Abstract
Agar has been widely used in the food, pharmaceutical and medical fieldsand has been traditionally extracted by soaking the macroalgae for up to 4 h at boiling temperatures. Traditional methods are often energy intensive and therefore extraction technologies are currently being investigated in order to pursue more sustainable form of extraction. This study focused on the extraction of agar from Gelidium sesquipedale by means a combination of an acid pre-treatment (citric or acetic acid) followed by autoclaving at different time/temperature combinations. It was found that among all the conditions tested (10, 15 and 20 min) and (100, 110, and 120 °C), the best condition was (20 min, 110 °C) by means of a citric acid pre-treatment, reporting a yield of 31.69 ± 3.5% (g extract per g dried G. sesquipedale), and a gel strength of 183.78 ± 36.80 g cm−2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agar is a hydrocolloid found in the cell walls of red macroalgae, more specifically in two genera, Gelidium and Gracilaria (McHugh 2003), and composed of agarose and agaropectin biopolymers (Zeece 2020). Agarose is formed by 3,6-anhydro-α-L-galactopyranosyl, which has linked 3-O- substituted β-D-galactopyranosyl units to it by (1 → 4) links (BeMiller 2019). Agaropectin consists of chains that are formed by galactopyranosyl residues connected by C-4 and C-6 linkages to pyruvic acid forming an acetal with agarose, and a sulfated galactan (Moldoveanu 2021). The content of each of the biopolymers determines the gel strength, among other factors, such as: macroalgae species, extraction methods, or the growing conditions of the algae species (Bertasa et al. 2020). One of the most applied uses of agar is for food applications, followed by bacteriological and biotechnological applications (Sousa et al. 2021). Thickening and gelling properties are the two of the main characteristics that make of agar a very versatile biopolymer (Stiger-Pouvreau et al. 2016). For example, the agar is used in yogurts to achieve the targeted texture and avoid whey separation (Sun et al. 2018). Low strength agar is mainly used in food applications such as thickener or fat replacer (Gómez-Mascaraque et al. 2019; Gomez et al. 2020). Another application attracting research interest is the development of food packaging materials based on agar. Agar is biodegradable and comes from a renewable source, which could help avoiding the accumulations of plastic-based materials (Mostafavi and Zaeim 2020). More applications have been reported for agar in the pharmaceutical field, such as wound dressing material, and controlled drug releasing (Rivadeneira et al. 2018). It is apparent that one of the challenges in commercial applications of seaweed biomass is the spatial and temporal heterogeneity in quality and among agarophytes, and in Gelidiales in particular, where agar yield and quality of Gelidiella acerosa were shown to vary seasonally (Roleda et al. 1997c) and among different reproductive status (Ganzon-Fortes, Roleda (Roleda et al. 1997a).
Commercially, agar has been extracted from Gelidium species and Gracilaria species by soaking the algae at temperatures above 85 °C in order to dissolve the agar in water. In the case of Gracilaria species, a pre-treatment with sodium hydroxide is used in order to improve the gelling properties by reducing the sulphate content. This pre-treatment is not performed for Gelidium species, as the sulphate content is lower than Gracilaria species and does not compromise the gelling properties of the agar extracts (Lomartire and Gonçalves 2022). This method is energy intensive as the extraction takes up to 4 h at high temperatures, therefore novel extraction methods are being tested to develop greener methods (Gomez et al. 2020). Among these novel extraction technologies, the use of higher temperatures (> 100 °C) is facilitated by increasing the pressure in the extraction vessel, such extraction protocol has not been as widely reported. Agar was extracted from Gracilaria salicornia using autoclave extraction at 120 °C for 1 h reported to produce high yield of agar (33.2 ± 0.8%) (Vuai and Mpatani 2019). Whereas, other studies did not show significant improvements, reporting lower yields for the agar extraction from Gelidium latifolium performed at 110 °C for 30 min (32.40 ± 0.57%), compared to hot water extraction at 95 °C for 6 h (34.26 ± 0.22%) with significant reduction in processing time (Öğretmen and Duyar 2018). Therefore, higher temperature extraction seems to be a promising alternative extraction technology that can allow extracting agar by using water as the solvent of extraction, while reducing the processing time and thus, the economic and environmental cost of the process, and disrupting the cell walls of the seaweed by the application of heat combined with pressure.
The main objective of this study was to assess the agar extraction yield from Gelidium sesquipedale using higher temperature at pressure, compared to a traditional agar extraction method. Additionally, two acid pre-treatments (acetic acid and citric acid) were compared. Acid pre-treatments were suggested in order to overcome the yield loss after applying the traditional alkaline pre-treatments, which was resulted successful as the yield obtained for acetic acid pre-treatment extraction was 29.8 ± 2.41% compared to 28.09 ± 2.55% for alkali pre-treatment extraction (Roleda et al. 1997b). Citric acid was also tested as it was expected that its cross-linking nature would enhance the extraction of agar (Uranga Gama et al. 2020). In order to select the most adequate process and to determine the impact of the extraction on the final agar extracts, these were characterized by using FT-IR, gel strength, molecular weight analysis, thermogravimetric analysis, and sugar quantification by HPLC.
Materials and methods
Raw seaweed material
Dried Gelidium sesquipedale (1000 g) was obtained from Hispanagar (Burgos, Spain). Gelidium sesquipedale was collected from the south of Europe’s Atlantic coast (Spain, Portugal, and France) during the summer months and left drying in the sun. The raw material was washed with tap water until no sand was found in the washing water. The raw material was then placed in aluminium trays in an oven at 60 °C overnight to dry until constant weight. The seaweed was milled (Laboratory Mill 3610, Perten Instruments, Ireland) and selected for a particle size between 0.7 – 1 mm by using a mechanical sieve (VWR Haver & Boecker, Germany) set for amplitude: 3.0 mm, time: 2 min, and interval: 5 s.
Chemicals
HCl 37% (CAS-No: 7647–01-0, Acros Organics, Germany). Distilled water was supplied by a MilliQ Direct 8 (France). Citric acid monohydrate ACS reagent ≥ 99.0% (CAS-No: 5949–29-1, Sigma-Aldrich, Japan). Acetic acid glacial, ReagentPlus®, ≥ 99% (CAS No: 64–19-7, Sigma-Aldrich, Ireland). Commercial Agar QSol® Soft 150 (Hispanagar, Spain). D-( +)-Glucose (CAS-No: 50–99-7, Sigma, France). D-( +)-Galactose (CAS-No:59–23-4, Sigma, Italy).
Agar extraction
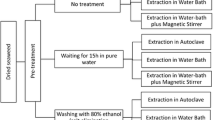
A sample (15 g) of milled G. sesquipedale was soaked in 450 mL of 0.5% (v/v) acetic acid, or 0.5% (w/v) citric acid for one hour at room temperature using a stirrer at 400 rpm (VOS 40 digital, VWR, USA). The mixture was then filtered using a muslin cloth and the seaweed was washed with tap water and rinsed with 1 L of distilled water until approximately pH = 5 was reached. The samples were soaked in 450 mL distilled water in a glass bottle to apply an autoclave treatment (Autoclave 4,002,424, JP SELECTA, SA, Barcelona, Spain). The autoclave treatment was carried out at three different temperatures (100, 110, and 120 °C) for 10, 15, and 20 min at a pressure of 1 atm. The control was performed by means of placing a round bottom flask into an oil bath and attached to a reflux condenser without acid pre-treatment (4 h, 95 °C). The following steps were the same as for both control and treated samples. Autoclaved samples were filtered in hot condition to avoid agar gelation with a muslin cloth. Filtred extract was cooled at room temperature followed by freezing overnight at -80 °C and freeze-dried in an industrial scale freeze-dryer (− 25 °C/30 °C, approximately 0 mbar) in an FD 80 model (Cuddon Engineering, New Zealand) to obtain a final solids agar crude extract. These extractions were performed in duplicates.
Determination of crude agar yield of extraction
The crude agar yield of extraction was calculated by using the formula in Eq. 1.
Fourier Transform InfraRed (FT-IR)
The algal extracts were analysed using Nicolet iS5 (Thermo Scientific, USA) Fourier transform mid-infrared spectrometer equipped with diamond crystal attenuated total reflectance (ATR) accessory (iD7 ATR, Thermo Scientific, USA). Single beam reflectance spectra (R) were collected over the wavenumber range 550—4000 cm−1 with a resolution of 4 cm−1; then were converted and recorded as absorbance spectra (log(1/R)). For each measurement, 64 scans were carried out and the average spectrum was extracted from the spectra of 64 scans. The average spectrum was calculated based on duplicate measurements for each sample.
Gel strength
Gel strength measurements of the agar extracts were carried out based on a penetration tests. For this purpose 1.5% (w/w) solutions of agar in distilled water prepared and heated at 90 °C until all the soluble compounds were dissolved and then transferred to a bloom jar and kept in the a fridge at 4 °C overnight. The next day, the samples were left to reach room temperature before the analysis. A texture analyser (Stable Micro Systems model – TA.HD plus C, UK) equipped with a radiused cylinder probe (P/0.5 R, 1.27 cm diameter) was operated at a penetration rate of 1 mm s−1 to a depth of 5 mm on the formed gel as performed by (Li et al. 2021). Each sample was measured in duplicate.
Molecular weight
Average molecular weight was estimated by High Performance Size Exclusion Chromatography and a Refractive Index detector (HPSEC—RID) as described by Gómez-Mascaraque et al. (2019). An Agilent 1200 Series HPLC separations equipment and an Agilent 1200 refractive index detector (Agilent, USA) controlled by Agilent ChemStation software with a pre-column (OHpak SB-G 6B, 8 × 50 mm) and a Shodex OHpak SB-804 HQ with 6% cross-linked HPLC carbohydrate column with 8 × 300 mm (length x I.D.) (Shodex, Japan) were used for the separation. Extracts were dissolved in the mobile phase that consisted of 0.05 M Na2SO4/0.01 M EDTA adjusted to pH 7, at a concentration of 1 mg mL−1 and filtered through 0.8 μm pore syringe filters before injection. The injection volume was 20 μL with a flow rate of 0.5 mL min−1. P-82 pullulan standards (Merck, Ireland) were used for calibration.
Thermogravimetric analysis
The study of the thermal stability of the alginate extracts were performed by TGA using a TGA/DSC 3+ STARe System. Approximately 10 to 12 mg of each sample (0.1 mg resolution) were placed in an aluminium pan and heated from 30 to 600 °C at a rate of 10 °C min−1 under nitrogen atmosphere with a nitrogen flow of 20 mL min−1 (Martínez-Sanz et al. 2021). The reference sample was an empty aluminium pan with lid. The mass loss sections expressed in percentages, and the temperature ranges were calculated at the end of each step (inflection point) by using the horizontal segment tool in the STARe software.
Sugar analysis
The galactose, glucose and xylose contents in the agar extracts were quantified by means of Ultra High Performance Liquid Chromatography with a Refractive Index detector (UHPLC-RI) with a BioRad Aminex HPX-87P column. The conditions used for the analysis were 10 µL of injection volume, 0.6 mL min−1 flow rate for 20 min, and 80 °C column temperature. RI detector temperature was set at 50 °C. Standards of galactose and glucose were used for calibration. In order to prepare the extracts for this analysis, 25 mg of each sample were mixed with 250 µL of 72% (w/w) sulphuric acid contained in 10 mL glass tubes. Then the samples were placed in a water bath at 30 °C for 1 h, mixing the samples every 10 min. After 1 h, the samples were added 7 mL of distilled water and placed in an autoclave at 121 °C for 1 h. Once the samples reached room temperature, these were neutralized by adding calcium carbonate. The samples were then filtered through a 0.2 µm filter and placed in vials (Van Wychen and Laurens 2016).
Statistical analysis
All extractions were carried out in duplicates, as well as the characterisation analysis. Average values and standard deviation are reported. Differences in the mean were carried out using Analysis of Variance (ANOVA) using Minitab (V 17) Statistical Package. Means were considered as significant at 95% confidence level (p ≤ 0.05). Means were grouped by using Tukey test (Post Hoc test). A 3-factors General Linear Model followed by a Tukey test was also performed to evaluate the differences among the three main factors: acid pre-treatment, time, and extraction temperatures.
Results
Agar yield of extraction
Figure 1 shows the crude agar extraction yields for the different protocols. According to the 3-factors GLM (General Linear Model), all citric acid pre-treatments reported statistically higher yields compared to the analogous acetic acid pre-treatments probably due to the cross-linking nature of this acid (Uranga Gama et al. 2020). Two trends are identified for both pre-treatments, i.e., the higher temperatures and longer times of autoclave treatment led, in all cases, to significant higher yields of extraction. According to the 3-factors GLM, (20 min, 120 °C) and (15 min, 120 °C) treatments were statistically higher than the rest of the treatments for both acids. A significant interaction was seen for time × acid, and for time × temperature, time × temperature × acid, meaning that the three factors affected significantly the yields of extraction (Table 1).
Crude agar yields (w/w, %) (n = 2) of extraction following the acetic acid, the citric acid and the control extraction protocols. Tukey test applied for acetic acid protocol and control are identified with lowercase letters, and Tukey test applied for citric acid protocol and control are identified with capital letters
FT-IR
In order to identify the chemical bonds found in the agar structure and the presence of impurities present in the different samples a FT-IR analysis was performed. The spectra of the samples and the commercial sample were collected from 400 to 4000 cm−1 for the citric acid pre-treated samples (Fig. 2a), and for the acetic acid pre-treated samples (Fig. 2b). The main chemical bonds found in agar, as found in all the samples, are the following:
-
S = O bond present in D-galactose as two peaks at 1042 and 1065 cm−1, of which only the second one is seen, and in sulphonamide (found in proteins) as a weak intensity peak at 1373 cm−1 (Li et al. 2021). The 1065 cm−1 peak becomes more evident in the samples seen as a “shoulder” compared to the commercial sample, where it was not seen.
-
C-H bond present in the β-1,3-linked galactopyranose and in the 3,6-anhydro-galactose residue at 891 cm−1 and 930 cm−1, respectively.
-
C-O and C–C bonds present in the pyranose ring are seen as a strong peak at 1041 cm−1 and a medium intensity peak at 1152 cm−1 (Pereira et al. 2003).
-
N–H bond found in proteins and that in this case represent an impurity is seen as a strong peak at 1629 cm−1 (Rasheed et al. 2019).
-
O–H bond as a broad peak between 3060 and 3560 cm−1 usually associated to the moisture content (Guerrero et al. 2014).
Gel strength
Gel strength tests were performed following a penetration test for the different agar extracts for both acid pre-treatments (Fig. 3). The 3-factor GLM showed that citric acid pre-treatment led to significantly higher gel strength extracts compared to acetic acid pre-treatment. Table 2 shows the effect of extraction time, temperature and acid pre-treatment on gel strength. Gel strength was observed to increase with increase in extraction time. Additionally, 120 and 110 °C treatments were significantly higher compared to 100 °C. A significant interaction was seen for temperature × acid, and for time × temperature × acid. The highest gel strength was reported for citric acid pre-treated (20 min, 110 °C) being 183.78 ± 36.80 g cm−2, and the lowest gel strength was reported for acetic acid pre-treated (10 min, 120 °C) being 6.06 ± 1.30 g cm−2. All of the samples reported a higher gel strength compared to the control sample (4 h, 95 °C) 13.92 ± 3.35 g cm−2, but lower than the commercial sample 1104.3 ± 113.93 g cm−2.
Molecular weight
Size exclusion chromatography was used to analyse the molecular weight of the different agar extracts (Table 3). Only citric acid samples reported a statistically higher molecular weight value than the commercial sample. No significant differences were found among the different treatments. A seen in Table 4, changes on temperature and acid led to significant differences. A significant interaction was seen only for time × temperature × acid.
Thermogravimetric analysis
ThermoGravimetric Analysis (TGA) was performed to assess the thermal stability and the ash content (%) of the different agar extracts. The mass losses corresponding to each onset temperature (°C) were also identified. Furthermore, Derivative ThermoGravimetric Analysis (DTG) was used to confirm the inflection point in each TGA curve by the appearance of peaks on the DTG plot. In the following Tables 5a and b, the onset temperatures and the mass losses can be seen. The first mass loss corresponds to the water loss by evaporation (T2) (T1 refers to the starting temperature of the TGA, that is 31 °C), the second mass loss corresponds to the degradation of agar (T3), and the third mass loss represents the mass loss until only inorganic material (ash) is left.
Sugar analysis
Galactose, glucose and xylose were quantified in the extracts (Table 6) and xylose was not detected. According to the 3-factors GLM, no significant differences were found between the citric acid and the acetic acid pre-treated samples, or among treatments. None of the factors, nor their interactions were significant (p > 0.05). It can be concluded that all treatments were as efficient for the galactose and glucose extraction. The one-way ANOVA analysis showed that all treatments extracted a similar quantity of galactose and glucose. Other study reported a galactose content in the range of 2.5 to 6.1% for agar extracts extracted from G. sesquipedale by means of different novel technologies, which are higher than the results shown on Table 6, with the exception of the (10 min, 100 °C) (Martínez-Sanz et al. 2021).
Discussion
Agar yield of extraction
Agar yields obtained for extraction conditions employed in this study (20 min, 120 °C), (20 min, 110 °C), and (15 min, 120 °C) corroborated with other reported studies where agar was extracted from G. salicornia by using an autoclave for 1 h without acid pre-treatment (30.7 ± 0.6%) for the 115 °C treatment, and 33.2 ± 0.8% for 120 °C treatment (Vuai and Mpatani 2019). Other study reported 32.40 ± 0.57% for the autoclave agar extraction from Gelidium latifolium at 110 °C for 30 min, similar to the yield reported in this study for the (20 min, 110 °C), which is 31.69 ± 3.5% (Öğretmen and Duyar 2018). The statistically lowest yields were reported for the 100 °C treatments. This finding can be explained as the higher temperatures led to more cell-wall degradation and a higher solubilisation of agar into the distilled water (i.e., solvent of extraction). Additionally, longer extraction times also contributed to a larger cell-wall degradation. Comparing control to the different treatments it was seen that the control extraction achieved statistically the same yields as the treatments that reported the higher yields. Some studies have reported higher yields at higher temperatures for the extraction of agar from G. sesquipedale; one study by employing of a combination of SWE (subcritical water extraction) and SWE-MEF (subcritical water extraction with modified electric fields) ranging from 20.10 ± 0.20% to 34.40 ± 0.90% (Pereira et al. 2003). Similar to the yields reported in our study for the treatments at 120 °C ranging from 22.58 ± 0.82% (acetic acid pre-treatment) to 33.53 ± 0.47% (citric acid pre-treatment). Other study by means of hydrothermal treatment, seeing that longer and higher temperature treatments lead to higher yields ranging from 10.8 ± 1.5% to 67.2 ± 2.0% (Gomes-Dias et al. 2022). The authors also highlight at a temperature of 190 °C agar degradation into smaller sugars is expected so higher yields are not necessarily due to a higher agar concentration in the extracts (Gomes-Dias et al. 2022). It can be concluded that the (20 min, 120 °C) and (15 min, 120 °C) treatments can effectively be a time saving alternative to control extraction in terms of agar yields of extraction.
FT-IR
The FT-IR spectra show that all the treatments for both acid pre-treatments have a very similar profile regardless the extraction conditions. Likewise, when the spectral profile is compared to the spectrum of the commercial agar sample, no main differences were observed. The main peaks associated to the chemical bonds found in agar were also found in the extracts. An additional peak at 1742 cm−1 was found only for the samples, which was reported to be associated to the non-cellulosic carbohydrates, or hemicelluloses (Seca et al. 2000; Sun et al. 2005).
Gel strength
Studies have reported higher gel strength compared to our study. Vuai and Mpatani (2019) reported 159.0 ± 2.5 g cm−2, for agar extracted from G. salicornia at 120 °C for 1 h treatment and pre-treated in 0.5% acetic acid, by using an autoclave compared to our values below 100 g cm−2 for acetic acid pre-treated samples. This difference could be due to the longer extraction time used by these authors that allowed extracting more agar, resulting therefore in a higher gel strength. We found no sulphate groups, which can be the reason for a lower gel strength compared to the commercial sample (Wang et al. 2017). Pereira et al. (2003) also reported notably higher gel strengths in the range of 748 to 885 g cm−2, and an opposite trend in the results compared to our study, reporting lower gel strengths as temperatures increased from 95 to 140 °C. Nevertheless, Gomes-Dias et al. (2022) reported unusually lower gel strengths compared to the rest of their treatments, i.e., 20 g cm−2 for hydrothermal extracted agar at 110 °C for 5 min of extraction similar to the gel strength of our acetic acid pre-treated samples with less than 50 g cm−2.
Molecular weight
In our study only citric acid treatments reported statistically significant higher molecular weight than the commercial sample. This finding can be explained as the citric acid is less acidic than the acetic acid, and therefore, it led to a lesser decomposition. Nevertheless, all of the sample values and control reported are lower than the ones reported in other studies. Gomes-Dias et al. (2022), performed a subcritical extraction of agar from G. sesquipedale reporting values of 165 ± 11 kDa for a 130 °C and 15 min treatment, and 183 ± 14 kDa for a control extraction performed for 3 h at 95 °C by means of a hot water bath. Others have reported values of 1112 kDa for a hot water bath extracted agar for 2 h at 95 °C, and 781 kDa for agar extracted by hot water bath and pre-treated with 2.5 M sodium hydroxide (Martínez-Sanz et al. 2019a). These low molecular weights are correlated to the low gel strength values reported earlier. No impact was found for the 120 °C temperature, as it would have been expected according to literature (Gomez et al. 2020). Gel strength is affected by the molecular weight, as well as by the concentration of 3,6- anhydro-α-L-galactopyranosyl found in the extracts (Martínez-Sanz et al. 2021). Due to this, it can be explained that the commercial sample had a higher gel strength despite of having a statistically similar molecular weight compared the samples.
Thermogravimetric analysis
As reported by Ouyang et al. (2018) and as seen in Table 2a and b, the first onset temperature occurred at about 100 °C, and the second onset temperature takes place in a range between 250 – 300 °C, which supports the evidence found in other characterization techniques that agar was found in the extracts. No significant differences were found among the different extraction conditions for citric acid samples, only for the first mass loss, which corresponds to the water loss, between the commercial sample and the control sample. In the case of acetic acid pre-treated samples, significant differences were found for T2, having the treatments (10 min, 100 °C), (15 min, 100 °C), (10 min, 110 °C), (15 min, 110 °C) a lower T1 than the commercial sample due to a higher presence of intermolecular bonds than of intramolecular bonds, and therefore, less energy as heat is required to break these bonds and free the water. According to the 3rd mass loss (%), (15 min, 110 °C) and (15 min, 120 °C) treatments had statistically the same mass loss as the commercial sample. The same was found for the ash content (%). The highest ash content (%) was given by the control sample, where no acid pre-treatment was used. This can be because the longer extraction time, 4 h, could have led to the extraction of carbonaceous co-products.
Sugar analysis
It has been reported that temperatures of 120 °C could decompose heat-sensitive polysaccharides, and therefore lead to a lower content in the samples (Gomez et al. 2020). A similar galactose content was found for our samples and the food packaging films developed with agar extracts from G. sesquipedale obtained by hot water extraction (31.96 ± 4.77%), and higher than the hot water extraction combined with ultrasound (22.78 ± 1.71%). The glucose content of the (10 min, 100 °C) citric acid pre-treated sample was similar to the hot water treated samples, pre-treated with sodium hydroxide, applying or not applying ultrasound treatment (2.56 ± 0.09%, and 2.50 ± 0.84%) (Martínez-Sanz et al. 2019b). It seems that the galactose content was not impacted by the autoclave extraction, whereas the glucose content was decreased considerably compared to this other study.
Conclusions
Different combinations of time (10, 15 and 20 min) and temperature (100, 110, and 120 °C) were tested to extract agar from G. sesquipedale, applying two different acid pre-treatments (citric acid and acetic acid). The set of parameters and acid pre-treatment that reported the highest yield were (20 min, 120 °C) after a citric acid pre-treatment, 33.53 ± 0.47%. Nevertheless, the citric acid pre-treatment followed by a milder extraction conditions (20 min, 110 °C), which reported a 31.69 ± 3.5% yield, had a higher gel strength 183.78 ± 36.80 g cm−2 compared to 110.03 ± 30.51 g cm−2 of the highest yield. It can be concluded that the temperature of 120 °C had a negative impact on gel strength; nevertheless, it did not affect the molecular weight or the sugar content. No significant differences were found in terms of molecular weight (20 ± 0.2 and 21 ± 0.2 kDa, respectively), TGA profile, ash content (41.01 ± 2.23 and 36.48 ± 1.48%), glucose (0.87 ± 0.58% and 1.02 ± 0.29%, mg mg−1 sample) and galactose (25.23 ± 13.63 and 30.74 ± 5.22%, mg mg-1 sample) content between these two treatments. Considering functionality, chemical properties and functionality, it can be concluded that the (20 min, 110 °C) citric acid pre-treatment was the best treatment to extract agar from G. sesquipedale.
Data availability
Data will be made available on request.
References
BeMiller JN (2019) Polysaccharides. In Carbohydrate Chemistry for Food Scientists, 3rd ed. Elsevier: New York, NY, USA, pp 103–157
Bertasa M, Dodero A, Alloisio M, Vicini S, Riedo C, Sansonetti A, Scalarone D, Castellano M (2020) Agar gel strength: A correlation study between chemical composition and rheological properties. Eur Polym J 123:109442
Gomes-Dias JS, Pereira SG, Teixeira JA, Rocha CM (2022) Hydrothermal treatments–A quick and efficient alternative for agar extraction from Gelidium sesquipedale. Food Hydrocoll 132:107898
Gómez-Mascaraque LG, Martínez-Sanz M, Hogan SA, López-Rubio A, Brodkorb A (2019) Nano- and microstructural evolution of alginate beads in simulated gastrointestinal fluids. Impact of M/G ratio, molecular weight and pH. Carbohydr Polym 223:115121
Gomez LP, Alvarez C, Zhao M, Tiwari U, Curtin J, Garcia-Vaquero M, Tiwari BK (2020) Innovative processing strategies and technologies to obtain hydrocolloids from macroalgae for food applications. Carbohydr Polym 248:116784
Guerrero P, Etxabide A, Leceta I, Peñalba M, De la Caba K (2014) Extraction of agar from Gelidium sesquipedale (Rhodopyta) and surface characterization of agar based films. Carbohydr Polym 99:491–498
Li Y, Zhao M, Gomez LP, Senthamaraikannan R, Padamati RB, O’Donnell CP, Tiwari BK (2021) Investigation of enzyme-assisted methods combined with ultrasonication under a controlled alkali pretreatment for agar extraction from Gelidium sesquipedale. Food Hydrocoll 120:106905
Lomartire S, Gonçalves AM (2022) Novel technologies for seaweed polysaccharides extraction and their use in food with therapeutically applications—A review. Foods 11:2654
Martínez-Sanz M, Gomez-Barrio LP, Zhao M, Tiwari B, Knutsen SH, Ballance S, Zobel HK, Nilsson AE, Krewer C, Östergren K (2021) Alternative protocols for the production of more sustainable agar-based extracts from Gelidium sesquipedale. Algal Res 55:102254
Martínez-Sanz M, Gómez-Mascaraque LG, Ballester AR, Martínez-Abad A, Brodkorb A, López-Rubio A (2019a) Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res 38:101420
Martínez-Sanz M, Martínez-Abad A, López-Rubio A (2019b) Cost-efficient bio-based food packaging films from unpurified agar-based extracts. Food Packag Shelf Life 21:100367
McHugh DJ (2003) A guide to the seaweed industry, FAO Technical Paper 441. Food and Agriculture Organization of the United Nations, Rome
Moldoveanu SC (2021) Analytical pyrolysis of polymeric carbohydrates. In: Moldoveanu SC (ed) Analytical Pyrolysis of Natural Organic Polymers, vol 20, 2nd edn. Elsevier, Amsterdam, pp 111–269
Mostafavi FS, Zaeim D (2020) Agar-based edible films for food packaging applications-A review. Int J Biol Macromol 159:1165–1176
Öğretmen ÖY, Duyar HA (2018) The effect of different extraction methods and pre-treatments on agar yield and physico-chemical properties of Gelidium latifolium (Gelidiaceae, Rhodophyta) from Sinop Peninsula coast of Black Sea, Turkey. J Appl Phycol 30:1355–1360
Ouyang Q-Q, Hu Z, Li S-D, Quan W-Y, Wen L-L, Yang Z-M, Li P-W (2018) Thermal degradation of agar: mechanism and toxicity of products. Food Cem 264:277–283
Pereira L, Sousa A, Coelho H, Amado AM, Ribeiro-Claro PJ (2003) Use of FTIR, FT-Raman and 13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol Eng 20:223–228
Rasheed I, Tabassum A, Khan U, Rehman A (2019) Fourier transform infrared (FT-IR) spectroscopy of agar from red seaweeds of Karachi coast. Int J Biol Biotechnol 16:59–63
Rivadeneira J, Audisio MC, Gorustovich A (2018) Films based on soy protein-agar blends for wound dressing: Effect of different biopolymer proportions on the drug release rate and the physical and antibacterial properties of the films. J Biomater Appl 32:1231–1238
Roleda MY, Ganzon-Fortes ET, Montaño NE (1998) Agar from vegetative and tetrasporic Gelidiella acerosa (Gelidiales, Rhodophyta). Oceanogr Lit Rev 8(45):1464
Roleda M, Montano N, Ganzon-Fortes E, Villanueva R (1997) Acetic acid pretreatment in agar extraction of Philippine Gelidiella acerosa (Forsskaal) Feldmann et Hamel (Rhodophyta, Gelidiales). Bot Mar 40:63-69
Seca AM, Cavaleiro JA, Domingues FM, Silvestre AJ, Evtuguin D, Neto CP (2000) Structural characterization of the lignin from the nodes and internodes of Arundo donax reed. J Agric Food Chem 48:817–824
Sousa AMM, Rocha CMR, Gonçalves MP (2021) Agar. In: Phillips GO, Williams PA (eds) Handbook of Hydrocolloids (Third Edition). Woodhead Publishing, Cambridge pp 731-765
Stiger-Pouvreau V, Bourgougnon N, Deslandes E (2016) Carbohydrates from seaweeds. In: Fleurence J, Levine I (eds) Seaweed in health and disease prevention. Elsevier, Amsterdam, pp 223–274
Sun J, Ren F, Chang Y, Wang P, Li Y, Zhang H, Luo J (2018) Formation and structural properties of acid-induced casein–agar double networks: Role of gelation sequence. Food Hydrocoll 85:291–298
Sun X, Xu F, Sun R, Fowler P, Baird M (2005) Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr Res 340:97–106
Uranga Gama J, Nguyen BT, Si TT, Guerrero Manso PM, De la Caba Ciriza MC (2020) The effect of cross-linking with citric acid on the properties of agar/fish gelatin films. Polymers 12:291
Van Wychen S, Laurens LM (2016) Determination of Total Solids and Ash in Algal Biomass: Laboratory Analytical Procedure (LAP). Technical Report NREL/TP-5100-60956. National Renewable Energy Lab, Golden, CO
Vuai SAH, Mpatani F (2019) Optimization of agar extraction from local seaweed species, Gracilaria salicornia in Tanzania. Phycol Res 67:261–266
Wang SZ, Mu H, Lin Y, Zhang J, Jiang X (2017) Impact of alkali pretreatment on yield, physico-chemical and gelling properties of high quality agar from Gracilaria tenuistipitata. Food Hydrocoll 70:356–362
Zeece M (2020) Carbohydrates. In: Zeece M. Introduction to the Chemistry of Food. Academic Press, London pp 81–125. https://doi.org/10.1016/b978-0-12-809434-1.00003-7
Funding
This work was supported by the European Union Horizon 2020 project BIOCARB-4-FOOD [grant number: 17RDSUSFOOD2ERA-NET1].
Author information
Authors and Affiliations
Contributions
Laura Pilar Gomez Barrio: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft. Dileswar Pradhan: Data curation, Formal analysis, Methodology. Carlos Álvarez García: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. Uma Tiwari: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. James Francis Curtin: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. Amit K. Jaiswal: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. Brijesh K. Tiwari: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gomez Barrio, L.P., Pradhan, D., Tiwari, U. et al. Pressure-based method for the extraction and characterisation of agar from Gelidium sesquipedale. J Appl Phycol 35, 2473–2483 (2023). https://doi.org/10.1007/s10811-023-03076-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03076-y