Abstract
Species of Gracilaria are some of the most useful algae in the world for the production of agar. As a consequence of its economic importance, the genus has been the subject of many studies worldwide. Color variants of Gracilaria birdiae have been found in the natural population on the Brazilian coast, and they have also been isolated from plants cultivated in laboratory. These findings raised new questions regarding intraspecific variation and the prospects of cultivating such variants for their agar production. Therefore, this work aimed to determine the mode of color inheritance for two G. birdiae strains: a greenish-brown strain (gb) found in a natural population and a green strain (gr) which had arisen as a spontaneous mutation in a red plant cultured in the laboratory. The pigment contents of these strains, as well as the red wild-type (rd), were also characterized. Crosses between female and male plants of the same color (rd, gr, or gb) and between different colors were performed. Crosses between plants of the same color showed tetrasporophytic and gametophytic descendents of the parental color. Recessive nuclear inheritance was found in the greenish-brown strain, and cytoplasmic maternal inheritance was found in the green strain; both had lower phycoerythrin and higher concentrations of allophycocyanin and phycocyanin than the wild-type. Chlorophyll a contents were similar among all strains. Taken together, our results contribute to knowledge about the variability of this important red algae. In addition, since greenish-brown and green strains showed stability of color, both could be selected and tested in experimental sea cultivation to evaluate if mutants have advantageous performance when compared with red strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of Gracilaria (Gracilariales, Rhodophyta) are some of the most useful algae in the world for the production of the polysaccharide agar (Kain and Destombe 1995; Oliveira et al. 2000). Recently, other uses have been suggested, such as abalone feed (Rothman et al. 2009). As a consequence of its economic importance, the genus has been the subject of many studies worldwide (Oliveira and Plastino 1994).
On the Brazilian coast, Gracilaria birdiae Plastino & E.C. Oliveira is the main raw material exploited for agar production (Plastino and Oliveira 2002). However, attempts at mariculture have failed in past decades, partly by the absence of previous studies in control conditions. Consequently, G. birdiae has been the subject of several investigations. Particularly, in order to study the phenotypic plasticity promoted by acclimation and adaptation processes the wild-type (red) and three color variants (greenish-brown, green, and light green) were isolated in our laboratory. The greenish-brown strain was collected from a natural population on the northeastern coast of Brazil, and a green strain originated from in vitro propagation of apical segments isolated from a green branch, which had arisen as a spontaneous mutation in a red plant (Costa and Plastino 2001). The light-green strain (deficient in both chlorophyll a and phycobiliprotein), which was collected from a natural population on the southeastern coast of Brazil, showed stability of color and a recessive nuclear transmission (Plastino et al. 2004). The ultrastructure has been described for both the wild-type and light-green strain. The wild-type showed the typical internal organization of the red algae (Plastino and Costa 1999). Although the light-green strain had similar general characteristics, it exhibited a distinct plastid organization, which was characterized by the few thylakoids surrounding one to many translucent round inclusions (Plastino and Costa 2001). Compared to wild-type, the light-green strain showed lower growth rates (Plastino et al. 2004). The best culture conditions in the laboratory for growth and reproduction were provided (Ursi and Plastino 2001; Ursi et al. 2008), making it possible to complete the life history of wild-type, greenish-brown, and green strains of G. birdiae in a laboratory culture (Costa and Plastino 2001). The photosynthetic, respiratory, and carotenoid concentrations were also investigated for these strains (Ursi et al. 2003).
A few color strains have been reported in natural populations of Gracilaria, including G. birdiae (Plastino et al. 2004), Gracilaria chilensis C.J. Bird, McLachlan & E.C. Oliveira (Santelices et al. 1996), Gracilaria cornea J. Agardh (Ferreira et al. 2006), Gracilaria domingensis (Kützing) Sonder ex Dickie (Plastino et al. 1999), and Gracilaria tikvahiae McLachlan (Ramus and van der Meer 1983). Three of them were collected from the Brazilian coast where a green strain of Hypnea musciformis (Wulfen) Lamouroux also has been reported (Yokoya et al. 2003). However, most color strains reported for red algae have arisen spontaneously in culture or were induced by chemical mutagenesis (see van der Meer 1990 for a review).
Color mutants have been used as visual markers to study spore coalescence (Santelices et al. 1996), life history (van der Meer 1987), mixed-phase reproduction (van der Meer 1986), pigment and phycobilisome composition (Kursar et al. 1983; Ramus and van der Meer 1983), and construction of polyploids (van der Meer 1981; Patwary and van der Meer 1984). Aside from their importance in the investigation of various biological processes, some of these strains have also been selected for commercial cultivation (Patwary and van der Meer 1992).
The stability and the mode of color inheritance were investigated mainly in strains of G. tikvahiae (van der Meer 1990) or, in a few cases, in G. birdiae (Plastino et al. 2004), G. domingensis (Plastino et al. 1999; Guimarães et al. 2003), and Gracilaria foliifera (Forsskål) Børgesen (van der Meer and Zhang 1988). Nuclear color transmission has been found in most taxa studied (van der Meer 1990; Plastino et al. 1999, 2004), although cytoplasmatic color transmission has also been observed (van der Meer 1978; Zhang and van der Meer 1988). While strain color variation has been determined to be recessive (e.g., van der Meer and Bird 1977; Zhang and van der Meer 1987; Plastino et al. 2004), both dominant and co-dominant nuclear transmissions have been reported (van der Meer and Todd 1977; van der Meer 1979; Plastino et al. 1999).
The aim of this work was to determine the mode of color inheritance in two color strains of G. birdiae, a greenish-brown strain found in a natural population and a green strain produced in the laboratory. The pigment contents of these strains were also characterized.
Material and methods
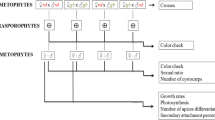
Gracilaria birdiae was collected from Paracuru Beach (3.4°S 39.07°W), Ceará State, Brazil, in March 1994. Red and greenish-brown individuals grow side by side in sandstone reef pools and are usually exposed during low tides. Branches of a greenish-brown female gametophyte with cystocarps and greenish-brown and red tetrasporophytes were transported to the laboratory, and unialgal cultures were established from apical segments, as described by Plastino and Oliveira (1990). Cultures were maintained in von Stosch-enriched seawater with modification (Ursi and Plastino 2001), diluted to 25% with sterile seawater (32 psu), and kept in a temperature-controlled room at 25 ± 1°C, under 100 μmol photons m−2 s−1 PAR provided by 40-W daylight fluorescent tubes, and on a 14-h light/10-h dark cycle. Another photoperiod was tested to induce tetrasporangium differentiation (10 h light/14 h dark cycle). Cultures were aerated for 30 min h−1, and the medium was renewed weekly. Voucher specimens were deposited in the herbarium of the University of São Paulo (SPF: 27930-33; 28049-53; 56492-94).
Color inheritance
Cultures of greenish-brown (gb) and red strains (rd; female and male gametophytes) were established from tetraspores obtained from a field-collected red tetrasporophyte. Cultures of green (gr) strain (female and male gametophytes) were established from tetraspores released by green apical segments isolated from a green sector which had spontaneously arisen on a red tetrasporophyte obtained in the laboratory from a rd female crossed with a gb male (Costa and Plastino 2001).
Tips cut from female and male rd, gb, and gr specimens were kept isolated for at least 2 months to ensure the absence of fertilized carpogonia before being utilized in the crosses.
Crosses between female and male individuals of the same color (rd, gr, or gb) and different colors (rd female X gb male, rd female X gr male, gb female X rd male, gb female X gr male, gr female X rd male, and gr female X gb male) were performed to determine the color inheritance pattern. During the experimental period, female branches of each color were kept isolated from male branches to check for the existence of hermaphroditic or parthenogenetic plants. For the crosses (n = 3), female and male branches were incubated together until cystocarps appeared on the female plants (14 days). The male branches were then removed and the female branches were cultured until the carpospores matured and were released. Carpospore liberation took 35 days.
Carpospores resulting from crosses were collected and grown in order to obtain fertile tetrasporophytes, as well as to verify color ratios. To induce tetrasporangium differentiation, tetrasporophytes were cultured in a 10-h light/14-h dark cycle. Fertile tetrasporophytes originating from each cross type were selected, and tetraspores were cultured in order to generate fertile gametophytes (Table 1 shows the numbers of cultivated plants). The color and sex ratios were then analyzed.
A chi-square (χ 2) test with Yates correction was applied to analyze sex and color ratio results in the gametophytic generation.
Pigment analysis
Pigment analysis was carried out on apical fragments from three female gametophytes of each color type (rd, gb, and gr). They were cultivated in the standard culture conditions in separate flasks with 0.9 L of enriched seawater for 2 months. The algae were weighed weekly, and the fresh biomasses were reduced at 0.3 g per flask, removing the basal parts of the branches. Both phycobiliprotein and chlorophyll a (Chl a) were analyzed from the same sample by spectrophotometry. Pigment extractions were carried out at 4°C, according to Kursar et al. (1983) as modified by Plastino and Guimarães (2001). Briefly, 300 mg of tissue were disrupted by grinding with liquid nitrogen and 50 mmol L-1 phosphate buffer, pH 5.5. Crude extracts were centrifuged at 36,000×g for 25 min to obtain the phycobiliproteins. Chl a was extracted after dissolving the pellet in 90% acetone and then centrifuged at 12,000×g for 15 min. Pigment concentration was calculated according to Kursar et al. (1983) for phycobiliproteins [phycoerythrin (PE), phycocyanin (PC), and alophycocyanin (APC)] and for Chl a, according to Jeffrey and Humphrey (1975). All pigment extractions were performed in triplicate.
Pigment concentrations were compared by one-way analysis of variance (ANOVA). A posteriori Newman–Keuls test was used to establish statistical differences. Statistical analyses were done using the Statistica 7 program.
Results
Color inheritance
All cross combinations originated cystocarps. No cystocarps were formed in the absence of males. Crosses between female and male of the same color strain showed tetrasporophytic and gametophytic descendents of the parental color (Table 1).
Carpospores resulting from all crosses showed red color. Carpospores resulting from crosses between green gametophytes developed into small light-red basal disks that soon gave rise to erect axes. Carpospores from other crosses gave rise to red basal disks. A strong color differentiation was observed only when the plantlets were 4–5 mm long and 60 days old (green individuals) or 3 cm long and 100 days old (greenish-brown individuals).
Tetrasporophytes resulting from the germination of carpospores and produced from the following crosses remained red: rd female X gb male, gb female X rd male, and rd female X gr male. Tetrasporophytes that originated from carpospores of the cross between gb female and gr male became greenish-brown, while those tetrasporophytes originating from the following crosses became green: gr female X rd male and gr female X gb male (Table 1).
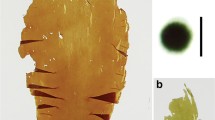
Tetrasporophytes resulting from all crosses showed “in situ” tetraspore germination. These structures developed into spherical or tetrahedral plantlets (Fig. 1), which differentiated erect branches later. The color of epiphytic gametophytes varied according to the cross from which the tetrasporophyte originated. Therefore, in some plants, different reproductive structures and colors appeared together.
Red tetrasporophytes that originated from the following crosses gave rise to red plantlets that developed red and greenish-brown gametophytes in a 1:1 proportion: rd female X gb male, rd female X gr male, and gb female X rd male. Greenish-brown tetrasporophytes that originated from the cross between gb female and gr male produced red plantlets, which developed into infertile greenish-brown plants. Green tetrasporophytes that originated from the cross between gr female and rd male gave rise to green plantlets, which developed into infertile green plants. Green plants that originated from the cross between gr female and gb male did not differentiate tetrasporangia (Table 1).
Independent of the thallus color, male and female gametophytes were obtained in a 1:1 proportion (rd, eight females–nine males, χ 2 = 0.058, P > 0.05, df = 1; gb, nine females–ten males, χ 2 = 0.052, P > 0.05, df = 1). Color and sex ratio of plants of different crosses are shown in Table 1. The occurrence of infertile individuals was greater in the green strain.
Pigment analysis
Since all pigments were observed in all strains, albeit with different concentrations, no qualitative differences were evident. The phycobiliprotein extracts of all strains had absorption peaks at 494, 564, and 614 nm (Fig. 2), whereas the Chl a extracts had absorption peaks at 494 and 564 nm (Fig. 3).
The red gametophytes had higher concentrations of PE than greenish-brown and green gametophytes, whereas the latter had lower concentrations of this pigment than greenish-brown gametophytes (Fig. 4, F = 198.407, p < 0.001). The green gametophytes had higher concentrations of PC and APC than greenish-brown and red gametophytes, whereas the latter had lower concentrations of these pigments than greenish-brown gametophytes (Fig. 4, PC (F = 36.532, p < 0.001) and APC (F = 46.046, p < 0.001)). No differences in concentration of Chl a were found among the three strains (Fig. 4, F = 4.561, p = 0.062).
Pigment concentrations of red (rd), greenish-brown (gb), and green (gr) individuals of Gracilaria birdiae. Bars indicate standard deviations (n = 3). Treatments with different letters indicate significant differences according to one-way ANOVA and Newman–Keuls test (P < 0.05). (Cl a) Chlorophyll a; (APC) allophycocyanin; (PC) phycocyanin; and (PE) phycoerythrin
Discussion
This study shows that both greenish-brown and green colors of G. birdiae are stable and genetically determinant. All spores were red and the color segregation only became apparent in older plantlets as observed in some color strains of G. tikvahiae and G. domingensis (van der Meer and Bird 1977; Plastino et al. 1999). However, in the light-green strain of G. birdiae, the color was already apparent in the spores (Plastino et al. 2004).
The greenish-brown phenotype of G. birdiae is recessive in relation to the phenotype that provides the red color because red is expressed in the heterozygous condition. This recessive nuclear transmission has been reported for the majority of color strains of red algae investigated to date (van der Meer 1990). Similar results were previously reported for the light-green strain of G. birdiae (Plastino et al. 2004).
The green phenotype of G. birdiae showed uniparental inheritance. Only female gametophytes expressed green color in the descendants. These results are in agreement with a cytoplasmic inheritance, as already described for other organisms (Kuroiwa and Uchida 1996). This pattern of maternal transmission is characteristic of organellar genes, and it was first reported for a multicellular marine alga in a color strain of G. tikvahiae. In this case, the action of chloroplast genes in pigment inheritance was suggested (van der Meer 1978). Since some previous studies reported the absence of these organelles in spermatia of Gracilaria, we also propose that the green color of G. birdiae is transmitted by chloroplast genes (Ryan and Nelson 1991; Bouzon et al. 2000). Furthermore, the discovery that most of the phycobilisome components in red algae were encoded by the chloroplast genome corroborates this hypothesis (Egelhoff and Grossman 1983; Apt and Grossman 1993; Reith and Munholland 1995)
The results of crosses between gb or rd female and gr male of G. birdiae suggested that the gr male employed in the crosses had a double mutation with organellar genome for the green color and nuclear genome for the greenish-brown color. The occurrence of a spontaneous cytoplasmic mutation in a somatic sector of a heterozygous tetrasporophyte, resulting in double mutants in the gametophytic generation has already been reported in G. tikvahiae (van der Meer 1977). As in G. birdiae, the mutation occurred in a tetrasporophyte with wild phenotype, but heterozygous for a Medelian recessive allele. The similarity of these events in both G. birdiae and G. tikvahiae suggests that spontaneous mutations may be more common than reported in the literature and may be, in part, the font of the chromatic variability observed in natural populations.
The pigment quantification did not reveal any differences in Chl a concentration among red, greenish-brown, and green strains of G. birdiae, indicating the absence of any alterations in these genes. However, both mutants are deficient in PE, and they both showed higher levels of APC and PC. These findings are in agreement with previous descriptions for other green variants of red algae (van der Meer and Bird 1977; Yokoya et al. 2003).
If the greenish-brown and green strains of G. birdiae had independent PE deficiencies determined by nuclear and cytoplasmic genes, respectively, the higher content of APC and PC in these strains could be a result of acclimation. It was reported that the photosynthetic units in G. tikvahiae are quiet plastics and that its plasticity is expressed as changes in light capturing pigment densities (Ramus and van der Meer 1983). This plasticity is related to a chromatic acclimation at high and low light intensities. Because both mutants of G. birdiae showed phycoerythrin deficiency, a stimulus in the phycobiliprotein synthesis would cause an increase only in PC and APC contents. An increment in these pigment concentrations could be explained by a photoacclimation mechanism that would result in the optimization absorbed light energy to mask the effects of low PE content. Higher values of PC and APC could explain the higher photosynthetic efficiency observed in green and greenish-brown strains when previously compared with the red strain (Ursi et al. 2003); these PE-deficient strains also showed similar or higher growth rates (Ursi 2005).
Although another photoperiod was tested, the infertility observed in green plants resulting from the germination of carpospores and produced from the cross between gr female and gb male could not be reverted. Moreover, green gametophytes that originated from the cross between gr female and rd male did not differentiate tetrasporangia. Therefore, it is plausible that this low fertility could, in part, explain why the green plants of G. birdiae have never been found in the natural population. In contrast, the greenish-brown plants showed higher fertility in laboratory conditions and were found in the field as part of the intertidal populations. In fact, the greenish-brown color may represent an adaptive advantage for G. birdiae at the population level, considering that these plants are frequently exposed to full sunlight and, under these environmental conditions, the loss of phycoerythrin may not be particularly disadvantageous.
In conclusion, since the greenish-brown and green strains showed stability of color, both could be selected and tested in experimental sea cultivation to evaluate if mutants have advantageous performance when compared with red strain. Although PE-deficient, they presented higher values of PC and APC, which apparently compensates for the lack of PE. Further studies could assess the frequencies of the greenish-brown variant in intertidal and subtidal populations and, hence, the possible advantages to the species in maintaining this variation. Finally, genetic differentiation between populations could be studied using neutral polymorphic genetic markers to estimate the gene flow along the coast.
References
Apt KE, Grossman AR (1993) Characterization and transcript analysis of the major phycobiliprotein subunit genes from Aglaeothamnion neglectum (Rhodophya). Plant Mol Biol 21:27–38
Bouzon ZL, Miguens F, Oliveira EC (2000) Male gametogenesis in the red algae Gracilaria and Gracilariopsis (Rhodophyta, Gracilariales). Cryptogam Algol 21:33–47
Costa VL, Plastino EM (2001) Histórico de vida de espécimes selvagens e variantes cromáticas de Gracilaria birdiae (Gracilariales, Rhodophyta). Rev Bras Bot 24(suppl):491–500
Egelhoff T, Grossman AR (1983) Cytoplasmic and chloroplast synthesis of phycobilissome polypeptides. Proc Natl Acad Sci USA 80:3339–3343
Ferreira LB, Barufi JB, Plastino EM (2006) Growth of red and green strains of the tropical agarophyte Gracilaria cornea (Gracilariales, Rhodophyta) in laboratory. Rev Bras Bot 29:185–190
Guimarães M, Plastino EM, Destombe C (2003) Green mutant frequency in natural populations of Gracilaria domingensis (Gracilariales, Rhodophyta) from Brazil. Eur J Phycol 38:165–169
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c 1 and c 2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanzen 167:191–194
Kain JM, Destombe C (1995) A review of life history, reproduction and phenology of Gracilaria. J Appl Phycol 7:269–281
Kuroiwa T, Uchida H (1996) Organelle division and cytoplasmic inheritance—the origin and basis of the transmission of organelle genomes. Bioscience 46:827–835
Kursar TA, van der Meer J, Alberte RS (1983) Light-harvesting system of the red alga Gracilaria tikvahiae. I. Biochemical analyses if pigment mutations. Plant Physiol 73:353–360
Oliveira EC, Plastino EM (1994) Gracilariaceae. In: Akatsuka I (ed) Biology of economic algae. SPB Academic, The Hague, pp 185–226
Oliveira EC, Alveal K, Anderson R (2000) Mariculture of agar-producing gracilarioid red algae. Rev Fish Sci 8:345–378
Patwary MU, van der Meer JP (1984) Growth experiments on autopolyploids of Gracilaria tikvahiae (Rhodophyceae). Phycologia 23:21–27
Patwary MU, van der Meer JP (1992) Genetic and breeding of cultivated seaweeds. Korean J Phycol 7:281–318
Plastino EM, Costa VL (1999) Ultrastructure of vegetative branches of the red macroalga Gracilaria birdiae (Gracilariales). Acta Microsc 8(suppl):793–794
Plastino EM, Costa VL (2001) Anomalous plastids in a light green strain of the red macroalga Gracilaria sp. (Gracilariales). Acta Microsc 3(Sup. C):315–316
Plastino EM, Guimarães M (2001) Diversidad Intraespecifica. In: Avelã KV, Antezana TJ (eds) Sustentabilidad de la Biodiversidad. Universidad de Concepción, Concepción, pp 19–27
Plastino EM, Oliveira EC (1990) Crossing experiments as an aid to the taxonomic recognition of the agarophytes Gracilaria. In: Oliveira EC, Kautsky N (eds) Cultivation of seaweeds in Latin America. Universidade de São Paulo, São Paulo, pp 127–133
Plastino EM, Oliveira EC (2002) Gracilaria birdiae (Gracilariales, Rhodophyta), a new species from the tropical South American Atlantic with terete frond and deep spermatangial conceptacles. Phycologia 41:389–396
Plastino EM, Guimarães M, Matioli SR, Oliveira EC (1999) Codominant inheritance of polymorphic color colors of Gracilaria domingensis (Gracilariales, Rhodophyta). Gen Mol Biol 22:105–108
Plastino EM, Ursi S, Fujii MT (2004) Color inheritance, pigment characterization, and growth of a rare light green strain of Gracilaria birdiae (Gracilariales, Rhodophyta). Phycol Res 52:45–52
Ramus J, van der Meer JP (1983) A physiological test of the theory of complementary chromatic adaptation. I. Color mutants of a red seaweed. J Phycol 19:86–91
Reith ME, Munholland J (1995) Complete sequence of the Porphyra purpurea chloroplast genome. Plant Mol Biol Report 13:333–335
Rothman MD, Anderson RJ, Boothroyd CJT, Kemp FA, Bolton JJ (2009) The gracilarioids in South Africa: long-term monitoring of a declining resource. J Appl Phycol 21:47–53
Ryan KG, Nelson WA (1991) Comparative study of reproductive development in two species of Gracilaria (Gracilariales, Rhodophyta). I—spermatogenesis. Crypt Bot 2/3:229–233
Santelices B, Correa JA, Meneses I, Aedo D, Varela D (1996) Sporeling coalescence and intraclonal variation in Gracilaria chilensis (Gracilariales, Rhodophyta). J Phycol 32:313–322
Ursi S (2005) Diversidade intraespecífica em Gracilaria birdiae (Gracilariales, Rhodophyta): crescimento, fotossíntese, pigmentos, polissacarídeos e genes da ficoeritrina de linhagens selvagens e variantes. Tese. São Paulo: Universidade de São Paulo
Ursi S, Plastino EM (2001) Crescimento in vitro de linhagens de coloração vermelha e verde clara de Gracilaria sp. (Gracilariales, Rhodophyta) em dois meios de cultura: análise de diferentes estádios reprodutivos. Rev Bras Bot 24:587–594
Ursi S, Pedersén M, Plastino EM, Snoeijs P (2003) Intraspecific variation of photosynthesis, respiration and photoprotective carotenoids in Gracilaria birdiae (Gracilariales, Rhodophyta). Mar Biol 142:997–1007
Ursi S, Guimarães M, Plastino EM (2008) Deleterious effect of TRIS buffer on growth rates and pigment contents of Gracilaria birdiae Plastino & E.C. Oliveira (Gracilariales, Rhodophyta). Acta Bot Bras 22:891–896
van der Meer JP (1977) Genetics of Gracilaria birdiae (Rhodophyceae, Gigartinales). II. The life history and genetic implications of cytokinetic failure during tetraspore formation. Phycologia 16:367–371
van der Meer JP (1978) Genetics of Gracilaria birdiae (Rhodophyceae, Gigartinales). III. Non-mendelian gene transmission. Phycologia 17:314–318
van der Meer JP (1979) Genetics of Gracilaria tikvahiae (Rhodophyceae, Gigartinales). V. Isolation and characterization of mutant strains. Phycologia 18:47–54
van der Meer JP (1981) Genetics of Gracilaria tikvahiae (Rhodophyceae). VII. Further observations and construction of polyploids. Can J Bot 59:787–792
van der Meer JP (1986) Genetics of Gracilaria tikvahiae (Rhodophyceae). XI. Further characterization of a bisexual mutant. J Phycol 22:151–158
van der Meer JP (1987) Marine algal genetics and genomes: using genetic markers in phycological research. Hydrobiologia 151/152:49–56
van der Meer JP (1990) Genetics. In: Cole K, Sheath R (eds) Biology of the red algae. Cambridge University Press, New York, pp 103–121
van der Meer JP, Bird NL (1977) Genetics of Gracilaria birdiae (Rhodophyceae, Gigartinales). I. Mendelian inheritance of two spontaneous green colors. Phycologia 16:159–161
van der Meer JP, Todd ER (1977) Genetics of Gracilaria sp. (Rhodophyceae, Gigartinales). IV. Mitotic recombination and its relationship to mixed phases in the life history. Can J Bot 55:2810–2817
van der Meer JP, Zhang X (1988) Similar unstable mutations in three species of Gracilaria (Rhodophyta). J Phycol 24:198–202
Yokoya NS, Plastino EM, Artel R (2003) Physiological responses and pigment characterization of two colour strains of the carrageenophyte Hypnea musciformis (Rhodophyta). In: Chapman ARO, Anderson RJ, Vreeland VJ, Davison R (eds) Proceedings of the 17th International Seaweed Symposium. Oxford University Press, New York, pp 425–433
Zhang X, van der Meer JP (1987) A study on heterosis in diploid gametophytes of marine red alga Gracilaria tikvahiae. Bot Mar 30:309–314
Zhang X, van der Meer JP (1988) A genetic study Gracilaria sjoestedtii. Can J Bot 66:2022–2026
Acknowledgments
This research was supported by FAPESP (98/11943-1; 00/12801-0; 10/00017-2) and CNPq (132845/98-9; 300148/93-3). The authors acknowledge Rosário Petti for assistance on cultivation and Maria Lucia Kawasaki for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, V.L., Plastino, E.M. Color inheritance and pigment characterization of red (wild-type), greenish-brown, and green strains of Gracilaria birdiae (Gracilariales, Rhodophyta). J Appl Phycol 23, 599–605 (2011). https://doi.org/10.1007/s10811-010-9642-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9642-3