Abstract
Microalgae play an important role during the tertiary treatment of municipal wastewater. Cell immobilization techniques have been developed in order to improve the quality of the treated wastewater and avoid wash out of the biomass. Since cell immobilization method may affect the nutrient removal efficiency, ten strains of microalgae were immobilized in sodium alginate gel in different-diameter circular screens, and orthophosphate removal efficiency from municipal wastewater was studied. Results indicate that the alginate immobilization screen size and contact surface with wastewater affects the microalgae synthesis activity and thus orthophosphate removal efficiency. Increasing the contact surface by making smaller alginate screens will increase the cation exchange rate and reduce the orthophosphate concentration in the medium. Among all microalgae treatments, Scenedesmus rubescens MCCS 018, Chlamydomonas sp. MCCS 026, and Chroococcus dispersus MCCS 006 had the highest PO 3-4 -P removal efficiency of 68.8%, 71.9%, and 72.3% within 12 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater treatment using microalgae has been studied widely and shown to be effective in nutrient removal (e.g., Aslan and Kapdan 2006; de Bashan et al. 2008; Zhang et al. 2008). The technology of using microalgae in wastewater treatment is based on natural ecosystems; thus it is not environmentally dangerous and even if the biomass produced is reused, causes no secondary pollution. Likewise, microalgae are highly effective at purifying the waste by producing oxygen and removing heavy metals and xenobiotic substances (Martínez et al. 2000).
Harvesting free cells from effluent is necessary to improve the quality of the treated wastewater and avoid wash out of the biomass which potentially can be used in food and pharmacy industries and/or as biogas (Rasoul-Amini et al. 2009; Muñoz and Guieysse 2006; Mallick 2002). Biomass immobilization is an efficient way of retaining biomass during wastewater treatment (Nicolella et al. 2000), and microalgae immobilization in polymeric material such as carrageenan, chitosan, or alginate has been reported (Chevalier and De la Noüe 1985; Lau et al. 1995; Robinson 1998). Alginate possesses the ideal immobilization matrix specification (Martinsen et al. 1989; Moreno-Garrido 2007; Moreira et al. 2006). Immobilization also enhances nutrient removal (Mallick 2002; Tam and Wong 2000; Jiménez-Pérez et al. 2004). Lau et al. (1997) immobilized Chlorella vulgaris in carrageenan and alginate to treat primary domestic wastewater. Free and immobilized cells removed about 95% and 50% of ammonium and 99% and 50% of phosphates from the wastewater in 3 days. Fierro et al. (2008) immobilized Scenedesmus sp. cells in chitosan. Free-living cells removed 20% and 30% of nitrates and phosphates within 36 h while immobilized cells removed 70% of nitrate and 94% of phosphate within 12 h of treatment. The physical shape of the immobilization system’s beads or screens should be considered as a technical parameter in this technologically oriented field (de Bashan and Bashan 2010). Kaya (1995) investigated the capacity of Scenedesmus bicellularis free and immobilized cells in alginate beads and screens to treat municipal wastewater. He found that higher nutrient removal efficiency was obtained by using immobilized cells on screens compared with free cells or bead-shaped alginate particles. Zhang et al. (2008) immobilized different cell density of Scendesmus sp. in calcium alginate screens of 2 or 3 mm thickness and measured nitrogen and phosphorus removal rates from artificial and real domestic secondary effluents. They included that cell density in gel is the key factor in the nutrient removal rather than gel thickness and that increasing cell density in the gel would decrease the removal efficiency.
The widely used microalgae cultures for nutrient removal are species of Chlorella, Scenedesmus, Chlamydomonas, and Phormidium (Aslan and Kapdan 2006; Mallick 2002). Alginate-immobilized C. vulgaris was more efficient than Scenedesmus bijugatus in removing N and P from wastewater (Megharaj et al. 1992). Shi et al. (2007) investigated nitrogen and phosphorus removal of C. vulgaris and Scenedesmus rubescens from secondary synthetic wastewater using the twin-layer immobilization system. These two algae removed ammonium, nitrate, and phosphate to less than 10% of their initial concentration within 9 days.
In this work, ten microalgae species were immobilized in sodium alginate gel in circular screens formed in different diameters, and orthophosphate removal efficiency from treated municipal wastewater of Shiraz were studied.
Materials and methods
Microorganism preparation
Microalgae were isolated during a screening program from soil samples collected from soil samples of paddy-fields of Fars province, south of Iran, from April to December 2004. Soil samples were suspended in a specific volume of distilled water. Surficial part (100 μL) was transferred to BG-11 solid culture medium (Borowitzka 1988), and Petri dishes were stored in a culture room under constant illumination (~25 μmol photons m−2 s−1) with white fluorescent lamps at 25 ± 2°C. After colonization, the isolation and purification were performed using plate agar method to obtain unialgal cultures (Ghasemi et al. 2003).
The algae were grown at room temperature in liquid BG-11 medium with shaking at 70 rpm. The taxonomic identification was done following the keys of Desikachary (1959) and John et al. (2003). In order to confirm and determine the species, the sequence of small subunit of rRNAs was studied using molecular markers. Genomic DNA of microalgal strains was prepared according to Rasoul-Amini et al. (2009). DNA fragments of ~800 and ~700 bp were amplified from genomic DNA of microalgal strains with polymerase chain reaction (PCR) by using universal primers against the 16S/18S rRNA genes, respectively. The forward 16S universal primer was 5′-CAGCCGCGGTAATAC-3′ and 5′-ACGGGCGGTGTG TAC-3′ using as the reverse primer (Billi et al. 2001). The universal eukaryotic primers 5′-GTCAGAGGTGAA ATTCTTGGATTTA-3′ as forward primer and 5′-AGGG CAGGGACGTAATCAACG-3′ as reverse primer, amplify a ~700-bp region of the 18S rRNA gene (Ghasemi et al. 2008). PCR amplifications were determined by 1% (w/v) agarose gel electrophoresis in Tris/Borate/EDTA buffer. PCR products were purified form agarose gel with the CoreBio PCR purification kit (Cat No. GE-100) and used as templates in sequencing reactions by CinnaGene Company. 16S/18S rRNA sequences were analyzed by using the BLAST program, and annotations of all microalgal strains were deposited in GeneBank (Table 1). Then, the isolated microalgae were kept in the liquid nitrogen and lyophilized in order to add into Microalgal Culture Collection of Shiraz University of Medical Science (MCCS) (Rasoul-Amini et al. 2010).
The so identified strains were C. vulgaris (MCCS 011, 013, 014, and 015), Synechococcus sp. (MCCS 034), Fischerella ambigua (MCCS 004), Oocystis sp. (MCCS 033), Chroococcus dispersus (MCCS 006), Chlamydomonas sp. (MCCS 026), and S. rubescens (MCCS 018; Table 1).
Cell immobilization
After 10 days of cultivation, cell number was determined using Neubauer hemocytometer, and 6 × 107 cells mL−1 of each strain were centrifuged at 2,000×g at 4°C for 25 min and washed twice in sterile saline (0.85% NaCl solution). The cells were suspended in 15 mL of saline and then mixed with 60 mL of 4% sodium alginate solution using magnetic stirrer for 15 min. Circular alginate screens were formed adding the algae–gel mixture to sterile 2% calcium chloride in a Petri dish for 1 h. For every replication of treatments, 20 mL of the alginate mixture was used. In the C. vulgaris MCCS 011, C. vulgaris MCCS 013, and C. vulgaris MCCS 014 treatments, this volume of gel formed one alginate screen with a height of 3–4 mm and mean diameter of 4.3 cm. For the other strains, 20 mL of the gel was divided and formed four smaller screens (5 mL of alginate mixture each) with a diameter of approximately 2.5 cm and 2–3 mm thickness. To investigate the effect of alginate, four blank alginate screens (5 mL of alginate gel each) were formed for every replication of alginate treatment (Table 1). Then, the screens were washed twice with sterile distilled water.
Municipal wastewater source and experimental condition
Wastewater from secondary effluent of Shiraz, Iran, wastewater refinery was collected, filtered, and then sterilized by autoclaving. Immobilized microalgae in alginate screens (ten strains/species) and also blank alginate screens were added to 500 mL of autoclaved wastewater in Erlenmayer flasks for every replication. The same volume of wastewater was added to flasks with no alginate screens and no microalgae as the Blank treatment. Then, the flasks were placed in culture room at 20 ± 1°C and irradiance of approx. 25 μmol photons m−2 s−1. All treatments and blank wastewater were in three replicates (12 treatments in total).
The experiment lasted 12 days. Using a sterile pipette, 10 mL of the wastewater was taken every 4 days for PO 3-4 -P measurement according to the Murphy–Riley method based on standard methods for the examination of water and wastewater (American Public Health Association 2005). The results were analyzed using F test at P ≤ 0.05.
Results
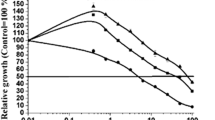
The concentration of PO 3-4 -P and percent of changes during every 4 days, and the whole period of the experiment are given in Tables 2 and 3 (positive sign indicates the decrease and negative sign indicates increase in PO 3-4 -P concentration). The trend of changes in measured data is shown in Fig. 1.
Orthophosphate concentrations of treatments in wastewater; a Blank, Alginate, F. ambigua MCCS 004; b Oocystis sp. MCCS 033, Synechococcus sp. MCCS 034, C. dispersus MCCS 006; c C. vulgaris MCCS 011, C. vulgaris MCCS 013, C. vulgaris MCCS 015; d C. vulgaris MCCS 014, S. rubescens MCCS 018, Chlamydomonas sp. MCCS 026. Error bars = mean ± SD, n = 3
The initial concentration of orthophosphate in the wastewater is 1.37 mg L−1 and decreased in almost all treatments to the minimum value of 0.38 mg L−1 in Chlamydomonas sp. MCCS 026 and 0.39 mg L−1 in C. dispersus MCCS 006 with the approximate removal efficiency of 72% over 12 days. A continuous increase and decrease in orthophosphate concentration occurred in Alginate and Blank, respectively, resulting in addition of 46% of PO 3-4 -P to and removal of 25% from the medium. In all treatments, except C. vulgaris MCCS 011, MCCS 013, and MCCS 014, orthophosphate concentration increases during the first 4 days and then decreases. In the C. vulgaris, MCCS 011, MCCS 013, and MCCS 014 treatments depletion of orthophosphate occurred over the whole period of the experiment.
For better comparison, orthophosphate removal rate was calculated by the following equation:
Where R P is PO 3-4 -P removal rate (mg L−1 day−1), C m is the maximum P concentration (mg L−1) during the experiment, C f is the final P concentration (mg L−1), t m is the time of maximum P concentration in the medium, and t f is the final day of the experiment. Results are given in Table 4. In Blank, C. vulgaris MCCS 011, MCCS 013, and MCCS 014 treatments, the duration of orthophosphate depletion is 12 days. Within this period, more orthophosphate is removed in the treatments containing microalgae in comparison with the Blank treatment. Among these four treatments, the highest and lowest removal rates are by C. vulgaris MCCS 011 (0.078 mg L−1 day−1) and Blank (0.028 mg L−1 day−1), respectively. In the treatment containing blank alginate screens, there is an increase in PO 3-4 -P concentration with the mean rate of 0.052 mg L−1day−1. In other microalgal treatments, the PO 3-4 -P removal period is 8 days, and the removal rate varies from the lowest 0.110 mg L−1day−1 for Oocystis sp. MCCS 033 to the highest 0.149 mg L−1day−1 for S. rubescens MCCS 018. The differences between the Microalgae treatments and Blank are statistically significant at the 5% level (Table 5).
Discussion
Wastewater samples were collected from secondary effluent of the wastewater treatment plant, before chlorination, so autoclaving was necessary to eliminate bacteria and pathogens. According to Hernandez et al. (2006), wastewater autoclaving causes the precipitation of orthophosphate. Since the concentration of calcium compounds in Shiraz wastewater is high, calcium phosphate complexes formed by autoclaving and remained in the samples.
The 25% of decrease in orthophosphate concentration in Blank treatment during the experimental time is due to the continual precipitation of orthophosphate.
In Alginate treatment, the 38% of increase in orthophosphate concentration occurred during the first period (days 1–4), and the rest of the total increase (8%) occurred in 8 days. The PO 3-4 -P concentration increased as a result of alginate presence in the medium. As described, the alginate screens were formed by adding the gel into calcium chloride solution (2%), and the screen was hardened as a result of cation exchange between the sodium ions in the structure of alginate (CyberColloids 2010) and calcium ions of the solution. Since the screen hardening process lasted 1 h, the internal sodium ions had not been exchanged by calcium ions and the inner screens layer had cation exchange capacity. By adding the screens into the wastewater, the cation exchange process continued and caused the re-solution of the precipitates, accumulation of Ca2+ into the alginate matrix, abandonment of PO 3-4 -P, and finally increase of orthophosphate concentration in the medium. Since there is no microalga in alginate screens of Alginate treatment to consume orthophosphate, the cation exchange between the precipitates and alginate matrix continued until the 12th day resulting in an increase of PO 3-4 -P concentration.
Inorganic nutrient ions such as phosphate would be available to the immobilized algae as freely as to their free counterparts (Chevalier et al. 2000). Jiménez-Pérez et al. (2004) measured the amount of P entrapped by 1,500 beads without algae during the experiment and concluded that the Ca–alginate matrix accumulated a maximum of ca. 100 mg P per culture in the first 10 h. In this work, as the P content of alginate screens was not measured, the effect of alginate in orthophosphate removal cannot be discussed.
In most of the microalgae treatments, PO 3-4 -P concentration increased in the first period, but it is 50 to 70% less than the increase of orthophosphate in Alginate treatment. In spite of the difference in immobilization method, the existence of microalgae caused this variation. While the precipitates were re-solved by alginate, microalgae biosorbed the orthophosphate, therefore the orthophosphate concentration in the microalgae treatments is less than alginate treatment.
According to Table 3, there is a difference in P removal efficiency of microalgae treatments in the first period. In the three treatments of C. vulgaris MCCS 011, MCCS 013, and MCCS 014, PO 3-4 -P concentration decreased during the first 4 days whereas, in other treatments, it increased. This difference is related to the immobilization method and contact surface of the screens with the wastewater. In the C. vulgaris MCSS 011, MCCS 013, and MCCS 014 treatments, 20 mL of alginate gel formed one circular screen with an approximate diameter of 4.3 cm and thickness of 3–4 mm while, in other immobilized microalgae treatments and also blank alginate treatment, this volume of gel formed four smaller and thinner screens with a mean diameter of 2.5 cm. The contact surface of alginate screens with the media is calculated according to the following equation:
Where A is the surface area (cm2), r is the radius (cm), h the thickness of screen (cm), and n number of screens in the flask. The contact surface of alginate screens in the C. vulgaris MCCS 011, MCCS 013, and MCCS 014 treatments is 123.74 cm2, and in the other treatments, it is 175.70 cm2.
The smaller contact surface of alginate screens in the C. vulgaris MCCS 011, MCCS 013, and MCCS 014 treatments means a smaller cation exchange at the surface of the screens, so that, within 4 days, the precipitate re-solution process had been completed; meanwhile, the microalgae had biosorbed the PO 3-4 -P from the medium. In the other treatments, the increase of PO 3-4 -P concentration continued after day 4 because of the higher contact surface of the alginate screens, and the P removal occurred later.
Kaya (1995) reported that immobilization of microalgae in alginate screens enhanced the nutrient removal efficiency compared with free or immobilized cells in alginate beads. Since increasing the number of beads per volume of effluent reduced light penetration and enhanced self-shading effects, the beads settled to the bottom of the reactor (Tam and Wong 2000; Abdel Hameed 2006). The light penetration in algal culture is affected directly by incident light and inversely by the depth and density of the algal culture (Zhang et al. 2008). Cell colonies located near the surface of the bead shade the internal ones, which produces a limitation of photosynthesis, thus decreasing nutrient uptake rate (Kaya 1995; Jiménez-Pérez et al. 2004). Microalgae immobilized in alginate screens receive more light thus algal anabolism and physiological activity would increase. Zhang et al. (2008) concluded that cell density in gel was the key factor affecting nutrient removal efficiency when algal sheet thickness was 2–3 mm.
Based on our results and comparison of orthophosphate removal rates in treatments, it can be concluded that the reduced thickness and increased contact surface area of the alginate screens prevents the self-shading effects of cells thus, increasing algal activity. Cells occupy a significant fraction of the useful volume of the matrix, and the cell density in the surface of immobilized matrix is high (Kuhn et al. 1991), and so the exit route for molecules is longer (Vichez and Vega 1994). Thus, as the surface area increases, more microalgae cells can contribute to nutrient removal and enhance the removal efficiency and rate.
The rates obtained here are markedly lower than those previously published as the initial cell density and immobilization method differed. Lau et al. (1997) obtained removal rates with C. vulgaris of 0.42 and 0.41 mg P L−1 day−1 for free and immobilized cells.
In the S. rubescens MCCS 018, Chlamydomonas sp. MCCS 026, C. dispersus MCCS 006, and C. vulgaris MCCS 011 treatments orthophosphate removal was statistically significantly different compared with the Blank treatment. Therefore, these strains have potential to be used as purifying agents in tertiary wastewater treatment in photobioreactors or high-rate algal ponds and beside that, produce a biomass which is enriched with proteins, fatty acids, etc., which might be used in the agriculture, pharmaceutical, and food industries.
Further research on initial cell concentration, immobilization method (different beads or screens diameter and/or thickness; hardening period, the solution, and its concentration), percent of alginate used for immobilization, and also initial nutrient concentration and environmental aspects should be done to determine the most effective immobilization shape and optimize the best removal conditions.
References
Abdel Hameed MS (2006) Continuous removal and recovery of lead by alginate beads, free and alginate-immobilized Chlorella vulgaris. Afr J Biotechnol 5:1819–1823
American Public Health Association (2005) Standard methods for the examination of water and wastewater 18th edition. Washington, DC
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28:64–70
Billi D, Friedmann EI, Helm RF, Potts M (2001) Gene transfer to the desiccation-tolerant cyanobacterium Chroococcidiopsis. J Bacteriol 183:2298–2305
Borowitzka MA (1988) Algal growth media and sources of algal cultures. In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal Biotechnology. Cambridge University Press, Cambridge, pp 456–465
Chevalier P, de la Noüe J (1985) Efficiency of immobilized hyperconcentrated algae for ammonium and ortho-phosphate removal from wastewaters. Biotechnol Lett 7:395–400
Chevalier P, Proulx D, Lessard P, Vincent WF, De la Noüe J (2000) Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J Appl Phycol 12:105–112
CyberColloids, Alginate. http://www.cybercolloids.net/library/alginate/introduction-alginate-structure. Accessed on: 2010
de Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bioresour Technol 101:1611–1627
de Bashan LE, Trejo A, Huss VAR (2008) Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour Technol 99:4980–4989
Desikachary TV (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi
Fierro S, Sanchez-Saavedra MDP, Copalcua C (2008) Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour Technol 99:1274–1279
Ghasemi Y, Tabatabaei Yazdi M, Shokravi S, Soltani N, Zarrini G (2003) Antifungal and antibacterial activity of paddy-field cyanobacteria from the north of Iran. J Sci IRI 14:203–209
Ghasemi Y, Rasoul-Amini S, Morowvat MH, Raee MJ, Ghoshoon MB, Nouri F, Negintaji N, Parvizi R, Mosavi-Azam SB (2008) Characterization of hydrocortisone biometabolites and 18S rRNA gene in Chlamydomonas reinhardtii culture. Molecules 13:2416–2425
Hernandez JP, de Bashan LE, Bashan Y (2006) Starvation enhances phosphorus removal from wastewater by the microalgae Chlorella spp. coimmobilized with Azospirillum brasilense. Enzyme Microb Technol 38:190–198
Jiménez-Pérez MV, Sanchez-Castillo P, Romera O, Fernandez-Moreno D, Perez-Martinenz C (2004) Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzyme Microb Technol 34:392–398
John DM, Whitton BA, Brook AJ (2003) The fresh water algal flora of the British Isles. An identification guide to fresh water and terrestrial algae. Cambridge University Press, Cambridge
Kaya VM (1995) The viability of Scenedesmus bicellularis cells immobilized on alginate screens following nutrient starvation in air at 100% relative humidity. Biotech Bioeng 46:459–464
Kuhn RH, Peretti SW, Ollis DF (1991) Microfluorimetric analysis of spatial and temporal patterns of immobilized cell growth. Biotech Bioeng 38:340–352
Lau PS, Tam NFY, Wong YS (1995) Effect of algal density on nutrient removal from primary settled. Waste Water Environ Pollut 89:59–66
Lau PS, Tam NFY, Wong YS (1997) Wastewater nutrients (N and P) removal by carrageenan and alginate immobilized Chlorella vulgaris. Environ Technol 18:945–951
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals 15:377–390
Martínez ME, Sánchez S, Jiménez JM, El Yousfi F, Muñoz L (2000) Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour Technol 73:263–272
Martinsen A, Skjak-Bræk G, Smidsrød O (1989) Alginate as immobilization material. I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol Bioeng 33:79–89
Megharaj M, Pearson HW, Venkateswarlu K (1992) Removal of nitrogen and phosphorus by immobilized cells of Chlorella vulgaris and Scenedesmus bijugatus isolated from soil. Enzyme Microb Technol 14:656–658
Moreira SM, Moriera-Santos M, Guilhermino L, Ribeiro R (2006) Immobilization of the marine microalga Phaeodactylum tricornutum in alginate for in situ experiments: bead stability and suitability. Enzyme Microb Technol 38:135–141
Moreno-Garrido I (2007) Microalgae immobilization: current techniques and uses. Bioresour Technol 99:3949–3964
Muñoz R, Guieysse B (2006) Algal-bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40:2799–2815
Nicolella C, van Loosdrecht MCM, Heijnen JJ (2000) Wastewater treatment with particulate biofilm reactors. J Biotechnol 80:1–33
Rasoul-Amini S, Ghasemi Y, Morowvat MH, Mohagheghzadeh A (2009) PCR amplification of 18S rRNA, single cell protein production and fatty acid evaluation of some naturally isolated microalgae. Food Chem 116:129–136
Rasoul-Amini S, Fotooh-Abadi E, Ghasemi Y (2010) Biotransformation of monoterpenes by immobilized microalgae. J Appl Phycol. doi:10.1007/s10811-010-9625-4
Robinson PK (1998) Immobilized algal technology for wastewater treatment purposes. In: Wong Y-S, Tam NFY (eds) Wastewater Treatment with Algae. Springer, New York, pp 1–16
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 5:417–423
Tam NFY, Wong YS (2000) Effect of immobilized microalgae bead concentrations on wastewater nutrient removal. Envir Pollut 107:145–151
Vichez C, Vega JM (1994) Nitrate uptake by Chlamydomonas reinhardtii cells immobilized in calcium alginate. Appl Microbiol Biotechnol 41:137–141
Zhang E, Wang B, Wang Q, Zhang S, Zhao B (2008) Ammonia–nitrogen and orthophosphate removal by immobilized Scenedesmus sp. isolated from municipal wastewater for potential use in tertiary treatment. Bioresour Technol 99:3787–3793
Acknowledgment
We would like to appreciate those who sincerely supported this research project, including the Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Shiraz University of Medical Sciences, and the Department of Water Engineering, College of Agriculture, Shiraz University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zamani, N., Noshadi, M., Amin, S. et al. Effect of alginate structure and microalgae immobilization method on orthophosphate removal from wastewater. J Appl Phycol 24, 649–656 (2012). https://doi.org/10.1007/s10811-011-9682-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-011-9682-3