Abstract
The agarophyte Gracilariopsis lemaneiformis is both important for biological research and of significant economic value. However, the genetic diversity of wild populations of the alga has not been studied. We used amplified fragment length polymorphism (AFLP) PCR and simple sequence repeat (SSR) analysis to investigate diversity in four field populations, three from the coast of Qingdao and one from Weihai, China. Forty G. lemaneiformis isolates collected from the four different geographical groups were analyzed using 16 pairs of SSR primers for PCR amplification. However, no polymorphisms were detected, indicative of a degree of genetic homogeneity. A total of 347 reproducible bands were then amplified using eight AFLP primer pairs, and genetic indices of diversity within and between populations were calculated. This analysis revealed only low levels of genetic diversity both within and between the four geographical groups of G. lemaneiformis. The Weihai population showed a higher within-population genetic diversity than any of the Qingdao populations. The data suggest that there is only limited gene flow between populations. An UPGMA dendrogram revealed two main clusters, and one of these included all the Qingdao isolates. The order of clustering was in accordance with their geographical distribution. These results suggest that the wild G. lemaneiformis populations are closely related and that there is little genetic diversity within wild germplasm in the regions sampled.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The red alga Gracilariopsis lemaneiformis (Bory) Dawson, Acleto, and Foldvik has long been adopted as a model organism due to several important traits that are generally absent from other macroalgae. The species is culturable under laboratory conditions throughout its complete life cycle and produces large numbers of tetraspores (haploid) and carpospores (diploid) that permit genetic investigations including tetrad analysis, multi-gene profiling, and the induction of mutations (Zhang and van der Meer 1988). Since the 1990s, genetic analysis has led to significant advances in our understanding of the life cycle, growth requirements, and basic molecular biology of the organism (Li 1999; Sui and Zhang 2002; Zhang and van der Meer 1988). Gracilariopsis lemaneiformis is well known as the major agarophyte in China because of its high yields and commercially valuable extracts (Lapointe and Ryther 1978; Troell et al. 1999; Wang 2002). Agar produced from G. lemaneiformis accounts for more than 90% of the total annual production in China (Fei et al 1999). In addition, G. lemaneiformis can remove nutrients including nitrogen and phosphate from the culture medium, and therefore has considerable potential in ecological restoration applications (Yang and Fei 2003). However, despite the importance of the alga in biological research and in commercial applications, the genetic backgrounds of field populations have not been studied in detail.
Amplified fragment length polymorphism (AFLP) PCR and simple sequence repeat (SSR) analysis have been widely used for genetic linkage mapping, and provide markers for genetic breeding, species identification, gene localization, and the identification of quantitative trait loci (Bruin et al 2003; Chen et al 2007), although AFLP and SSR have both advantages and disadvantages compared to other parallel techniques (Xu et al. 2007). In the present study, we used AFLP and SSR to analyze the genetic diversity of four field populations of G. lemaneiformis in China. Three population samples were collected from sites several miles apart on the coast of Qingdao, whereas the fourth group of samples was from Weihai, several hundred kilometers away. The aim of the study was to investigate the genetic background and diversity of wild populations in China in order to facilitate further biological studies on this species.
Materials and methods
Forty Gracilariopsis lemaneiformis isolates were collected from four different geographical sites: Zhanshan Bay (QDZS), Huiquan Bay (QDHQ), and Taiwan Road (QDTW) of the Qingdao region, and the Shidao area of the Weihai region (WHSD), China. Ten isolates were collected from the inter-tidal zones at each site, and isolates were randomly selected within a moderate distance. The three Qingdao sampling sites were located several kilometers apart. The sampling site in Weihai was several hundred kilometers away. After collection, isolates were washed in tap water to remove excess salts and sand, and then dried to remove surface water prior to nucleic acid extraction.
Nucleic acid extraction
Genomic DNA was extracted using DNA MagExtractor TIANamp Genomic Kit (TianGen, Beijing, China) according to the instructions of the manufacturer. Extracted genomic DNA was stored at −20°C.
SSR analysis
Genomic DNA libraries enriched for (GA)n repeats were constructed by a modification of a published method for AFLP PCR amplification of sequences containing repeats (Zane et al. 2002). In brief, genomic DNA was sheared by ultrasound treatment, fragments were converted to blunt ends by a standard technique, ligated with synthetic adaptors, and hybridized to a (GA)n probe bound to nylon membrane in order to enrich for fragments containing microsatellite sequences. Bound fragments were eluted, PCR amplified, and ligated into a cloning vector. Clones containing microsatellite sequences were PCR-amplified and their DNA sequences were determined (Pang et al. 2009). Based on these G. lemaneiformis sequences, 16 PCR primer pairs were randomly selected that gave reproducible amplification products (Pang et al. 2009). The sequences of the primers used in SSR analysis are shown in Table 1. PCR was performed using 20 ng of template DNA, 50 ng primers, 1.0 μl dNTP (2.0 mM), 1.2 μL MgCl2 (25 mM), and 1 U Taq DNA polymerase in a final volume of 20 μL. PCR amplification products were separated on 6% denaturing polyacrylamide gels, and electrophoresis was performed at constant power of 60 W for 1.5 h. Following electrophoresis, DNA bands were visualized using the Silver Sequence DNA Sequencing System (Saini et al. 2004).
AFLP analysis
AFLP reactions were according to the protocol of Vos et al. (1995) with the modification that 500 ng of DNA were digested at 37°C for 6 h with 5 U each of EcoRI and MseI. The resulting fragments were ligated to EcoRI and MseI adaptors; pre-amplification was performed using primers based on the EcoRI and MseI adaptors plus one selective nucleotide (EcoRI-A, MseI-C) under the conditions described by Vos et al. (1995) except that 1 U of Taq polymerase (MBI) was used. Selective amplification was then conducted using eight primer combinations, each with three nucleotides for MseI- and two nucleotides for EcoRI-specific primers. PCR products was denatured at 95°C for 3 min then separated on 5% denaturing polyacrylamide gels. Electrophoresis was performed at a constant power of 60 W for 2.5 h including a 0.5 h pre-run. Following electrophoresis, DNA bands were visualized using the Silver Sequence DNA Sequencing System (Saini et al. 2004).
Data analysis
Ten G. lemaneiformis isolates from each of the four different geographical groups (QDZS, QDHQ, QDTW, and WHSD) were analyzed for genetic diversity by SSR and AFLP. Individual AFLP bands were scored for presence (1) or absence (0) and entered into a binary matrix representing the AFLP profile of each sample. Analysis was restricted to bands that were generated reproducibly. Statistical analysis employed PopGen32 and MEGA3.1 software.
Results
SSR analysis
Ten G. lemaneiformis isolates from each of the four different geographical populations were analyzed for genetic polymorphisms using 16 different PCR primer pairs. All of the 16 SSR primer pair PCR products were monomorphic, and consistent SSR products of the same sizes were generated in all displays. This analysis indicates a degree of genetic homogeneity between the different G. lemaneiformis samples.
To refine our analysis, isolates were subdivided according to agar content. Ten isolates with a high agar (∼20%), ten with moderate content (15–20%), and ten with low content (∼15%) were also analyzed using four of the 16 primer pairs. No genetic variation was detected by this method.
We also investigated whether the primer pairs selected were able to amplify bands from other closely related Gracilaria species. Twelve of the 16 primers consistently generated PCR products from 981 G. lemaneiformis, a strain obtained from wild-type G. lemaneiformis by selective breeding (Zhang and Fei 2008), while only one primer pair of the 16 (6.25%) consistently generated PCR products from the more distantly related Gracilaria verrucosa and Gracilaria textorii.
AFLP analysis
Eight of the 16 primer pairs that gave consistent AFLP results were selected for the analysis of the 40 G. lemaneiformis isolates from four different geographical locations. This generated a total of 347 reproducible bands. The polymorphism rates (P) identified using eight primer pairs are shown in Tables 2 and 3. Polymorphism rates identified using the different primer pairs ranged from 36.2% to 62.7%, and ranged from 9.8% to 20.4% within different groups, a much lower extent of polymorphism than has been reported for other algae including Undaria pinnatifida (∼80%; Zhang et al. 2009a, b) and Laminaria japonica (80.95%; our unpublished data).
The diversity indices of the four geographical groups are shown in Table 4. The observed mean number of alleles (Na) ranged from 1.1066 to 1.3026 while the effective number of alleles (Ne) ranged from 1.0477 to 1.1130. The Nei measure of genetic diversity (H) ranged from 0.0292 to 0.0715 whereas Shannon diversity indices (I) ranged from 0.0457 to 0.1151. Furthermore, the indices of diversity were significantly higher within the Weihai isolates than within the Qingdao isolates, and were also increased when all individual isolates were considered. Overall, these data suggest that the levels of genetic diversity within the four geographical groups of G. lemaneiformis are very low. The Weihai population showed the highest within-population genetic diversity, and inter-group genetic diversity was significantly higher than intra-group diversity.
The genetic diversity between the four groups was analyzed mathematically (Table 5). The values of the mean genetic diversity index (Ht) and within-population genetic diversity index (Hs) of the three Qingdao groups ranged from 0.0804 to 0.1078 and from 0.0346 to 0.05, respectively, while those between Weihai and Qingdao groups were 0.2106 and 0.0909, respectively. The Ht and Hs values between all of the groups were 0.1887 and 0.0502, respectively. The gene flow as reflected by the Nm value ranged from 0.1812, when all of the groups were considered, to 0.6498 between two of Qingdao groups. Values were all less than 1.000, indicating little gene flow between the four groups. The coefficient of gene differentiation (Gst) ranged from 0.5368 to 0.8246, indicating that the majority of genetic diversity was represented by inter-group differences (Sokal and Michener 1958).
Genetic distances and similarities between populations are shown in Table 6. The genetic similarity coefficient ranged from 0.6797 to 0.9388. The coefficient between the Qingdao populations was higher than between each Qingdao group isolate and the Weihai isolates. The genetic distance ranged from 0.0632 to 0.3861, however, the distance between the Weihai isolates and any of the Qingdao isolates was larger than the distance between different Qingdao group isolates.
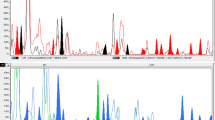
A distance tree was then constructed using clustering with the unweighted pair group method with arithmetic mean (UPGMA; Fig. 1). Two principal clusters were generated, and one included all of the Qingdao isolates. One isolate, No 31 collected from Weihai, did not cluster strongly with any other isolates. QDZS clustered most strongly with QDTW, then with QDHQ, and lastly with WHSD. The order of clustering was in accordance with their geographical distributions.
Discussion
Sixteen SSR primers were developed from G. lemaneiformis by a random selection method (Pang et al. 2010). However, this failed to reveal any genetic polymorphism between the 40 isolates, including between isolates of different agar contents. A similar low rate of SSR polymorphism was also reported in a recent study on G. lemaneiformis (Zhang et al. 2009b) and in another red alga, Porphyra (our unpublished data), although this technique has demonstrated high rates of genetic polymorphism in terrestrial plants and marine animals (Guo et al. 2009; Waldbieser et al. 2001; Zhang et al. 2008).
The core sequences of the SSR sites employed in this study were short, and were repeated from seven to 38 times. It has been reported that the rate of SSR polymorphism is proportional to the repeat interval of the core sequence (Rico et al. 1997), and this may explain our SSR findings in G. lemaneiformis. However, it is possible that simple repeat sequences in this alga are stable, with little copy number variation, and this could potentially reflect differences in the DNA replication machinery of these algae compared to other species.
In addition, only one of 16 SSR primers (6.25%) tested consistently generated PCR products in other Gracilaria species, showing a similar level of transferability that genome-derived (normally randomly picked) SSR primers instead of conserved region-derived ones may produce (Yang et al. 2008).
The AFLP results suggest that there are only low levels of genetic diversity in wild G. lemaneiformis populations from northern yellow sea of China. Genetic diversity indices indicated that the different isolates are closely related at the genetic level. This low level of diversity differs from findings in other cultivated macroalgae including U. pinnatifida (Zhang et al. 2009a) and L. japonica (Shi et al 2007), where a far higher level of genetic diversity was reported.
G. lemaneiformis is distributed across North America, South Africa, Asia, and the Hawaiian Islands (Abbott 1985a, b, 1994; Ganesan 1990; Norris 1985; Xia and Zhang 1999). In China, the species has been reported to be distributed in the North of the country (Zhang et al. 2004; Qingdao, Weihai, and Dalian, personal observations). It is possible that more genetic variation might have been revealed by analysis of isolates from Dalian, a coastal city about 160 km in Liaoning province of Northern Huanghai. However, we have no data on this possibility due to sampling failure from Dalian.
Each local geographical population of G. lemaneiformis is normally restricted to a limited site with areas of inter-tidal rock and sand substrata ranging from several tens to several hundreds of square meters. In view of the large number of progeny of each carposporophyte or tetrasporophyte, ten samples were collected from different sites within the area in order to maximize genetic diversity. Nevertheless, only low levels of genetic polymorphism were detected within each G. lemaneiformis population (Table 4). It is notable that these species are known to have been present at the sampling sites for many years (Xia and Zhang 1999), and it is unlikely that these populations reflect recent colonization by a genetically restricted substrain; a recent population bottleneck is unlikely to explain the low levels of genetic diversity. It is possible that isolates from each sampling site derive from a single ancestral strain; however, we cannot exclude that possibility of genetic homogenization following establishment of the population. The low levels of genetic diversity between the different geographical groups indicates a paucity of wild germplasm resources in this species, and this is likely to impact negatively on selective breeding programs based on wild isolates of G. lemaneiformis.
References
Abbott IA (1985a) Gracilaria from California: key list and distribution of the species. In: Abbott IA, Norris JN (eds) Taxonomy of economic seaweeds with reference to some Pacific and Caribbean species, vol. 1. California Sea Grant College Program, pp 97–99
Abbott IA (1985b) Gracilaria from Hawaii: key, list and distribution of the species. In: Abbott IA, Norris JN (eds) Taxonomy of economic seaweeds with reference to some Pacific and Caribbean species, vol 1. California Sea Grant College Program, pp 85–87
Abbott IA (1994) New records and a reassessment of Gracilaria (Rhodophyta) from the Philippines. In: Abbott IA (ed) Taxonomy of economic seaweeds with reference to some Pacific species, vol 4. California Sea Grant College, pp. 111–118
Bruin A, Belings BW, Donk EV (2003) Molecular techniques in phytoplankton research: from allozyme electrophoresis to genomics. Hydrobiologia 491:47–63
Chen CY, Gu CH, Mensah C, Nelson RL, Wang D (2007) SSR marker diversity of soybean aphid resistance sources in North America. Genome 50:1104–1111
Fei XG, Bao Y, Lu S (1999) Seaweed cultivation: traditional way and its reformation. Chin J Oceanol Limnol 7:193–199
Ganesan EK (1990) A catalog of benthic marine algae and seagrasses of Venezuela, 15 maps. Fondo Editorial Conicit, Caracas, p 237
Guo SS, Zou GW, Yang GP (2009) Development of microsatellite DNA markers of grass carp (Ctenopharyngodon idella) and their cross-species application in black carp (Mylopharyngodon piceus). Conserv Genet 10(5):1515–1519
Lapointe BE, Ryther JH (1978) Some aspects of the growth and yield of Gracilaria tikvahiae in culture. Aquaculture 15:185–193
Li RZ (1999) Physiological character and functional studies on the creeping tissue of Gracilaria lemaneiformis. Oceanol Limnol Sinica 30:41–44
Norris JN (1985) Gracilaria and Polycavernosa from the Caribbean and Florida: key and list of the species of economic potential. In: Abbott IA, Norris JN (eds) Taxonomy of economic seaweeds, vol T-CSGCP-011. California Sea Grant College Program, La Jolla, California, pp 101–113
Pang Q Q, Sui Z H, Zhang X C, Kong F N (2010). The preliminary study on the microsatellite DNA of Gracilaria lemaneiformis. J Ocean Univ China (in press)
Rico C, Ibrahim KM, Rico I, Hewitt GM (1997) Stock composition in North Atlantic populations of whiting using microsatellite marker. J Fish Biol 51:462–475
Saini N, Jain N, Jain S, Jain RK (2004) Assessment of genetic diversity within and among Basmati and non-Basmati rice varieties using AFLP, ISSR and SSR markers. Euphytica 140:133–146
Shi YY, Yang GP, Liu YJ, Liao MJ, Li XJ, Cong YZ (2007) Development of 18 polymorphic microsatellite DNA markers of Laminaria japonica (Phaeophyceae). Mol Ecol Notes 7:620–622
Sokal RR, Michener CD (1958) A statistical method for evaluating systematic relationships. Univ Kansas Sci Bull 38:1409–1438
Sui ZH, Zhang XC (2002) Comparison of phycobiliproteins from Gracilaria lemaneiformis (Rhodophyceae) and its pigment mutants in spectral and molecular respects. Acta Botanica Sinica 44:557–561
Troell M, Ronnback P, Halling C, Kautsky N, Buschmann A (1999) Ecological engineering in aquaculture: use of seaweeds for removing nutrients from intensive mariculture. J Appl Phycol 11:89–97
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M (1995) AFLP: a new technique for DNA fingerprinting. Nucl Acids Res 23:4407–4411
Waldbieser GC, Bosworth BG, Nonneman DJ, Wolters WR (2001) Microsatallite based genetic linkage map for channel catfish, Ictalurus punctatus. Genetics 158:727–734
Wang GC (2002) Isolation and purification of phycoerythrin from red alga Gracilaria verrucosa by combined expanded bed adsorption and ion exchange chromatography. Chrommatographia 56:509–513
Xia B, Zhang J (1999) Flora Algarum Marinarum Sinicarum Tomus II Rhodophyta No. V Ahnfeltiales Gigartinales Rhodymeniales (In Chinese). Science, Beijing
Xu LF, Chen JY, Luo GM (2007) Molecular marker technology and its application in plant breeding. Food Drug 9:43–46
Yang YF, Fei XG (2003) Prospects for bioremediation of cultivation of large sized seaweed in eutrophic mariculture areas. J Ocean Univ Qingdao 33:53–57
Yang YL, Zhang YD, Zhang XY (2008) Transferability analysis of Populus SSR markers in Salix. Mol Plant Breeding 6:1134–1138
Zane L, Bargelloni L, Patarnello T (2002) Strategies for microsatellite isolation: a review. Mol Ecol 11:1–16
Zhang XC, Fei XG (2008) Qualified breeding species by National Marine Origin Breed and Fine Breed Committee—an introduction to 981 Gracilariopsis lemaneiformis and its cultivation. Sci Fish Farming 6:21–22
Zhang XC, van der Meer JP (1988) A genetic study on Gracilaria sjoestedtii. Can J Bot 66:2022–2026
Zhang XC, Qin S, Ma JH, Xu P (2004) The genetics of marine algae (in Chinese). Agriculture Press of China, Beijing, p 237
Zhang YY, Wu J, Zhang SL (2008) Genetic diversity of germplasm resources of Pyrus based on SSR marker. J Agri Biotechnol 16:983–989
Zhang D, Sui ZH, Yi H, Tong Z, Bao ZM (2009a) The genetic diversity analysis of the sporophytes of Undaria pannatifida (Phaeophyceae) of Qingdao population by means of AFLP. Period Ocean Univ China 39:664–668
Zhang XC, He Y, Xu D (2009b) Screening microsatellite sequences from Gracilaria lemaneiformis and its phylogenetic analysis. Period Ocean Univ China 39:259–264
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 30771647) and the National High Technology Project (863 Plan; Grant No. 2006AA10A413). We also thank International Science Editing for word process, and constructive suggestions from two anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pang, Q., Sui, Z., Kang, K.H. et al. Application of SSR and AFLP to the analysis of genetic diversity in Gracilariopsis lemaneiformis (Rhodophyta). J Appl Phycol 22, 607–612 (2010). https://doi.org/10.1007/s10811-009-9500-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-009-9500-3