Abstract
Chemical investigation of polar lipids from the marine eustigmatophyte microalga Nannochloropsis granulata led to the isolation of six betaine lipid diacylglyceryltrimethylhomoserine (DGTS), namely, (2S)-1,2-bis-O-eicosapentaenoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (1), (2S)-1-O-eicosapentaenoyl-2-O-arachidonoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (2), (2S)-1-O-eicosapentaenoyl-2-O-myristoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (3), (2S)-1-O-eicosapentaenoyl-2-O-palmitoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (4), (2S)-1-O-eicosapentaenoyl-2-O-palmitoleoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (5), and (2S)-1-O-eicosapentaenoyl-2-O-linoleoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (6). Structures of the isolated DGTSs were elucidated based on both spectroscopic technique and degradation methods. This is the first report of isolation of 1 in pure state, and 2–6 are all new compounds. The isolated betaine lipids showed dose-dependent nitric oxide (NO) inhibitory activity against lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Further study suggested that these betaine lipids (1–6) inhibit NO production in RAW264.7 macrophage cells through downregulation of inducible nitric oxide synthase expression, indicating the possible use as an anti-inflammatory agent. This is the first report of DGTS with anti-inflammatory activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipids are a large group of naturally occurring organic compounds that are related by their solubility in nonpolar organic solvents. Fats, waxes, sterols, vitamins, monoglycerides, diglycerides, triglycerides, glycolipids, sulfolipids, phospholipids, and pigments are all examples of lipids found in nature. The biological functions of lipids include energy storage and as structural components of cell membranes. Lipids also act as important signaling molecules between cells. Betaine lipids are complex lipids which have positively charged trimethylammonium groups; they constitute a group of polar lipids in plants, together with phospholipids and glycolipids. They are widespread but limited to nonflowering plants, and three different types are known at the present time, namely, diacylglyceryl-N,N,N-trimethylhomoserine, diacylglycerylhydroxymethyl-N,N,N-trimethyl-β-alanine (DGTA), and diacylglycerylcarboxy-N-hydroxymethyl-choline (DGCC). A recent study suggested that eukaryotic phytoplankton and cyanobacteria have the ability to decrease their cellular phosphorus content when phosphorus in their environment is scare. Sulfur- and nitrogen-containing membrane lipids including sulphoquinovosyldiacylglycerol and betaine lipid can substitute for phospholipids that allow phytoplankton to maintain growth in the face of phosphorus limitation (Mooy et al. 2009). The only known role of diacylglyceryltrimethylhomoserine (DGTS) is to act as a carrier of acyl groups in fatty acid desaturation (Sato 1992); no biological study has been conducted so far on DGTS.
Nitric oxide (NO) is an important signaling molecule that acts in many tissues to regulate a diverse range of physiological processes. It has both inflammatory and anti-inflammatory properties. Endothelium-derived NO has anti-inflammatory activity, which inhibits adhesion and migration of inflammatory leukocytes (Gils 2006). However, excessive production of NO catalyzed by inducible nitric oxide synthase (iNOS) is pathogenic for host tissue (Aktan 2004). Thus, effective inhibition of NO accumulation represents a beneficial therapeutic strategy for the treatment of NO-mediated disorders. In our continuing effort to identify anti-inflammatory agents from microalgal sources, we further examined NO inhibitory activity of DGTS isolated from a marine microalga, Nannochloropsis granulata. In this article, we describe the isolation, structure elucidation, and NO inhibitory activity of new DGTS in lipopolysaccharide (LPS)-induced NO production in RAW264.7 cells.
Material and methods
The NMR spectra were measured on Bruker 700 MHz spectrometer with deuterated solvents. Both analytical and preparative HPLC were carried out on an Agilent 1200 Series HPLC equipped with a diode array detector and the HPLC coupled with 6100B Series Single Quadrupole LC/MS systems for LCMS analysis. High-resolution mass spectra (HRMS) were recorded on Thermo Fisher Scientific (USA) Exactive mass spectrometer. GC analysis was carried out on an Agilent Technologies 7890A GC spectrometer using an Omegawax 250 fused silica capillary column (30 m × 0.25 mm × 0.25 μm film thickness). Supelco® 37 component FAME mix and PUFA-3 (Supelco, USA) were used as standards. Specific rotations were measured on Perkin-Elmer-141 Polarimeter. HPLC grade solvents were used for the extraction and purification processes.
Nannochloropsis granulata Karlson et Potter (a marine eustigmatophyte, CCMP 535; Provasoli-Guillard National Center for Culture of Marine Phytoplankton, West Boothbay Harbor, Maine) was grown under a continuous photoperiod in a 1,000-L Brite-Box photobioreactor as described in our previous report (Banskota et al. 2012b).
Extraction and isolation
The lyophilized microalgal biomass (100 g) was extracted with MeOH/CH2Cl2 (1:1, 1 L × 3) by stirring at room temperature for 1 h followed by sonication for 15 min. The organic fraction was separated by filtration and evaporated under reduced pressure yielding 24.2 g extract. The extract was subjected to silica gel column (11 × 13 cm, 70–230 mesh, Sigma-Aldrich, USA) and eluted with EtOAc (1 L), EtOAc/MeOH (9:1, 1 L), EtOAc/MeOH (1:1, 1 L), EtOAc/MeOH (1:3, 1 L), and MeOH (3 L), obtaining seven fractions (L/fraction). Fractions 6–7 (3.3 g) containing primarily DGTS were combined, dissolved in MeOH (100 mg mL−1), and subjected to HPLC separation after filtering through a 0.20-μm, 13-mm nylon membrane syringe filter (VWR, USA). The semi-preparative HPLC was performed on Agilent 1200 series HPLC using Synergi Max-RP column (4 μm, 10 × 250 mm; Phenomenex, USA) under isocratic condition with mobile phase MeOH/H2O (19:1) for 50 min, with UV detection at 205 nm. (2S)-1,2-bis-O-Eicosapentaenoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (1, 6.1 mg), (2S)-1-O-eicosapentaenoyl-2-O-arachidonoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (2, 1.1 mg), (2S)-1-O-eicosapentaenoyl-2-O-myristoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (3, 2.2 mg), (2S)-1-O-eicosapentaenoyl-2-O-palmitoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (4, 3.6 mg), (2S)-1-O-eicosapentaenoyl-2-O-palmitoleoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (5, 3.7 mg), and (2S)-1-O-eicosapentaenoyl-2-O-linoleoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (6, 1.4 mg) were obtained from multiple injections, eluting at 29.0, 35.7, 32.0, 42.1, 34.1, and 45.1 min, respectively, as follows:
-
1:
[α]D +3.5° (c = 0.05, MeOH); UV (λ max) 200 nm; mass spectroscopy (MS) [electrospray ionization (ESI) in positive mode] m/z 804.5 (M + H)+; HRMS 804.5742 calculated (calcd) for C50H78NO7 (M + H)+ 804.5773. The 1H and 13C NMR are reported in Table 1.
Table 1 1H- and 13C-NMR data of betaine lipids 1–6 measured in CD3OD -
2:
[α]D +3.5° (c = 0.05, MeOH); UV (λ max) 200 nm; MS (ESI in positive mode) m/z 806.5 (M + H)+; HRMS 806.5898 calcd for C50H80NO7 (M + H)+ 806.5929. The 1H- and 13C-NMR data are reported in Table 1.
-
3:
[α]D +2.7° (c = 0.07, MeOH); UV (λ max) 200 nm; MS (ESI in positive mode) m/z 730.5 (M + H)+; HRMS 730.5592 calcd for C44H76NO7 (M + H)+ 730.5616. The 1H- and 13C-NMR data are reported in Table 1.
-
4:
[α]D +2.1° (c = 0.09, MeOH); UV (λ max) 200 nm; MS (ESI in positive mode) m/z 758.5 (M + H)+; HRMS 758.5904 calcd for C46H80NO7 (M + H)+ 758.5929. The 1H- and 13C-NMR data are reported in Table 1.
-
5:
[α]D +2.0° (c = 0.09, MeOH); UV (λ max) 200 nm; MS (ESI in positive mode) m/z 756.5 (M + H)+; HRMS 756.5745 calcd for C46H78NO7 (M + H)+ 756.5773; The 1H- and 13C-NMR data are reported in Table 1.
-
6:
[α]D +10.0° (c = 0.01, MeOH); UV (λ max) 200 nm; MS (ESI in positive mode) m/z 784.5 (M + H)+; HRMS 784.6062 calcd for C48H82NO7 (M + H)+ 784.6086. The 1H- and 13C-NMR data are reported in Table 1.
Acid hydrolysis of DGTS
Combined fractions 6–7 (122 mg) were dissolved in 2 % HCl in MeOH (4.0 mL), and the resulting mixture was kept at 85 °C for 45 min. The reaction was stopped by adding water (2.0 mL) and partitioned with hexane (10.0 mL). The hexane fraction (13.2 mg) was subjected to GC analysis, which showed the presence of methyl ester of eicosapentaenoic, arachidonoic, linoleic, palmitoleic, palmitic, and myristic acids. The aqueous/MeOH fraction was dried, redissolved in water (5.0 mL), and subjected to a solid phase extraction using a C18 cartridge (Strata C18-E, 10 g; Phenomenex, USA) by gradient elution with MeOH/H2O (20 mL per fraction) to give four fractions [fraction 1, MeOH elute, 0.6 mg; fraction 2, MeOH/H2O (3:1) elute, 3.3 mg; fraction 3, MeOH/H2O (1:1) elute, 2.5 mg; fraction 4, H2O elute, 3.3 mg]. Fractions 3 and 4 proved to be pure 4-N,N,N-trimethylhomoserine (Abe and Kaneda 1975).
Enzymatic hydrolysis of DGTS
Fractions 6–7 (10 mg) dissolved in 1,4-dioxane (1.5 mL) was mixed with lipase (4.0 mg, from Pseudomonas sp., 24 units/mg; Sigma-Aldrich, USA) dissolved in water (1.5 mL). The reaction mixture was stirred at 37 °C for 4 h and quenched by adding 5 % AcOH (0.5 mL). The resulting mixture was dried under reduced pressure, suspended in water (5.0 mL), and extracted with EtOAc (10.0 mL). The EtOAc fraction was dried, redissolved in MeOH (0.2 mL), and subjected to LC/MS analysis using Synergi Max-RP column (4.6 × 250 mm, 4 μm; Phenomenex, USA) under isocratic conditions using mobile phase of MeOH/0.025 M H2SO4 in water (19:1) at 1.0 mL/min flow rate for 30 min and UV detection at 205 nm. Eicosapentaenoic acid was eluted at 6.0 min with pseudo-molecular ion peaks at m/z 303.2 (M + H)+.
Cell culture
The murine macrophage RAW246.7 cell line was purchased from American Type Culture Collection and cultured in DMEM, high glucose (HyClone, USA) supplemented with 2 mM glutamine (Sigma, USA), penicillin (50 units mL−1) (MP Biomedicals, USA), streptomycin (50 μg mL−1) (MP Biomedicals, USA), and 10 % FCS (HyClone, USA), at 37 °C in a humidified atmosphere of 5 % CO2. The cells were grown to 80 − 90 % confluence, harvested by scraping, and diluted in fresh medium to desired concentration of cells mL−1. RAW264.7 cell cultures were not used for assays beyond passage 12.
Nitrite production in macrophage RAW264.7 cells
The RAW246.7 cells were seeded in 48-well plastic plates (Corning, USA) with 1.25 × 105 cells well−1 and allowed to adhere for 22 h. After cell adherence, the medium was replaced with fresh medium (without phenol red), containing LPS alone (1 μg mL−1) or LPS together with test compound at various concentrations dissolved in dimethyl sulfoxide (DMSO), and the cells were incubated for 24 h. The final concentration of DMSO was less than 0.2 %, which has no effect on NO production or on cell viability. NO production was determined by measuring the accumulation of nitrite in the culture supernatant using Griess reagent (Sigma, USA). Briefly, 50 μL of the supernatant from incubates was mixed with an equal volume of Griess reagent (0.5 % sulfanilamide and 0.05 % naphthalene diamine dihydrochloride in 2.5 % H3PO4) and allowed to stand for 10 min at room temperature. Absorbance at 540 nm was measured using a Spectramax Plus microplate reader (Molecular Devices, USA). The nitrite concentration in the medium was determined from the calibration curve obtained using different concentrations of sodium nitrite (Sigma, USA) as standard. The percentage inhibition was calculated as follows: % inhibition = [(N c–N s)/N c] × 100, where N c and N s are the nitrite concentrations of control group treated with LPS alone and nitrite concentration of the group treated with LPS plus sample, respectively. The data are expressed as mean ± SD of three determinations, and significance of the test compounds was calculated from the LPS-treated control group using Student’s t test.
Cell viability
After taking 200 μL of supernatant from 48-well plate for NO determination, 40 μL of MTS solution from Cell Titer 96 AQueous One Solution Cell Proliferation Assay (Promega, USA) was added to the remaining 200 μL of medium and incubated for 2 h at 37 °C in a humidified atmosphere containing 5 % CO2. The amount of formazan was measured spectrophotometrically at 490 nm using Victor X5 2030 multilabel microplate reader (Perkin Elmer, USA). The cell viability was calculated as follows: % cell viability = (A s/A c) × 100, where A c and A s are the absorbances of LPS-treated group and LPS plus sample-treated group, respectively. None of the compound showed any significant cytotoxic effect at tested concentrations.
Effect of DGTSs on iNOS expression
The RAW246.7 cells were seeded in 48-well plastic plates (Corning, USA) with 1.25 × 105cells well−1 and allowed to adhere for 24 h at 37 °C in a humidified atmosphere containing 5 % CO2. The medium was replaced with fresh medium (without phenol red) either containing LPS alone (1 mg mL−1) or LPS with test compound (100 μM) and incubated for 24 h. Cells were rinsed 1× posttreatment with ice-cold phosphate-buffered saline and lysed on ice with lysis buffer (50 μM Tris, pH 8.0, 150 mM NaCl, 10 mM Na pyrophosphate, 10 mM NaF, 10 mM β-glycerophosphate, 5 mM Na vanadate, 1 % Triton X-100, 0.1 % SDS, and 1 % HALT™ protease inhibitor cocktail; Pierce, USA). Cell lysate protein concentration was determined using the bicinchoninic acid reagent kit supplied by Pierce (USA) according to manufacturer’s instructions. Equal amounts of protein were subjected to SDS-PAGE and transferred onto PVDF membrane. Membranes were blocked for 1 h at room temperature with blocking solution (1× Tris-buffered saline, 0.1 % Tween, and 5 % skim milk powder) and incubated with rabbit anti-mouse iNOS primary antibody (Cell Signaling Technology, USA) overnight with shaking at 4 °C. After washing with wash solution (1× Tris-buffered saline and 0.1 % Tween), membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Cell Signaling Technology, USA) in blocking solution for 1 h at room temperature, wash step repeated, and membranes were then developed using ECL Western Lightning plus-ECL Enhanced Chemiluminescent Substrate (GE Healthcare Biosciences, USA).
Results and discussion
In our previous study, we reported the nitric oxide inhibitory activity of the monogalactosyldiacylglycerols (MGDGs) and digalactosyldiacylglycerols (DGDGs) from the marine microalga N. granulata (Banskota et al. 2012b). Further purification of the polar fractions of MeOH/CH2Cl2 extract led to the isolation of six DGTSs (Fig. 1) as major components. Five (2–6) out of six DGTSs isolated from N. granulata were new compounds, and their structure was elucidated based on spectral analyses and both chemical and enzymatic hydrolysis.
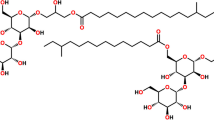
(2S)-1,2-bis-O-Eicosapentaenoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (1) was isolated as a colorless film with molecular formula C50H77O7 determined by HRMS. The 1H- and 13C-NMR spectra of 1 (Table 1) displayed signals corresponding to 20 unsaturated protons, 2 methine protons, 20 methylene groups including 3 oxygenated methylenes, 5 primary methyl signals, and 3 carbonyl signals (174.7, 174.3, and 171.6 ppm). A strong singlet signal corresponding to three methyl group at δ H 3.20 in 1H-NMR spectrum suggested the presence of a trimethylammonium group. In depth analyses of the 2D NMR spectra including 1H–1H correlation spectroscopy, together with heteronuclear single quantum coherence and heteronuclear multiple bond coherence (HMBC), led to the identification of a trimethylhomoserine unit with 13C-NMR signals at δ C 68.4 (C-1′), 29.1 (C-2′), 77.4 (C-3′), 171.6 (C-4′), and 52.3 (N-CH3). Similarly, a glycerol moiety also revealed with 13 N-NMR signal at δ C 63.9 (C-1), 71.5 (C-2), and 70.5 (C-3). The HMBC correlation (Fig. 2) between methylene protons of glycerol moiety at δ H 4.41 and 4.16 with methylene carbon of homoserine at δ C 68.4 and vice versa suggested the homoserine unit is attached to the glycerol moiety at C-3 via an ether bond. The remaining signals including two carbonyl carbons having HMBC correlations (Fig. 2) with glycerol protons at δ H 5.23 and 3.61, respectively, suggested the presence of two fatty acids attached to glycerol via ester bond. Thus, compound 1 was identified as diacylglyceryltrimethylhomoserine. Twenty unsaturated protons together with eight bis-allylic methylene groups and identical methyl signal at δ H 0.97 strongly suggested the presence of two eicosapentaenoyl moieties, which was further confirmed by the fact that acid hydrolysis of the mixture of DGTS yielded eicosapentaenoic acid. Thus, the planar structure of 1 was elucidated as 1,2-bis-O-eicosapentaenoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine. Acid hydrolysis of DGTS containing a fraction gave ulvaline (Fushiya et al. 1997), which was considered as derived from l-series amino acid (Abe and Kaneda 1975). Moreover, the identical coupling constants of methylene protons and chemical shift of methane proton of glycerol moiety of DGTS with galactolipids (MGDG and DGDG) isolated from nonpolar fraction of the same extract (Banskota et al. 2012b) suggested that the relative stereochemistry of C-2 of both DGTS and galactolipids is the same; accordingly, the structure of 1 was concluded as (2S)-1,2-bis-O-eicosapentaenoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine. The compound had been previously described in the literature and was believed to be one of the major components in the mixture of DGTS purified from the marine green alga Chlorella minutissima based on the high content of eicosapentaenoic acid (EPA) (Haigh et al. 1996; Guschina and Harwood 2006). To the best of our knowledge, this is the first report of isolation of 1 in its pure state along with complete spectral data measurement including 13C-NMR spectrum.
(2S)-1-O-Eicosapentaenoyl-2-O-arachidonoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (2) was also isolated as a colorless film with [α]D +3.5°. HRMS analysis of 2 showed the molecular ion peak at m/z 806.5898 [M + H]+ (calcd 806.5929) corresponding to a molecular formula C50H79NO7. The 1H- and 13C-NMR spectra of 2 were almost identical to those of 1 except the absence of one double bond. The high-field shift of one primary methyl group at δ H 0.90 having HMBC correlation with two methylene carbons at δ C 23.6 and 30.4 suggested that one of the fatty acid should be arachidonoic acid. Lipase-mediated regioselective hydrolysis of DGTS which gave only eicosapentaenoic acid suggested that EPA was in sn-1 position; accordingly, structure of 2 was elucidated as (2S)-1-O-eicosapentaenoyl-2-O-arachidonoyl-3-O-4′-(N,N,N-trimethyl)-homoserine.
(2S)-1-O-Eicosapentaenoyl-2-O-myristoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (3) showed molecular ion peak at m/z 730.5592 [M + H]+ (calcd 730.5616) corresponding to molecular formula C44H75NO7. The 1H- and 13C-NMR spectra of 3 displayed signals corresponding to 10 unsaturated protons, 2 methine protons, 24 methylene groups including 3 oxygenated methylenes, 5 primary methyl signals including 3 methyl from trimethylammonium group at δ C 3.20, and 3 carbonyl signals at δ C 174.9, 174.4, and 171.6, indicating that 1 was a DGTS. Close comparison of both 1H- and 13C-NMR data of 3 with 1 and molecular mass revealed that 3 was DGTS having eicosapentaenoyl and myristoyl-acyl groups at sn-1 and sn-2 positions, respectively. This was further confirmed by the HMBC correlation observed between methylene protons of glycerol moiety at δ H 4.41 and 4.17 with carbonyl carbon of eicosapentaenoic acid at δ C 174.9. The remaining myristoyl group then should be at C-2; therefore, the structure of 3 was determined as (2S)-1-O-eicosapentaenoyl-2-O-myristoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine.
(2S)-1-O-Eicosapentaenoyl-2-O-palmitoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (4) was also isolated as a colorless film with molecular formula C46H79NO7 determined by HRMS data. The 1H- and 13C-NMR spectra of 4 were identical to those of 3 except for the difference in the fatty acid attached at C-2 of glycerol. The two additional methylene groups in the acyl side chain attached at C-2 suggested that the fatty acid should be palmitic acid, which was confirmed by both HMBC correlations observed between oxymethine proton at δ H 5.23 and palmitoyl carbonyl carbon at δ C 174.3. Moreover, GC analysis of the hydrolyzed product gave methyl eater of palmitic acid. Accordingly, the structure of 4 was elucidated as (2S)-1-O-eicosapentaenoyl-2-O-palmitoyl-3-O-4′-(N,N,N-trimethyl)-homoserine.
(2S)-1-O-Eicosapentaenoyl-2-O-palmitoleoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (5) was also isolated as colorless film with molecular formula C46H77NO7. The 1H- and 13C-NMR signals of 5 were identical to those of 4 except the presence of an additional double bond instead of two methylene groups. Both NMR and MS data suggested that the fatty acid attached at C-2 should be palmitoleic acid, which was further confirmed by GC analysis of fatty acid methyl ester derived from DGTS fraction, which contain methyl ester of palmitoleic acid as a major component. Moreover, the HMBC correlation observed between methylene protons (δ 4.41 and 4.17) of the glycerol moiety and the carbonyl carbon of EPA (δ 174.8) suggested the position of EPA at C-1 joined via ester bond. The remaining fatty acid, i.e., palmitoleic acid, was therefore confirmed to be at C-2. Accordingly, the structure of 5 was determined as (2S)-1-O-eicosapentaenoyl-2-O-palmitoleoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine.
(2S)-1-O-Eicosapentaenoyl-2-O-linoleoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine (6) was isolated as a colorless film with molecular formula determined as C48H81NO7 by HRMS analysis which showed molecular ion peak at m/z 784.6062 (calcd 784.6086). The 1H- and 13C-NMR spectral data (Table 1) and MS data suggested that 6 should be a DGTS. Close comparison of both 1H- and 13C-NMR spectra of 6 and 1 revealed that the differences were only on the acyl group at C-2 of the glycerol moiety. In depth spectral analysis led to conclusion that the acyl group at C-2 was linoleic acid, further confirmed by the fact that HCl/MeOH-mediated hydrolysis of DGTS fraction gave methyl ester of linoleic acid. Thus, the structure of 6 was elucidated as (2S)-1-O-eicosapentaenoyl-2-O-linoleoylglyceryl-3-O-4′-(N,N,N-trimethyl)-homoserine.
Betaine lipids including DGTS, DGTA, and DGCC represent a third group of membrane lipids beside phospholipids and glycolipids. Nichols and Appleby (1969) first noted the presence of betaine lipids in Ochromonas danica, and the chemical structure was established later by Brown and Elovson (1974). Several groups of scientist studied both the biosynthesis and chemotaxonomy of betaine lipids. Eichenberger et al. (1993) reported that the occurrence of phosphatidylcholine and DGTA partly follows the brown algal taxonomy. They observed the presence of DGTA in the orders Tilopteridales, Dictyotales, Notheiales, Fucales, and Durvillaeales. Müller and Eichenberger (1994) later found that DGTA was strongly correlated with certain species in the genera Hincksia and Ectocarpus. Kato et al. (1996) examined the distribution of betaine lipids in marine algae and found that DGCC is one of the common constituents of Haptophyceae; DGTA was detected in 5 out of 16 species of Haptophyceae, whereas DGTS was not detected at all. Kunzler and Eichenberger (1997), on the other hand, studied 110 plants and fungi for betaine lipids and zwitterionic phospholipids, and their results revealed that betaine lipids are present exclusively in nonflowering plants as well as lichens and fungi. In [14C]-methionine labeling experiments in intact cells of Chlamydomonas reinhardtii, Sato (1988) observed that C4 backbone and the S-methyl group of L-methionine are precursors to the C4 backbone and N-methyl groups of DGTS, respectively. The results of biosynthetic studies of betaine lipids have revealed that DGTA is synthesized from DGTS (Sato 1992). More interestingly, fatty acids esterified at the C-1 and C-2 of glycerol on DGTS were mostly asymmetric (Sato 1992; Guschina and Harwood 2006) except for DGTS isolated from C. minutissima (Haigh et al. 1996) in which EPA appeared to be present at both positions based on the high content of EPA (over 90 % of total fatty acids). It is worthwhile to mention here that the present study represents the second example of DGTS (1) having identical fatty acid at both positions, i.e., EPA isolated as a major betaine lipid from N. granulata. Interestingly, all the isolated DGTSs (1–6) have EPA in sn-1 position, which is in line with results from our previous study, i.e., the MGDGs and DGDGs isolated from relatively nonpolar fraction of the MeOH/CH2Cl2 extract of N. granulata all have EPA in sn-1 position (Banskota et al. 2012b).
No biological activity has been reported for the betaine lipids to our knowledge, even though they were isolated and identified two decades ago. Based on the fact that both galactolipids MGDG and DGDG isolated from N. granulata having similar fatty acids at sn-1 and sn-2 positions of glycerol moiety (Banskota et al. 2012b) showed potent NO inhibitory activity, we tested these newly isolated betaine lipids for their NO inhibitory activity against lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. The tested DGTS 1–6 showed strong and dose-dependent NO inhibitory activity (Fig. 3). Their potency on NO inhibition was equal or stronger than N G-methyl-l-arginine acetate salt (L-NMMA), a well-known NO inhibitor used for positive control. DGTS with both fatty acids as EPA (1) and with EPA and arachidonoic acid (2) possessed superior NO activity compared to the other DGTS, suggesting that increased unsaturation of the fatty acid side chain may enhance NO inhibitory activity, similar to what was previously observed in the MGDGs isolated from Tetraselmis chui (Banskota et al. 2012a). Moreover, we further tested the effect of DGTS towards inducible nitric oxide synthase enzyme expression in RAW264.7 cells to understand the mechanism of action. As shown in Fig. 4, all the tested DGTS downregulated the iNOS protein levels in LPS-stimulated RAW264.7 macrophage cells, suggesting that these betaine lipids inhibited NO production through the downregulation of iNOS expression not by just reacting with unsaturated bond of the fatty acid, as suggested earlier by O’Donnell et al. (1999). These results demonstrate for the first time that the betaine lipids would be a potential candidate for anti-inflammatory agents in the pharmaceutical, nutraceutical, and functional food industries.
References
Abe S, Kaneda T (1975) Studies on effect of marine products on cholesterol metabolism in rat-XI, isolation of a new betaine, ulvaline, from a green laver Monostroma nitidum and its depressing effect on plasma cholesterol levels. Bull Jap Soc Sci Fish 41:567–571
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75:639–653
Banskota AH, Gallant P, Stefanova R, Melanson R, O’Leary SJB (2012a) Monogalactosyldiacylglycerols, potent nitric oxide inhibitors from the marine microalga Tetraselmis chui. Nat Prod Res. doi:10.1080/14786419.2012.717285
Banskota AH, Stefanova R, Gallant P, McGinn P (2012b) Mono- and digalactosyldiacylglycerols; potent nitric oxide inhibitors from the marine microalga Nannochloropsis granulata. J Appl Phycol. doi:10.1007/s10811-012-9869-2
Brown AE, Elovson J (1974) Isolation and characterization of a novel lipid, 1(3),2-diacylglyceryl-(3)-O-4′-(N, N, N-trimethyl)homoserine, from Ochromonas danica. Biochemistry 13:3476–3482
Eichenberger W, Araki S, Müller DG (1993) Betaine lipids and phospholipids in brown algae. Phytochemistry 34:1323–1333
Fushiya S, Komatu Y, Nozoe S (1997) Two betaine type amino acid derivatives of Lampteromyces japonicus. Nat Med 51:558
Gils TD (2006) Aspects of nitric oxide in health and disease: a focus on hypertension and cardiovascular disease. J Clin Hypertens 8(12 Suppl 4):2–16
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Haigh WG, Yoder TF, Ericson L, Pratum T, Winget RR (1996) the characterization and cyclic production of a highly unsaturated homoserine lipid in Chlorella minutissima. Biochim Biophys Acta 1299:183–190
Kato M, Sakai M, Adachi K, Ikemoto H, Sano H (1996) Distribution of betaine lipids in marine algae. Phytochemistry 42:1341–1345
Kunzler K, Eichenberger W (1997) Betaine lipids and zwitterionic phospholipids in plants and fungi. Phytochemistry 46:883–892
Mooy BASV, Fredricks HF, Pedler BE, Dyhrman ST, Karl DM, Koblížek M, Lomas MW, Mincer TJ, Moore LR, Moutin T, Rappe MS, Webb EA (2009) Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458:69–72
Müller DG, Eichenberger W (1994) Betaine lipids content and species delimitation in Ectocarpus, Feldmannia and Hincksia (Ectocarpales, Phaeophyceae). Eur J Phycol 29:219–225
Nichols BW, Appleby RS (1969) The distribution and biosynthesis of arachidonic acid in algae. Phytochemistry 8:1907–1915
O’Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA (1999) Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol 12:83–92
Sato N (1988) Dual role of methionine in the biosynthesis of diacylglyceryltrimethylhomoserine in Chlamydomonas reinhardtii. Plant Physiol 86:931–934
Sato N (1992) Betaine lipids. Bot Mag Tokyo 105:185–197
Acknowledgments
The authors are thankful to J. Milley, J. Hui, and Dr. J. Melanson for their technical support. This is NRC publication no. 50515.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banskota, A.H., Stefanova, R., Sperker, S. et al. New diacylglyceryltrimethylhomoserines from the marine microalga Nannochloropsis granulata and their nitric oxide inhibitory activity. J Appl Phycol 25, 1513–1521 (2013). https://doi.org/10.1007/s10811-012-9967-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9967-1