Abstract
The effect of several alkali treatments on the yield, gel strength, rheology, and chemical characteristics (quality) of the agar obtained from Gracilariopsis lemaneiformis from the Gulf of California was analyzed using different alkali concentrations, temperatures and treatment times. In the first stage of the experiment, all treatments lasted 60 min and the NaOH concentrations (2.5, 3.0, 4.0, 5.0, 6.0%) and temperature (80, 90, 100°C) varied. At constant time, temperature played the predominant role, promoting an increase in agar gel strength. Based on the best treatment conditions found (4% and 5% NaOH, and 90°C and 100°C temperature), in the second stage different treatment times (15, 30, 60, 90, 120 min) were used. Since agar yields were not significantly different among temperatures and times, the optimal conditions to obtain best quality agar were those providing the highest gel strength. Treatment time played an important role in increasing gel strength. Maximum gel strength (Nikan, 954 g cm−2) was obtained with 5% NaOH at 100°C after 90 min of treatment, though these conditions resulted in an agar yield reduction of 25.5% relative to native agar. This treatment proved to efficiently yield G. lemaneiformis agar that will meet the commercial quality requirements regarding gel strength, 3,6 anhydrogalactose and sulfate content, as well as rheology and hysteresis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At least 55 economically important seaweed species are found in the Gulf of California, 19 of which have high commercial harvest potential; the most abundant are those of the genus Gracilariopsis (Espinoza-Ávalos 1993; Pacheco-Ruíz and Zertuche-González 1996; Zertuche-González 1988; Pacheco-Ruíz et al. 2003).
Agar is the main component of the cell matrix and wall of some red algal species, particularly from the families Gelidiaceae and Gracilariaceae (Painter 1983; Craigie 1990); however, due to their poor gel strength native agars from Gracilaria and other species require molecular modification in order to meet commercial agar specifications (Armisen 1995).
The agar molecule is composed of an alternating sequence of (1–3) β-d-galactopyranose and (1–4) 3,6 anhydro α-l-galactopyranose units, and the quality of the agar is maximized when as much as possible of the α-l-galactopyranose 6 sulfate is converted to 3,6 anhydro galactose (3,6 AG) by a sulfohydrolytic enzymatic reaction (Rees 1961; Wong and Craigie 1978) or alkali treatment (Armisen 1995).
Alkali treatment (Duckworth and Yaphe 1971; Rees 1972) has been widely applied by agar producers using Gracilaria as raw material (Armisen 1995; Minghou 1990). This species, however, yields agars with different degrees of sulfate hemiester substitution, so the final agar yield and chemical characteristics will depend on the strength of the treatment (e.g., alkali concentration, temperature, and duration) (Minghou 1990; Hurtado-Ponce 1992; Lai and Lii 1998). Despite the increase in gel strength due to the elimination of the sulfate group and concomitant formation of 3,6 AG, under certain conditions the alkali treatment may produce severe depolymerization that affects the yield and properties of the final product (Myslabodski 1990).
This study aimed to determine the optimal treatment conditions (time, temperature, and alkali concentration) that would provide the highest yield and quality of the agar obtained from G. lemaneiformis from the Gulf of California.
Materials and methods
Plants of Gracilariopsis lemaneiformis (Bory) Dawson, Acleto et Foldvik were obtained in March 2001 from the commercial harvest at Las Ánimas Bay, on the NW coast of the Gulf of California, Mexico (28° 49′ 00″ N, 113° 21′ 50″ W; Fig. 1).
The optimal alkali treatment conditions were determined in two stages. The first factorial design involved 15 treatments: five NaOH concentrations (2.5, 3.0, 4.0, 5.0, 6.0%), three temperatures (80, 90, 100°C), and one treatment time (60 min). Based on the alkali treatment conditions that resulted in the best agar yield and gel strength in the first stage, a second factorial design involved 20 different treatments: two NaOH concentrations (4.0, 5.0%), two temperatures (90, 100°C), and five treatment times (15, 30, 60, 90, 120 min).
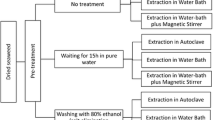
For native agar extraction, a modification of the method proposed by Craigie and Leigh (1978) was employed as follows: 5 g whole dry algae was washed twice with 100 mL distilled water and extracted in 0.1 M phosphate buffer at pH 6.3 for 1 h at 100°C with constant stirring. The resulting extract was vacuum-filtered (Whatman paper and diatomaceus earth), frozen for 12 h then thawed, and the resulting liquid was discarded. The gel was washed twice with 70% ethanol for 15 min and twice with concentrated ethanol for 30 min; finally, it was dried at 60°C for 72 h, weighed, and the agar yield was calculated relative to the initial 5-g sample. In all cases, agar extraction was carried out with five replicates.
For the extraction of alkali-treated agar, 5 g whole dry algae was treated at different times with 150 mL alkali solution at the predetermined concentrations and temperatures. Once the sample had cooled, the alkali solution was removed and the algae were washed under running tap water for 5 min, and rinsed for 10 min with 0.02% H2SO4 and three times with distilled water. After settling overnight in distilled water, the liquid was discarded. The agar extraction was carried out in 150 mL phosphate buffer, pH 6.3, in an autoclave at 121°C for 60 min, and the resulting agar was collected as described for native agar.
Gel strength, expressed as g cm−2, was evaluated in 1.5% agar gels using a Nikan gel meter (Armisen and Galatas 1987). The melting and gelling temperatures were determined according to Armisen (1995). The 3,6 AG content was determined by the colorimetric method of Yaphe and Arsenault (1965), as modified by Craigie and Leigh (1978). The sulfate content was quantified by the turbidimetric method using BaCl2 (Tabatabai 1974; modified by Craigie and Wen 1984).
Differences between the results of the agar treatments were assessed by a Cochran test, followed by a two-way ANOVA (α = 0.05). When differences were found, a multiple post hoc Tukey test (Wayne 2002; Zar 1999) was applied. Sulfate and 3,6 AG contents, and gel strength were analyzed using the Pearson correlation test.
Results
Gracilaria lemaneiformis yielded 25.8% of native agar with low gel strength (< 100 g cm−2), while the alkali treatment applied for 60 min reduced the yield by 5.5–8.0% (20.3–17.8%) (Fig. 2a), with no significant differences regarding the NaOH concentration (P = 0.344). The application of the alkali treatment at constant time yielded agar with gelling characteristics; maximum gel strength (1,116 g cm−2) was achieved with 5.0% NaOH at 90°C. Temperature was the most important factor, especially at low NaOH concentrations (2.5–4.0%) (Fig. 3b). The gel strength values obtained at 90°C and 100°C were similar but higher than those obtained at 80°C (Tukey test). Thus, the best conditions found in the first experimental stage at a constant time of 60 min were 4% and 5% NaOH and temperatures of 90 and 100°C.
In the second stage, the mean agar yield of G. lemaneiformis varied between 24.4% and 15.5%. There was a reduction in agar yield as the treatment time increased, with no significant differences among alkali treatments regarding time, temperature, and NaOH concentration (P > 0.05) (Fig. 3a). As opposed to agar yield, the mean gel strength values increased as the treatment time increased, and maximum gel strength (954 g cm−2) was obtained with 5.0% NaOH at 100°C and 90 min treatment. There were significant differences among alkali treatments at different temperatures and treatment times (P = 0.011), but not regarding NaOH concentration (P = 0.284) (Fig. 3b).
The 3,6 AG content in native agar was 26.9%, but increased to 40.2% in the 90-min alkali treatment with 5.0% NaOH at 100°C (Fig. 4b). The sulfate content value obtained for native agar was much higher (5.1%) than the minimum value (1.0%) recorded in 120-min alkali-treated agar (Fig. 4b). It is worth mentioning, however, that both the 3,6 AG and sulfate contents in the agar were not statistically different (P > 0.05) with regard to the alkali treatment time after 15 min of treatment (Fig. 4b).
The yield and sulfate content of the G. lemaneiformis agar showed a high correlation (r = 0.85). A negative correlation (r = −0.72) was found between gel strength and yield and between gel strength and sulfate content (r = −0.66) but low correlation values were found between 3,6 AG and sulfate content (r = 0.23), gel strength and 3,6 AG content (r= −0.16), and yield and 3,6 AG content (r = 0.09).
The highest (94°C) and lowest (81°C) melting points were achieved with the 60- and 120-min alkali treatments, respectively, whereas gelling temperature fluctuated between 32°C and 36°C, corresponding to the 120- and 90-min alkali treatments, respectively (Table 1).
Discussion
The alkali treatment had a positive effect on the quality of the G. lemaneiformis agar, increasing the gel strength and 3,6 AG content by decreasing the sulfate content. The resulting agar showed acceptable commercial agar characteristics, forming clear and strong gels. During the alkali treatment, temperature played the predominant role, followed by alkali concentration and treatment time, similar to that reported by Lai and Lii (1998) and Villanueva et al. (1997).
The optimal alkali treatment conditions found in this study corresponded to 5.0% NaOH at 100°C for 90 min. Even though the G. lemaneiformis agar yield obtained after the alkali treatment did not surpass the values reported in other studies, it was more than double that recommended for industrial purposes (>8.0%) using agar-producing species (Armisen 1995).
In the alkali treatment, gel strength was observed to increase, peaking (954 g cm−2) at 90 min. It then decreased by 11% (848 g cm−2) at 120 min, indicating some degradation by depolymerization of the agar molecule (Lai and Lii 1998; Nishinari and Watase 1983). Protective mechanisms, however, can be employed to minimize degradation (Myslabodski 1990).
The maximum gel strength obtained in this study (954 g cm−2) was slightly higher (892 g cm−2) than that reported by Arellano-Carbajal et al. (1999) for the same species and study area. The difference may be attributed to the different extraction conditions used, or to seasonal variations in the agar structure of the species.
Most of the increment in the 3,6 AG content and reduction in the sulfate content of G. lemaneiformis agar occurred in the first 15 min of the alkali treatment (Fig. 4), whereas the 3,6 AG peak value (40.2%) coincided with a low content of the sulfate groups (1.0%) and the highest gel strength, as reported for the agar of Gracilaria eucheumoides (Villanueva et al. 1997).
The gelling (32–36°C) and melting point (81–94°C) ranges obtained for the G. lemaneiformis agar are comparable to those reported by Arellano-Carbajal et al. (1999) for this species (34.3–37°C and 92–98°C, respectively). It is important to mention that the gelling point range found in both studies was lower than that reported for Gracilaria (42°C) and other Gracilariopsis (40°C) species (Armisen 2000), perhaps because the G. lemaneiformis agar shows a lower degree of methylation (particularly low 6-O-methylgalactose content; Guiseley 1970). Proving this, however, is beyond the scope of this study.
The optimal conditions for the alkali treatment in this study aimed to reduce the excessive consumption of reagents and treatment time, avoiding an over processing that would result in degradation of the agar molecule (Nishinari and Watase 1983; Lai and Lii 1998), but without compromising agar quality. These conditions were obtained with 5% NaOH at 100°C for 90 min. However, our results show that it is possible to use a less expensive treatment by reducing the time, with 15 min of treatment and 5% NaOH at 100°C. This would yield an agar with a gel strength >700 g cm−2, a value close enough to fulfill commercial requirements (>750 g cm−2) (Armisen 1995).
References
Arellano-Carbajal F, Pacheco-Ruíz I, Correa-Díaz F (1999) Seasonal variation in agar yield and quality of Gracilariopis lemaneiformis (Bory) Dawson, Acleto et Foldvik, from the Gulf of California, México. Cienc Mar 25:51–62
Armisen R (1995) Word-wide use and importance of Gracilaria. J Appl Phycol 7:231–243 doi:10.1007/BF00003998
Armisen R (2000) Ficocoloides. Polisacáridos de algas marinas. Memorias Hispanagar, España, p 132
Armisen R, Galatas F (1987) Production, properties and uses of agar. In: McHugh DJ (Ed) Production and utilization of products from commercial seaweeds. Fish Tech Paper FAO No 288, pp 1–57
Craigie JS (1990) Cell walls. In: Cole KM, Sheath RG (eds) Biology of the red algae. Cambridge University Press, Cambridge, pp 221–257
Craigie JS, Leigh C (1978) Carrageenans and agars. In: Hellebust JA, Craigie JS eds Handbook of phycological methods. Physiological and biochemical methods. Cambridge University Press, Cambridge, pp 109–131
Craigie JS, Wen ZC (1984) Effects of temperature and tissue age on gel strength and composition of agar from Gracilaria tikvahiae (Rhodophyceae). Can J Bot 62:1665–1670
Duckworth M, Yaphe W (1971) The structure of agar. Part 1. Fractionation of a complex mixture of polysaccharides. Carbohydr Res 16:359–366
Espinoza-Ávalos J (1993) Macroalgas Marinas del Golfo de California. In: Salazar-Vallejo SI, González NE (eds) Biodiversidad Marina y Costera de México. Com Nal Biodiversidad y CIQRO, México, pp 328–357
Guiseley KB (1970) The relationship between methoxyl content and gelling temperature of agarose. Carbohydr Res 13:247–256 doi:10.1016/S0008–6215(00)80831–9
Hurtado-Ponce AQ (1992) Influence of extraction time on the rheological properties of agar from some Gracilaria species from the Philippines. Bot Mar 35:441–445
Lai M-F, Lii C (1998) Effect of extraction conditions on structural and rheological characteristics of agar from Pterocladia capillacea and carrageenan from Grateloupia filicina. Bot Mar 41:223–234
Minghou J (1990) Processing and extraction of phycocolloids. In: FAC/ACA. Technical Resource Papers. Regional Workshop on the Culture and Utilization of Seaweeds. Vol. II (Regional Sea Farming Development & Demonstration Project RAS/901002. 27–31 August 1990. Cebu City, Philippines. Regional Sea Farming Development & Demonstration Project/Network of Aquaculture Centres in Asia. Bangkok, Thailand, pp 85–96
Myslabodski DE (1990) Red-Algae galactans: isolation and recovery procedures-effects on the structure and rheology. PhD Thesis. University of Trondheim, Norway, 225
Nishinari K, Watase M (1983) Effect of alkali pre-treatment on the rheological properties of concentrated agar-agar gels. Carbohydr Polym 3:39–52 doi:10.1016/0144–8617(83)90011–5
Painter TJ (1983) Algal polysaccharides. In: Aspinall GO (ed) The polysaccharides. Vol. II. Academic, New York, pp 2:195–285
Pacheco-Ruíz I, Zertuche-González JA (1996) The commercially valuable seaweeds of the Gulf of California. Bot Mar 39:201–206
Pacheco-Ruíz I, Zertuche-González JA, Che-Barragán A (2003) Commercial exploitation of Gracilariopsis lemaneiformis in the Gulf of California. Proc XVIIth Int Seaweed Symp 101–105
Rees DA (1961) Enzymic desulphation of porphyran. Biochem J 80:449–453
Rees DA (1972) Shapely polysaccharides. Biochem J 126:257–273
Tabatabai MA (1974) Determination of sulphates in water samples. Sulphur Inst J 10:11–13
Villanueva RD, Pagba CV, Montaño NE (1997) Optimized agar extraction from Gracilaria eucheumoides Harvey. Bot Mar 40:369–372
Wayne WD (2002) Bioestadística. Base para el análisis de las ciencias de la salud, 4th edn. Limusa Wiley, Mexico, p 755
Wong KF, Craigie JS (1978) Sulfohydrolase activity and carrageenan biosynthesis in Chondrus crispus (Rhodophyceae). Plant Physiol 61:663–666
Yaphe W, Arsenault GP (1965) Improved resorcinol reagent for the determination of fructose and of 3,6 anhydrogalactose in polysaccharides. Anal Biochem 13:143–148 doi:10.1016/0003–2697(65)90128–4
Zar JH (1999) Biostatistical Analysis. Prentice-Hall, New Jersey, p 718
Zertuche-González JA (1988) In situ life history, growth and carrageenan characteristics of Eucheuma uncinatum (Setchell & Gardner) Dawson from the Gulf of California. PhD Dissertation, University State of New York at Stony Brook, 162
Acknowledgments
The authors acknowledge the support of the Universidad Autónoma de Baja California and the Instituto Nacional de Pesca. We thank Dr. Felipe Correa-Díaz and Dr. Yolanda Freile-Pelegrín for their valuable comments and suggestions, Manuel Gardea and Christine Harris for translation of the document, as well as the anonymous reviewers whose remarks helped to significantly improve this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enrique Hernández-Garibay holds a CONACyT scholarship.
Rights and permissions
About this article
Cite this article
González-Leija, J.A., Hernández-Garibay, E., Pacheco-Ruíz, I. et al. Optimization of the yield and quality of agar from Gracilariopsis lemaneiformis (Gracilariales) from the Gulf of California using an alkaline treatment. J Appl Phycol 21, 321–326 (2009). https://doi.org/10.1007/s10811-008-9370-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-008-9370-0