1737–1746
Hinnebusch BF, Siddique A, Henderson JW, Malo MS, Zhang W, Athaide CP, Abedrapo MA, Chen X, Yang VW, Hodin RA (2004) Enterocyte differentiation marker intestinal alkaline phosphatase is a target gene of the gut-enriched Krüppel-like factor. Am J Physiol - Gastro L 286:G23–G30
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Hoseinifar SH, Yousefi S, Capillo G, Paknejad H, Khalili M, Tabarraei A, Van Doan H, Spano N, Faggio C (2018) Mucosal immune parameters, immune and antioxidant defence related genes expression and growth performance of zebrafish (Danio rerio) fed on Gracilaria gracilis powder. Fish Shellfish Immunol 83:232–237
Husni A, Ustadi U, Wijaya H (2013) The use of Gracilaria sp. extract on refrigerated red tilapia fillet. J Biol Sci 13:640–644
Hutchinson TH, Manning MJ (1996) Seasonal trends in serum lysozyme activity and total protein concentration in dab (Limanda limanda L.) sampled from Lyme Bay, U.K. Fish Shellfish Immun 6:473–482
Jonsson, Sigurgisladottir S, Hafsteinsson, Kristbergsson K (2001) Texture properties of raw Atlantic salmon (Salmo salar) fillets measured by different methods in comparison to expressible moisture. Aquac Nutr 7:81–89
Kim JJ, Khan WI (2013) Goblet cells and mucins: role in innate defense in enteric infections. Pathogens 2:55–70
Kiron V, Phromkunthong W, Huntley M, Archibald I, De Scheemaker G (2012) Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac Nut 18:521–531
Kiron V, Sørensen M, Huntley M, Vasanth GK, Gong Y, Dahle D, Palihawadana AM (2016) Defatted biomass of the microalga, Desmodesmus sp., can replace fishmeal in the feeds for Atlantic salmon. Front Mar Sci 3
Lee J-C, Hou M-F, Huang H-W, Chang F-R, Chi-Chen Y, Tang J-Y, Chang H-W (2013) Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int 13:55
Lu SC (2009) Regulation of glutathione synthesis. Mol Asp Med 30:42–59
Magnoni LJ, Martos-Sitcha JA, Queiroz A, Calduch-Giner JA, Goncalves JFM, Rocha CMR, Abreu HT, Schrama JW, Ozorio ROA, Perez-Sanchez J (2017) Dietary supplementation of heat-treated Gracilaria and Ulva seaweeds enhanced acute hypoxia tolerance in gilthead sea bream (Sparus aurata). Biol Open 6:897–908
Maynard LA, Loosli JK, Hintz HF, Warner RG (1979) Animal nutrition. 7th edn., New York
Messina M, Bulfon C, Beraldo P, Tibaldi E, Cardinaletti G (2019) Intestinal morpho-physiology and innate immune status of European sea bass (Dicentrarchus labrax) in response to diets including a blend of two marine microalgae, Tisochrysis lutea and Tetraselmis suecica. Aquaculture 500:660–669
Mohamed S, Hashim SN, Rahman HA (2012) Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci Technol 23:83–96
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ (1984) Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res 44:5086–5091
Molino A, Iovine A, Casella P, Mehariya S, Chianese S, Cerbone A, Rimauro J, Musmarra D (2018) Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int J Environ Res Public Health 15:E2436
Moreda-Piñeiro A, Peña-Vázquez E, Bermejo-Barrera P (2012) Significance of the presence of trace and ultratrace elements in seaweeds. In: Kim SK (ed) Handbook of marine macroalgae: biotechnology and applied phycology. John Wiley & Sons, Chichester, pp 116–170
Moutinho S, Linares F, Rodríguez JL, Sousa V, Valente LMP (2018) Inclusion of 10% seaweed meal in diets for juvenile and on-growing life stages of Senegalese sole (Solea senegalensis). J Appl Phycol 30:3589–3601
Mustafa MG, Umino T, Nakagawa H (1997) Limited synergistic effect of dietary Spirulina on vitamin C nutrition of red sea bream Pagrus major. J Mar Biotechnol 5:129–132
Nakagawa H, Montgomery L (2007) Algae. In: Nakagawa H, Sato M, Gatlin D III (eds) Dietary supplements for the health and quality of cultured fish. CABI, pp 133–167
Nakagawa H, Mustafa MG, Takii K, Umino T, Kumai H (2000) Effect of dietary catechin and Spirulina on vitamin C metabolism in red sea bream. Fish Sci 66:321–326
Natrah FMI, Yusoff F, Shariff M, Abas F, Mariana NS (2007) Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J Appl Phycol 19:711–718
NRC (2011) Nutrient requirements of fish and shrimp. The National Academies Press
Patterson D, Gatlin DM (2013) Evaluation of whole and lipid-extracted algae meals in the diets of juvenile red drum (Sciaenops ocellatus). Aquaculture 416-417:92–98
Peixoto MJ, Salas-Leitón E, Pereira LF, Queiroz A, Magalhães F, Pereira R, Abreu H, Reis PA, Gonçalves JFM, Ozório ROA (2016a) Role of dietary seaweed supplementation on growth performance, digestive capacity and immune and stress responsiveness in European seabass (Dicentrarchus labrax). Aquac Rep 3:189–197
Peixoto MJ, Svendsen JC, Malte H, Pereira LF, Carvalho P, Pereira R, Gonçalves JFM, Ozório ROA (2016b) Diets supplemented with seaweed affect metabolic rate, innate immune, and antioxidant responses, but not individual growth rate in European seabass (Dicentrarchus labrax). J Appl Phycol 28:2061–2071
Peixoto M, Magnoni L, Gonçalves JFM, Twijnstra RH, Kijjoa A, Pereira R, Palstra AP, Ozorio R (2018) Effects of dietary supplementation of Gracilaria sp. extracts on fillet quality, oxidative stress, and immune responses in European seabass (Dicentrarchus labrax). J Appl Phycol 31:761–770
Peixoto MJ, Ferraz R, Magnoni LJ, Pereira R, Gonçalves JF, Calduch-Giner J, Pérez-Sánchez J, Ozório ROA (2019) Protective effects of seaweed supplemented diet on antioxidant and immune responses in European seabass (Dicentrarchus labrax) subjected to bacterial infection. Sci Rep 9:16134
Qiao H, Wang H, Song Z, Ma J, Li B, Liu X, Zhang S, Wang J, Zhang L (2014) Effects of dietary fish oil replacement by microalgae raw materials on growth performance, body composition and fatty acid profile of juvenile olive flounder, Paralichthys olivaceus. Aquac Nutr 20:646–653
Qiao H, Hu D, Ma J, Wang X, Wu H, Wang J (2019) Feeding effects of the microalga Nannochloropsis sp. on juvenile turbot (Scophthalmus maximus L.). Algal Res 41:101540
Quade MJ, Roth JA (1997) A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol 58:239–248
Radha P (2018) Proximate analysis and mineral composition of seaweeds of Manamelkudi Coast, Pudukkottai District, India. Int J Curr Microbiol App Sci 7:3121–3128
Rahimnejad S, Lee S-M, Park H-G, Choi J (2017) Effects of dietary inclusion of Chlorella vulgaris on growth, blood biochemical parameters, and antioxidant enzyme activity in olive flounder, Paralichthys olivaceus. J World Aquac Soc 48:103–112
Rajapakse N, Kim S-K (2011) Chapter 2 - nutritional and digestive health benefits of seaweed. In: Kim S-K (ed) Advances in food and nutrition research. Academic Press, pp 17–28
Ramos MA, Batista S, Pires MA, Silva AP, Pereira LF, Saavedra MJ, Ozorio ROA, Rema P (2017) Dietary probiotic supplementation improves growth and the intestinal morphology of Nile tilapia. Animal 11:1259–1269
Reka P, Azeez T, Seethalakshmi M (2017) Elemental composition of selected edible seaweeds using SEM- energy dispersive spectroscopic analysis. Int Food Res J 24:600–606
Robertson JB, Van Soest PJ (1981) The detergent system of analysis and its application to human foods. In: James WPT, Olof T (eds) The analysis of dietary fiber in food. Marcel Dekker Inc., New York, pp 123–158
Sabeena Farvin KH, Baron CP, Nielsen NS, Otte J, Jacobsen C (2010) Antioxidant activity of yoghurt peptides: part 2 – characterisation of peptide fractions. Food Chem 123:1090–1097
Sarker PK, Gamble MM, Kelson S, Kapuscinski AR (2016) Nile tilapia (Oreochromis niloticus) show high digestibility of lipid and fatty acids from marine Schizochytrium sp. and of protein and essential amino acids from freshwater Spirulina sp. feed ingredients. Aquac Nutr 22:109–119
Schubert N, Garcia-Mendoza E, Pacheco-Ruiz I (2006) Carotenoid composition of marine red algae. J Phycol 42:1208–1216
Sheikhzadeh N, Heidarieh M, Karimi Pashaki A, Nofouzi K, Ahrab Farshbafi M, Akbari M (2012) Hilyses®, fermented Saccharomyces cerevisiae, enhances the growth performance and skin non-specific immune parameters in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 32:1083–1087
Silva DM, Valente LMP, Sousa-Pinto I, Pereira R, Pires MA, Seixas F, Rema P (2015) Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. J Appl Phycol 27:1671–1680
Sørensen M, Gong Y, Bjarnason F, Vasanth GK, Dahle D, Huntley M, Kiron V (2017) Nannochloropsis oceania-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PLoS One 12:e0179907
Sotoudeh E, Mardani F (2018) Antioxidant-related parameters, digestive enzyme activity and intestinal morphology in rainbow trout (Oncorhynchus mykiss) fry fed graded levels of red seaweed, Gracilaria pygmaea. Aquac Nutr 24:777–785
Specian RD, Oliver MG (1991) Functional biology of intestinal goblet cells. Am J Phys 260:C183–C193
Spence C, Levitan C, Shankar M, Zampini M (2010) Does food color influence taste and flavor perception in humans? Chemos Percept 3:68–84
Stadtlander T, Khalil WKB, Focken U, Becker K (2013) Effects of low and medium levels of red alga Nori (Porphyra yezoensis Ueda) in the diets on growth, feed utilization and metabolism in intensively fed Nile tilapia, Oreochromis niloticus (L.). Aquac Nutr 19:64–73
Sunyer J, Tort L (1995) Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are effected by the alternative complement pathway. Vet Immunol Immun 45:333–345
Teimouri M, Yeganeh S, Mianji GR, Najafi M, Mahjoub S (2019) The effect of Spirulina platensis meal on antioxidant gene expression, total antioxidant capacity, and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 45:977–986
Tibaldi E, Hakim Y, Uni Z, Tulli F, de Francesco M, Luzzana U, Harpaz S (2006) Effects of the partial substitution of dietary fish meal by differently processed soybean meals on growth performance, nutrient digestibility and activity of intestinal brush border enzymes in the European sea bass (Dicentrarchus labrax). Aquaculture 261:182–193
Tibaldi E, Chini Zittelli G, Parisi G, Bruno M, Giorgi G, Tulli F, Venturini S, Tredici MR, Poli BM (2015) Growth performance and quality traits of European sea bass (D. labrax) fed diets including increasing levels of freeze-dried Isochrysis sp. (T-ISO) biomass as a source of protein and n-3 long chain PUFA in partial substitution of fish derivatives. Aquaculture 440:60–68
Tibbetts S (2018) The potential for ‘next-generation’, microalgae-based feed ingredients for salmonid aquaculture in context of the blue revolution. In: Jacob-Lopes E, Zepka LQ, Queiroz MI (eds) Microalgal Biotechnology. Intechopen, Riejeka, pp 151–175
Torres MD, Florez-Fernandez N, Dominguez H (2019) Integral utilization of red seaweed for bioactive production. Mar Drugs 17:e314
Tulli F, Chini Zittelli G, Giorgi G, Poli BM, Tibaldi E, Tredici MR (2012) Effect of the inclusion of dried Tetraselmis suecica on growth, feed utilization, and fillet composition of European sea bass juveniles fed organic diets. J Aquat Food Prod Technol 21:188–197
USEPA (1995) EPA method 3051: microwave assisted acid digestion of sediments, sludges, soils, and oils. Test methods for evaluating solid waste, 3rd edn. USEPA, Washington, D.C
Valente LMP, Gouveia A, Rema P, Matos J, Gomes EF, Pinto IS (2006) Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 252:85–91
Valente LMP, Rema P, Ferraro V, Pintado M, Sousa-Pinto I, Cunha LM, Oliveira MB, Araújo M (2015) Iodine enrichment of rainbow trout flesh by dietary supplementation with the red seaweed Gracilaria vermiculophylla. Aquaculture 446:132–139
Valente L, Custódio M, Batista S, Fernandes H, Kiron V (2019) Defatted microalgae (Nannochloropsis sp.) from biorefinery as a potential feed protein source to replace fishmeal in European sea bass diets. Fish Physiol Biochem 45:1067–1081
Van Soest PJ, Robertson JB (1985) Analysis of forages and fibrous foods a laboratory manual for animal science. Department of Animal Science, Ithaca
Varghese T, Mathew S (2017) Assessment of the textural variation of iced stored Anabas testudineus (Bloch, 1792) muscle tissue with emphasis on their collagen and myofibrillar protein content. J Food Sci Technol 54:2512–2518
Vizcaíno AJ, Mendes SI, Varela JL, Ruiz-Jarabo I, Rico R, Figueroa FL, Abdala R, Moriñigo MÁ, Mancera JM, Alarcón FJ (2016) Growth, tissue metabolites and digestive functionality in Sparus aurata juveniles fed different levels of macroalgae, Gracilaria cornea and Ulva rigida. Aquac Res 47:3224–3238
Vizcaíno A, Rodiles A, López G, Saez M, Herrera M, Hachero-Cruzado I, Martínez Moya T, Cerón-García MC, Alarcón F (2018) Growth performance, body composition, and digestive functionality of Senegalese sole (Solea senegalensis Kaup, 1858) juveniles fed diets including microalgae freeze-dried biomass. Fish Physiol Biochem 44
Walker AB, Berlinsky DL (2011) Effects of partial replacement of fish meal protein by microalgae on growth, feed intake, and body composition of Atlantic cod. N Am J Aquac 73:76–83
Younis E-SM, Al-Quffail AS, Al-Asgah NA, Abdel-Warith A-WA, Al-Hafedh YS (2018) Effect of dietary fish meal replacement by red algae, Gracilaria arcuata, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi J Biol Sci 25:198–203
Zheng L-X, Chen X-Q, Cheong K-L (2020) Current trends in marine algae polysaccharides: the digestive tract, microbial catabolism, and prebiotic potential. Int J Biol Macromol 151:344–354
Acknowledgments
Work supported by Project “MARINALGAE4AQUA - Improving bio-utilisation of marine algae as sustainable feed ingredients to increase efficiency and quality of aquaculture production”, financed by FCT-Portugal through the research project ERA-NET COFASP/004/2015. The authors thank Tiago Sá, Catarina Ribeiro, Alexandra Marques, and Marianna Dourou for their technical support during the feeding trial chemical and chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The present study was performed by accredited scientists, in compliance with the guidelines of the European Union (directive 2010/63/EU) and Portuguese law (Decreto-Lei no. 113/2013, de 7 de Agosto) on the protection of animals used for scientific purposes. All animal procedures were subject to an ethical review process carried out by CIIMAR animal welfare body (ORBEA-CIIMAR) and further approved by national competent authorities.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Batista, S., Pereira, R., Oliveira, B. et al. Exploring the potential of seaweed Gracilaria gracilis and microalga Nannochloropsis oceanica, single or blended, as natural dietary ingredients for European seabass Dicentrarchus labrax. J Appl Phycol 32, 2041–2059 (2020). https://doi.org/10.1007/s10811-020-02118-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02118-z