Abstract
The amino acid profile of the red microalga Porphyridium cruentum and its protein extract have been determined in order to assess the nutritional quality of this biomass for human consumption. Total protein determined by elemental analysis represented 56 % of its dry weight. Hydro-soluble proteins extracted at pH 12 and 40 °C were analysed by the Lowry method giving 47 %, which represented 84 % of total protein per dry weight. The amino acid sequence of the biomass and the protein extract was composed of a set of essential (39 % for the former and 37 % for the latter) and non-essential amino acids (61 % for the former and 63 % for the latter) that compares favourably with the standard protein/amino acid requirements proposed by Food and Agricultural Organisation and World Health Organisation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae have been thoroughly studied for their impressive richness in multiple important components that can be valorised for numerous products such as biofuel, animal feed, food supplements, dyes and pharmaceuticals. For instance, in Japan and Chile, Chlorella vulgaris is added to food, such as noodles and pasta (Fradique et al. 2010), to improve the nutritional quality of the meal. Spirulina platensis is also used as food supplement in France, Mexico, USA and other countries (FAO 2008).
Porphyridium cruentum is a unicellular microalga with cell diameter ranging between 6 and 10 μm (Durmaz et al. 2007). It grows in saline water and lacks a cell wall, but instead, it excretes a sulphurized polysaccharide that encapsulates the cell (Arad et al. 1985, 1988; Adda et al. 1986; Geresh and Arad 1991; Geresh et al. 2002), therefore accumulating a high amount of polysaccharides 40–57 % (Fuentes et al. 2000). The source of red colour of this microalga is phycoerythrin, which plays an important role together with other phycobiliproteins present as an accessory photosynthetic pigment (Ley et al. 1977). In addition, P. cruentum is a rich source of lipids and polyunsaturated fatty acids, such as eicosapentanoic acid, docosahexaenoic acid and arachidonic acid (AA) (Shiran et al. 1996; Cohen et al. 1997; Khozin-Goldberg et al. 2000) that have health benefits by reducing the risk of cardiovascular disease, high cholesterol levels (Ginzberg et al. 2000) and inflammation and also reduce the risk of cancer (Patil et al. 2007). Moreover, this microalga accumulates high quantity of proteins (39 % according to Fuentes et al. 2000).
Some studies have focused on amino acid profile of phycocyanin (Ducret et al. 1994) or on extracellular protein-polysaccharide linkage analysis (Heaney-Kieras and Chapman 1976). Other studies worked on the nutritional importance of this microalga without taking into consideration its protein quality and only relying on fatty acids, carbohydrates and phycobilisomes (Fuentes et al. 2000; Berge et al. 2002). González López et al. (2010) measured the nitrogen to protein conversion factor of five different microalgae, including P. cruentum, by taking into account protein nitrogen by Lowry method and total nitrogen by elemental analysis and Kjeldahl method. Nevertheless, no study has been carried out on the amino acid profile of the crude P. cruentum in order to evaluate the quality of proteins present in the biomass.
Therefore, this study focuses on evaluating the protein quality of the microalga by investigating the amino acid profile of P. cruentum to compare it with standard protein source proposed by World Health Organisation (WHO) and Food and Agricultural Organisation (FAO) (WHO 2007; Becker 1994). The profile of the protein extract of this microalga was also performed to evaluate the potential of both substrates as an unconventional protein source for food consumption and food supplement.
Materials and methods
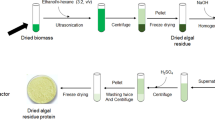
The microalga Porphyridium cruentum (strain UTEX 161) was grown in batch mode in an indoor tubular air-lift photobioreactor (PBR, 10 L) at 25 °C (Loubiere et al. 2011), inoculated from a prior culture in a flat panel air-lift PBR (1 L). Hemerick culture medium was used (Hemerick 1973). Culture circulation was by sterile air injection at the bottom of the PBR. The pH and temperature were recorded by a pH/temperature probe, and the pH was regulated at pH 7.5 with CO2 bubbling. P. cruentum was harvested during the exponential phase and concentrated by centrifugation (11 % dry matter) and was furnished as frozen paste from Alpha Biotech (Asserac, France).
The frozen paste of crude microalga was freeze dried in a Fisher Bioblock Scientific Alpha 2-4 LD Plus device (France). The pressure was reduced to 0.010 bar, and the temperature was further decreased to −80 °C, and freeze-drying was under vacuum for 48 h.
Protein extraction
For protein determination, stock solutions were prepared with approximately 500 mL of ultrapure water and some drops of NaOH 2 N to adjust the pH to the desired value from 7 to 14. A sample of 1 g of freeze-dried biomass was added to 50 mL of stock solution. The mixture was heated at 40 °C with stirring for 1 h. The sample was then centrifuged at 10,000 × g for 10 min. Samples were taken for analyses: colorimetric method of Lowry, elemental analysis and amino acid analysis.
Lowry method (Lowry et al. 1951)
The procedure involves reaction of proteins with cupric sulphate and tartare in an alkaline solution, leading to the formation of tetradentate copper protein complexes. The addition of the Folin–Ciocalteu reagent leads to the oxidation of the peptide bonds by forming molybdenum blue with the copper ions. Therefore, a calibration curve was prepared using a concentration range of bovine serum albumin from 0 to 1,500 μg mL−1. In order to measure the protein content, 0.2 mL of each standard or samples containing the crude protein extract were withdrawn, and 1 mL of modified Lowry reagent was added to each sample. Each sample was then vortexed and incubated for exactly 10 min. After incubation, 100 μL of Folin–Ciocalteu Reagent (1 N) were added and again vortexed and incubated for exactly 30 min. The blue colour solution is then measured at 750 nm.
Elemental analysis
Total nitrogen of the freeze-dried biomass was evaluated using a PerkinElmer 2400 series II elemental analyser. Samples of 2 mg were placed in thin capsules and then heated at 925 °C using pure oxygen as the combustion gas and pure helium as the carrier gas, then evaluating the nitrogen percentage and converting it into protein percentage using the standard 6.25 as the conversion factor.
Amino acid analysis
Amino acid composition of the biomass was determined according to the method of Moore and Stein (1948). The samples were hydrolysed with 6 N HCl at 103 °C for 24 h. Then, the hydrolysed material was adjusted to pH 2.2 with 6 N NaOH and then stabilised with a pH 2.2 citrate buffer solution. The final solution was then filtered through a 0.45-μm PTFE membrane to remove any residual solid remaining in the solution. The analysis was performed using an amino acid analyser Biochrom Ltd 30 (Cambridge, UK) equipped with a system “column + pre-column (PEEK)” (size 200 × 4.6 mm) with ion exchange resins containing sodium. The separation of amino acids is carried out by elution with loading buffers at different pH, with a flow rate of 20 μL. After reaction with ninhydrin, amino acids are detected at a wavelength of 570 nm, with the exception of proline, for which detection was at 440 nm. Ammonia was automatically added to compensate the value of some less resistant amino acids that disappeared after the strong acid hydrolysis.
Results
All results in this section are presented as percent dry weight of the biomass. Aqueous extraction of P. cruentum was carried out at different pH. The highest hydro-soluble protein content obtained was 47 % at pH 12 and 47 % for pH 14, and the lowest was 8 % at pH 7 followed by 33 % at pH 10 (Fig. 1). On the other hand, total protein obtained by elemental analysis was 56 % from which hydro-soluble proteins represented 84 % with 16 % as non-soluble proteins.
The amino acid sequence of P. cruentum and its protein extract obtained at pH 12 (Table 1) included a set of essential and non-essential amino acids (Table 2) representing a total of 38.7 % essential, 61.0 % non-essential and 0.3 % ornithine, which is an unclassified amino acid. As for the protein extract, 36.8 % were essential, 62.9 % non-essential and 0.2 % ornithine. The major amino acids of the crude biomass were aspartic acid 11.2 % and serine 8.1 % as well as glutamic acid 8.2 %. The major amino acids of the protein extract were aspartic acid 10.7 %, glutamic acid 9.0 % and alanine 10.5 %. It is noteworthy that the alanine content in the protein extract was 36 % higher compared to crude microalga.
Discussion
Total nitrogen content obtained by elemental analysis was required to calculate total protein content and, then through it, the exact percentage of hydro-soluble and non-hydro-soluble proteins content of the biomass. Surprisingly, total protein content (56 %) was higher than the results (34 %) reported by Fuentes et al. (2000) and (39 %) Becker (1994), where the standard conversion factor 6.25 was used. This is probably due to the growing conditions of the microalga or to the standard conversion factor used, which can overestimates the exact protein content. For instance, multiple studies (Lourenço et al. 1998, 2004; Mariotti et al. 2008; González López et al. 2010) recommended a conversion factor lower than the standard 6.25.
Furthermore, cell disruption was not necessary since P. cruentum does not have a cell wall, and instead, it is encapsulated with a fragile polysaccharide layer surrounding the cell. Thus, the extraction of the internal proteins was not mechanically hindered. Only the chemical environment controls the extraction process. Hence, extraction of proteins was optimal at pH 12 since it gave similar results at pH 14, 1.4 times higher than pH 10 and 6.5 times higher than pH 7. Therefore, pH 12 was sufficient to extract the maximum amount of proteins that have been quantified by the Lowry method. This colorimetric method is considered as one of the most accurate methods to quantify proteins. Nevertheless, it takes only into consideration hydro-soluble proteins (Crossman et al. 2000; Diniz et al. 2011), which represents the major part of proteins present in the microalga. In our case, soluble protein content (84 %) was higher than results reported by González López et al. (2010), where it was estimated to represent 75 % of total protein. The variability observed in total and hydro-soluble protein content, compared to literature values, depends on environmental and culture conditions, which are key factors for the growth of the microalga and its nutrient content. Moreover, the high value for hydro-soluble proteins is explained by the possible presence of small peptides and free amino acids in the reaction with Lowry's reagents (Barbarino and Lourenço 2005). Protein nutritional quality for food consumption is evaluated by the amino acid profile and by the proportion and availability of amino acids (Becker 1994). It should be noted that plants are capable of synthesising essential and non-essential amino acids, while humans and other animals are only capable of synthesising non-essential amino acids, and the rest are obtained from food (Mahan and Escott-Stump 2004). P. cruentum shows an interesting combination of ten essential and nine non-essential amino acids in its proteins. A difference has been detected between the fractions of amino acids of the crude biomass and its protein extract, indicating that the extractable hydro-soluble proteins have a different primary structure. Fifteen out of 19 amino acids of the protein extract showed statistical significant difference (α = 0.05) compared to the amino acid profile of the crude biomass (Table 1). There was no high difference between the total percentage of essential and non-essential amino acids of crude P. cruentum (Table 2) and its protein extract, proving again that the cell wall of this microalga was not a barrier that prevents the extraction of intracellular proteins.
Furthermore, the fragility of its pseudo-cell wall favours the digestibility of the proteins compared to C. vulgaris and Scenedesmus sp., which are considered as important protein sources but have a rigid cell wall mainly composed of cellulose. Hence, P. cruentum does not require a previous unit operation to liberate the internal proteins.
The protein quality assessed in this study compares favourably to standard protein food recommended by WHO and FAO (Becker 1994), and it can be considered as an additional key element for this unicellular microalga, and together with its remarkable richness in polyunsaturated fatty acids (Berge et al. 2002) accompanied by its anticancer and immunomodulating activities of the phycobiliproteins (Bermejo Roman et al. 2002) and its high content in polysaccharides (Fuentes et al. 2000), it could make P. cruentum a special unconventional food source that covers all the necessary nutritional primary requirements. Of course, other aspects, such as toxicity, must be evaluated to give a final assessment on appropriateness for human consumption.
References
Adda M, Merchuk JC, Arad S (1986) Effect of nitrate on growth and production of cell-wall polysaccharide by the unicellular red alga Porphyridium. Biomass 10:131–140
Arad S, Adda M, Cohen E (1985) The potential of production of sulfated polysaccharides from Porphyridium. Plant Soil 89:117–127
Arad S, Friedman O, Rotem A (1988) Effect of nitrogen on polysaccharide production in a Porphyridium sp. Appl Environ Microbiol 54:2411–2414
Barbarino E, Lourenço SO (2005) An evaluation of methodologies for extraction and quantification of protein of marine macro-and microalgae. J Appl Phycol 17:447–460
Becker EW (1994) Microalgae biotechnology and microbiology. Cambridge University Press, Cambridge, p 180
Berge JP, Debiton E, Dumay J, Durand P, Barthomeuf C (2002) In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J Agric Food Chem 50:6227–6232
Bermejo Roman RB, Alvarez-Pez JM, Acien Fernandez FG, Molina Grima E (2002) Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J Biotechnol 93:73–85
Cohen Z, Shiran D, Khozin I, Heimer YM (1997) Fatty acid unsaturation in the red alga Porphyridium cruentum. Is the methylene-interrupted nature of polyunsaturated fatty acids an intrinsic property of the desaturases? BBA-Lipids Lipid Met 1344:59–64
Crossman DJ, Clements KD, Cooper GJS (2000) Determination of protein for studies of marine herbivory: a comparison of methods. J Exp Mar Biol Ecol 244:45–65
Diniz GS, Barbarino E, Oiano-Neto J, Pacheco S, Lourenço SO (2011) Gross chemical profile and calculation of nitrogen-to-protein conversion factors for five tropical seaweeds. Am J Plant Sci 2:287–296
Ducret A, Sidler W, Frank G, Zuber H (1994) The complete amino acid sequence of R-phycocyanin-I α and β subunits from the red alga Porphyridium cruentum. Eur J Biochem 221:563–580
Durmaz Y, Monteiro M, Bandarra N, Gkpinar O (2007) The effect of low temperature on fatty acid composition and tocopherols of the red microalga, Porphyridium cruentum. J Appl Phycol 19:223–227
FAO (2008) A review on culture, production and use of Spirulina as food for humans and feeds for domestic animals and fish. FAO Fish Aquacult, Rome, p 20
Fradique M, Batista AP, Nunes MC, Gouveia L, Bandarra NM, Raymundo A (2010) Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: preparation and evaluation. J Sci Food Agric 90:1656–1664
Fuentes MM, Fernández GG, Pérez JA, Guerrero JJ (2000) Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem 70:345–353
Geresh S, Arad S (1991) The extracellular polysaccharides of the red microalgae: chemistry and rheology. Bioresour Technol 38:195–201
Geresh S, Mamontov A, Weinstein J (2002) Sulfation of extracellular polysaccharides of red microalgae: preparation, characterization and properties. J Biochem Biophys Methods 50:179–187
Ginzberg A, Cohen M, Sod-Moriah UA, Shany S, Rosenstrauch A, Arad SM (2000) Chickens fed with biomass of the red microalga Porphyridium sp. have reduced blood cholesterol level and modified fatty acid composition in egg yolk. J Appl Phycol 12:325–330
González López CV, García MDCC, Fernández FGA, Bustos CS, Chisti Y, Sevilla JMF (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591
Heaney-Kieras J, Chapman D (1976) Structural studies on the extracellular polysaccharide of the red alga, Porphyridium cruentum. Carbohyd Res 52:169–177
Hemerick G (1973) Culture methods and growth measurements. In: Stein JR (ed) Handbook of phycological methods. Cambridge University Press, Cambridge, pp 259–260
Khozin-Goldberg I, Yu H, Adlerstein D, Didi-Cohen S, Heimer Y, Cohen Z (2000) Triacylglycerols of the red microalga Porphyridium cruentum can contribute to the biosynthesis of eukaryotic galactolipids. Lipids 35:881–889
Ley AC, Butler WL, Bryant DA, Glazer AN (1977) Isolation and function of allophycocyanin B of Porphyridium cruentum. Plant Physiol 59:974–980
Loubiere K, Pruvost J, Aloui F, Legrand J (2011) Investigations in an external-loop airlift photobioreactor with annular light chambers and swirling flow. Chem Eng Res Des 89:164–171
Lourenço SO, Barbarino E, Marquez UML, Aidar E (1998) Distribution of intracellular nitrogen in marine microalgae: basis for the calculation of specific nitrogen-to-protein conversion factors. J Phycol 34:798–811
Lourenço SO, Barbarino E, Lavan PL, Lanfer Marquez UM, Aidar E (2004) Distribution of intracellular nitrogen in marine microalgae: calculation of new nitrogen-to-protein conversion factors. Eur J Phycol 39:17–32
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mahan LK, Escott-Stump S (2004) Krause's Food, nutrition and diet therapy. Saunders Elsevier, Pennsylvania, p 66
Mariotti F, Tomé D, Mirand PP (2008) Converting nitrogen into protein: beyond 6.25 and Jones' factors. Crit Rev Food Sci 48:177–184
Moore S, Stein WH (1948) Photometric ninhydrin method for use in the chromatography of amino acids. J Biol Chem 176:367–388
Patil V, Källqvist T, Olsen E, Vogt G, Gislerød H (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult Int 15:1–9
Shiran D, Khozin I, Heimer Y, Cohen Z (1996) Biosynthesis of eicosapentaenoic acid in the microalga Porphyridium cruentum: the use of externally supplied fatty acids. Lipids 31:1277–1282
WHO (2007) Protein and amino acid requirements in human nutrition. World Health Organisation, Geneva, p 284
Acknowledgments
The authors would like to thank Alpha Biotech for providing the biomass. This work was supported by the French National Research Agency (ANR) in the context of Algoraffinerie project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safi, C., Charton, M., Pignolet, O. et al. Evaluation of the protein quality of Porphyridium cruentum . J Appl Phycol 25, 497–501 (2013). https://doi.org/10.1007/s10811-012-9883-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9883-4