Abstract
The enhancing effect of three marine bioactive substances (MBS) – EXT1116, NA9158 and 251104 – on the absorption of ammonium and potassium by the root system and the growth of potted grapevine (Vitis vinifera L.) plants is reported. Root ion influxes were determined in vivo by the non-invasive vibrating probe technique. Treatment with MBS generally enhanced nutrient absorption only in the root region between 0.8 and 1.7 mm from the root apex. Among the three substances tested, EXT1116 was the most effective in terms of enhancing the absorption of both ions, with significantly higher values than those of the other two substances and the control. NA9158 and 251104 were more effective in improving ammonium absorption than potassium absorption, while NA9158 was the most effective MBS in enhancing both biomorphometric parameters (shoot length, number of leaves, visual assessment of root system) and dry weight. Based on these results, we suggest that a combination of NA9158 and EXT1116 may be useful in enhancing plant growth by combining the capacity of NA9158 to increase root biomass and that of EXT1116 to enhance mineral absorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Any improvement in culture practices that results in roots becoming more effective in terms of nutrient capture should reduce the negative environmental impacts of agriculture and increase crop production and sustainability in reduced input systems. One such approach is the use of biostimulants that can enhance the effectiveness of conventional mineral fertilizers (Frankenberger and Arshad 1995). Marine bioactive substances (MBS) extracted from seaweeds have been used for several decades to enhance plant growth and productivity. Research in this field is therefore strongly oriented towards searching and testing the effectiveness and efficiency of new products. MBS have been reported to induce higher yields, a better efficiency of nutrient use and resistance to various abiotic and biotic stresses in a wide range of annual and perennial crops, including grapevine (Mancuso et al. 2006; Turan and Köse 2004; Norrie et al. 2001). The mechanisms by which MBS affect cell metabolism are mainly through the physiological action of major and minor nutrients, amino acids, vitamins, and also cytokinins, auxin, and absicisic acid (ABA)-like growth substances (Ördög et al. 2004; Stirk et al. 2003; Durand et al. 2003; Crouch and van Staden 1993; Crouch et al. 1992). Therefore, their effect is a result of many components that may work synergistically at different concentrations, although the mode of action of seaweed extracts still remains quite unknown (Vernieri et al. 2005; Fornes et al. 2002). In recent years, MBS have gained importance due to their potential use in organic and sustainable agriculture, especially in viticulture, as a means to avoid excessive fertilizer applications and to improve mineral absorption, both through leaves and roots. To date, no studies have been conducted aimed at investigating in depth the role of MBS in enhancing both ion fluxes and nutrient absorption rates at the root level. Moreover, while there are a few reports describing the response of grapevines to the foliar application of seaweed extracts with the aim of enhancing the action of the fertilizers (Mancuso et al. 2006; Turan and Köse 2004), there is a total lack of research on the effects of applying MBS to the roots of the grapevines as a means of enhancing growth.
The main objective of this study, therefore, was to evaluate different MBS in enhancing mineral absorption by grapevine (Vitis vinifera) roots and to assess their positive effect on plant growth. In a first step, we measured ammonium (NH4 +) and potassium (K+) fluxes at the root level on intact and healthy root apices by the use of selective microelectrodes in order to test the effectiveness of MBS in improving nutrient absorption at the root level. We subsequently evaluated the effect of the different substances on plant growth and the development of the root system.
Materials and methods
Plant materials and treatments
Experiments were carried out during the spring–summer period (May–September) at the Department of Horticulture of the University of Florence. One-year-old rooted cuttings of Vitis vinifera cv. Sangiovese grafted on Vitis berlandieri X riparia 420A were planted in plastic containers (diameter: 20 cm) filled with a peat-pumice mixture (1:1, v:v), placed on benches in a climate-controlled greenhouse (28/20°C day/night, 70% RH, ambient illumination and photoperiod) and fert-irrigated by an ebb-and-flow system with a modified Hoagland’s solution (pH 5.5, EC 1.3 mS cm−1; NO3 −, 102 ppm; NH4 +, 5 ppm; PO4 3 −, 29 ppm; K+, 153 ppm; Ca2+ 88 ppm; Mg2+,18 ppm; SO4 2− −, 37 ppm). Three different MBS (251104, NA9158 and EXT1116) provided by BiotechMarine (Pontrieux, France) were separately compared with a control treatment (distilled water).
251104 was extracted from Laminariales and contained an auxin-rich fraction (1200 μg L−1) including IAA (indole-3-acetic acid), IAAme (indole-3-acetic acid methyl ester), IBA (indole-3-butyric acid) and I2CA (indole-2-carboxylic acid). The extract was fractionated on a solid-phase extraction (SPE)-C18 cartridge to isolate the auxin fraction, according to Dobrev and Miroslav (2002); quantification was performed by high-performance liquid chromatography (HPLC; Alliance 2695–Waters, Sunfire C18 column; Waters, Mass.) associated with a mass spectrometer in a MS/MS system (Quattro Micro API; Waters), according to Kowalczyk and Sandberg (2001).
NA9158 was a water extract obtained after acid hydrolysis from Fucales.
EXT1116, extracted from Ulvales, contained a polysaccharide (glucuroxylorhamnan)-rich fraction in the water extract. The glucuroxylorhamnans were precipitated in ethanol, then freeze-dried before their identification on an infra-red (IR) spectrophotometer (Perkin Elmer model 6000; Calif.), according to Pengzhan et al. (2003).
All of the biostimulants were applied at root level as a 0.1% solution in distilled water, as suggested by the company providing the MBS, once a week for the entire period of the experiment.
Ion flux measurements
Ammonium and K+ flux measurements were performed using the vibrating probe technique, the only method available for measuring actual ion fluxes in a plant cell in a non-invasive manner. A detailed description of the technique is provided by Shabala et al. (2006) and Mancuso et al. (2000) or in the review by Mugnai et al. (2006). Briefly, Vitis vinifera healthy root apices (5–6 mm long) were cut, carefully washed with deionized water and placed one at a time at the bottom of the measuring chamber containing a nutrient solution (NH4 +, 5 ppm; K+, 153 ppm) and a MBS (0.1%). Flux measurements were performed at 25 ± 0.25°C, with the ion-selective microelectrode positioned close to the root surface. During recording, the microelectrode oscillated in a square wave parallel to the electrode axis over a distance of 10 μm (frequency: 0.1 Hz), moving along the entire root length. The computer calculated the difference between each electrode position voltage and the previous one at the other extreme position and evaluated a moving average of these differences over any desired time period, computing in this way the potential difference. NH4 + and K+ influxes were finally calculated using Fick’s first law of diffusion, assuming a cylindrical diffusion geometry. The flux measurements were made on at least five different root apices per each treatment (n ≥ 5).
Growth analysis
Growth analysis was performed using (1) biomorphometric and (2) dry weight measurements. The biomorphometric measurements consisted of weekly measurements of shoot elongation and number of leaves, starting from the end of May until mid-September. Dry weight measurements were carried at the end of the experimental period (mid-September) on leaf, stem and root samples dried in an oven at 70°C for at least 48 h. Visual assessment of root health was conducted after having gently washed the root system.
Statistical analysis
The whole experiment was in a completely randomized design. Each treatment consisted of 25 plants, with each plant representing a replicate. Data were subjected to one-way ANOVA, and means were separated by Tukey’s test (P < 0.05, n = 5). Statistical analysis was carried out using GraphPad Prism ver. 4.0 (GraphPad Software, San Diego, Calif.).
Results
Ion flux measurements
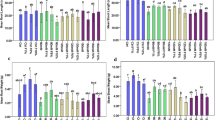
In comparison to the control treatment, MBS generally enhanced and improved root nutrient absorption in the part of the root 0.8 and 1.7 mm from the root apex, but the different MBS showed varying degrees of effectiveness (Fig. 1). EXT1116 was the most effective of the three MBS in terms of enhancing both NH4 + and K+ influx. The maximum influx value for NH4 + was observed to occur between 0.8 and 1.4 mm from the root apex in EXT1106-treated plants (120–130 pmol cm−2 s−1), which is significantly higher than the values observed for NA9158- (90 pmol cm−2 s−1) and 251104-treated (75–80 pmol cm−2 s−1) plants (Fig. 1a). Significant differences were also found between the latter two MBS, but only in the region between 1.1 and 1.4 mm from the root apex, whereas significant differences between 251104 and the control treatment were observed only in the region between 1.0 and 1.7 mm, indicating a very low effectiveness.
Differences among treatments in terms of K+ absorption rate values were less evident than those for NH4 + (Fig. 1b) and were only observable in a smaller region (1–1.2 mm from the root apex). EXT1116 confirmed its effectiveness as a MBS, showing a significantly enhanced effect in comparison with the other substances. No differences were found among NA9158, 251104 and control, except for 251104 in the region between 0.4 and 0.6 mm from the root apex.
Growth analysis
NA9158 was the most effective MBS in enhancing both biomorphometric and dry weight parameters (Table 1). NA9158 strongly and significantly improved shoot elongation (Fig. 2a) compared to the other substances, especially during the summer period (mid-July and August) when growth was partially slowed down due to high temperatures. Plants treated with the other MBS almost ceased shoot elongation starting from mid-July (no differences between EXT1116, 251104 and control were observed), whereas shoots of NA9158-treated plants continued their growth. The number of leaves was also affected by the use of a MBS (Fig. 2b), but only during the last period of growth, as no significant differences were found between treatments until mid-August, after which time NA9158 and 251104 continued to enhance new leaf formation compared to the control and EXT1104 treatments, which had no improving effect in terms of leaf formation from the beginning of July. Total and organ dry weight (root, stem and leaves) was measured at the end of the experimental period. Among the tested MBS, only NA9158 affected total dry weight compared to control (Table 1). The dry weights of the separate plant organs showed no differences between the MBS treatments and the control, except for root dry weight. NA9158 significantly improved root size, a finding also confirmed by visual assessment (Fig. 3). Visual assessment also revealed a general improvement in the size, health and presence of fine roots in MBS-treated plants compared to the controls. Among the MBS, NA9158 emerged as the most effective in enhancing root improvement, both in terms of dry weight and structure, as few primary roots were detectable despite a general development of young roots.
Discussion
Biostimulants, even those containing varying levels of mineral fertilizers, are not able to supply all of the essential nutrients in the quantities the plant needs (Schmidt et al. 2003), but one of their main function is to increase plant mineral uptake, thereby improving the efficiency of nutrient use both at the root (Vernieri et al. 2005) and leaf levels (Mancuso et al. 2006). Although evidence for the uptake of most major and micro-nutrients by the root apex has been empirically verified, the signalling pathways involved in regulating these transport mechanisms remain elusive (Gilroy and Jones 2000). The driving force for most nutrient uptake in plants is the electrochemical gradient across the plasma membrane, a major proportion of which is generated by H+–ATPase. In support of the theory that root apices and root hairs are involved in active nutrient uptake, high levels of expression of H+–ATPase genes have been detected in Nicotiana (Moriau et al. 1999), with the strongest expression occurring in developing root apices and root hairs and reduced expression in the region of the mature root. Although the spatial localization of H+–ATPase proteins is still unknown, evidence obtained with vibrating pH-sensitive microelectrodes indicates a strong H+ efflux from the base of the root apex and an apparent tip-localized H+ influx (Palmgren 2001). Our data clearly indicate that NH4 + and K+ influxes in grapevine root apices were mainly located in the so-called “transition zone (TZ)” of the root apex, a root area showing very active metabolism (Baluska et al. 2004) that is positioned between the dividing zone (meristem) and the elongating region. As such, the TZ seems to be strongly involved in nutrient absorption in Vitis vinifera roots, as previously shown both in other perennial plants (copper in Olea europea, Papeschi et al. 2000) and herbaceous plants (K+ in Hordeum vulgare, Pang et al. 2006). As the plasma membrane-located H+–ATPase is extensively regulated by both IAA (Tanimoto 2005) and polysaccharides (Camoni et al. 2006), we hypothesize that MBS should directly affect H+–ATPase activity in the TZ region due to their high levels of auxin-like and polysaccharide components. All of the MBS tested here enhanced ion influxes, but they showed a different effectiveness, as 251104 and NA9158 led to a slight improvement in the ion uptake peaks compared to the control, whereas EXT1116 application strongly enhanced NH4 + uptake (and K+ to a less extent, Fig. 1). In our case, the EXT1116 polysaccharide fraction seems to be more implicated and effective in the regulation of H+–ATPase activity than the 251104 auxin compounds. This observation was also made by Camoni et al. (2006), who reported that sugar signalling regulates the H+–ATPase, the enzyme being maintained in an activated state by a non-limiting sugar supply.
Biostimulants are normally able to affect root development, increasing both lateral root formation (Vernieri et al. 2005; Atzmon and van Staden 1994) and the overall size of the root system (Mancuso et al. 2006; Slàvik 2005; Thompson 2004). Unfortunately, the role of biostimulants in plant growth enhancement is still under study, mainly due to the multitude of products and combinations thereof and to the fact that the effects of biostimulant application seems to be species-related. For example, while the use of biostimulants has been reported to increase the rate of development of different plant species (Coffea, Alnus, Pinus; see Berlyn and Sivaramakrishnan 1996), but there is as yet no report of statistically significant differences between non-treated and biostimulant-treated seedlings of Betula papylifera (Richardson et al. 2004). Ferrini and Nicese (2002) found no differences in the leaf fresh/dry weight ratio and specific leaf weight in Quercus robur seedlings, while leaf area and leaf dry weight were higher in the treated plants. Kelting et al. (1998), who tested different biostimulants on Acer rubrum and Crataegus phaenopyrum, noticed no differences in height, stem diameter, top dry mass or root length in Acer, but they did observed significant differences in top dry mass in Crataegus. In the present study, there was a clear increment of growth in potted V. vinifera plants in terms of dry weight only when NA9158 was applied. Moreover, NA9158 was very effective in enhancing shoot length and number of leaves, especially during the hot summer period. Following this period, this product also stimulated a quick and effective growth reprise, which is usually very important in V. vinifera plants before ripening and harvesting. On the contrary, both 251184 and EXT1116 did not show any clear effectiveness in enhancing plant growth, despite their high content of promoting (auxins, 251104) and energetic (polysaccharides, EXT1116) compounds. This apparent discrepancy related to the known effects of these compounds on plant growth is probably due to the low concentrations used during the experiment being inadequate to stimulate any growth improvement, or to a local mode of action without any translocation to the aerial part, as the two biostimulants were clearly able to enhance both nutrient absorption and root biomass development (Fig. 3). NA9158 was also capable of increasing root biomass, both in dimensions (root dry weight) and quality (more root hairs compared to the other treatments). EXT1116 was less effective in increasing root biomass and growth, but roots treated with this MBS showed a very high enhancement of mineral root absorption, both for NH4 + and, to a lesser extent, for K+. Based on our results and under our experimental conditions, we suggest that a combination of NA9158 and EXT1116 would be a useful and helpful supplement as it combines the capacity of NA9158 to increase root biomass with the ability of EXT1116 to enhance mineral absorption. Consequently, a mix of these two MBS mostly likely would lead to a synergistic advantage.
Abbreviations
- MBS:
-
Marine Bioactive Substances
- TZ:
-
Transition Zone
References
Atzmon N, van Staden J (1994) The effect of seaweed concentrate on the growth of Pinus pinea seedlings. New For 8:279–288
Baluska F, Mancuso S, Barlow P, Volkmann D (2004) Root apices as plant command centres: the unique ‘brain-like’ status of the root apex transition zone. Biologia 13:1–13
Berlyn GP, Sivaramakrishnan S (1996) The use of organic biostimulants to reduce fertilizer use, increase stress resistance, and promote growth. In: Landis TD, South DB (eds) Natl Proc For Conserv Nursery Assoc Meet. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland
Camoni L, Marra M, Garufi A, Visconti S, Aducci P (2006) The maize root plasma membrane H+–ATPase is regulated by a sugar-induced transduction pathway. Plant Cell Physiol 47:743–747
Crouch IJ, van Staden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul 13:21–29
Crouch IJ, Smith MT, van Staden J, Lewis MJ, Hoad GV (1992) Identification of auxins in a commercial seaweed concentrate. J Plant Physiol 139:590–594
Dobrev PI, Miroslav K (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr 950:21–29
Durand N, Briand X, Meyer C (2003) The effect of marine bioactive substances (N PRO) and exogenous cytokinins on nitrate reductase activity in Arabidopsis thaliana. Physiol Plant 119:489–493
Ferrini F, Nicese FP (2002) Response of English oak (Quercus robur L.) trees to biostimulants application in the urban environment. J Arb 28:70–75
Fornes F, Sànchez-Perales M, Guadiola JL (2002) Effect of a seaweed extract on the productivity of ‘de Nules’ clementine mandarin and navelina orange. Bot Mar 45:486–489
Frankenberger WT, Arshad M (1995) Phytohormones in soils. Marcel Dekker, New York
Gilroy S, Jones DL (2000) Through form to function: root hair development and nutrient uptake. Trends Plant Sci 5:56–60
Kelting MP, Harris JR, Fanelli J, Appleton B (1998) Biostimulants and soil amendments affect two-year posttransplant growth of red maple and Washington hawthorn. HortScience 33:819–822
Kowalczyk M, Sandberg G (2001) Quantitative analysis of indole-3-acetic acid metabolites in Arabidopsis. Plant Physiol 127:1845–1853
Mancuso S, Azzarello E, Mugnai S, Briand X (2006) Marine bioactive substances (IPA extract) improve ion fluxes and water stress tolerance in potted Vitis vinifera plants. Adv Hortic Sci 20:156–161
Mancuso S, Papeschi G, Marras AM (2000) A polarographic, oxygen-selective, vibrating-microelectrode system for the spatial and temporal characterisation of transmembrane oxygen fluxes in plants. Planta 211:384–389
Moriau L, Michelet B, Bogaerts P, Lambert L, Michel A, Oufattole M, Boutry M (1999) Expression analysis of two gene subfamilies encoding the plasma membrane H+-ATPase in Nicotiana plumbaginifolia reveals the major transport functions of this enzyme. Plant J 19:31–41
Mugnai S, Pandolfi C, Azzarello E, Masi E, Mancuso S (2006) Using vibrating selective microelectrodes for flux measurements in roots. In: Texeira Da Silva J (ed.) Floriculture, ornamental and plant biotechnology: advances and topical issues Global Science Books, Kyoto, pp 176–182
Norrie J, Branson T, Keathley PE (2001) Marine plant extracts impact on grape yield and quality. Acta Hortic 594:315–319
Ördög V, Stirk WA, Lenobel R, Bancìřova M, van Staden J, Szigeti J, Németh L (2004) Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J Appl Phycol 16:309–314
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845
Pang JY, Newman I, Mendham N, Zhou M, Shabala S (2006) Microelectrode ion and O2 fluxes measurements reveal differential sensitivity of barley root tissues to hypoxia. Plant Cell Environ 29:1107–1121
Papeschi G, Mancuso S, Marras AM (2000) Electrochemical behaviour of a Cu/CuSe microelectrode and its application in detecting temporal and spatial localisation of copper(II) fluxes along Olea europaea roots. J Solid State Electrochem 4:325–329
Pengzhan Y, Quanbin Z, Ning L, Zuhong X, Yanmei W, Zhi’enet L (2003) Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J Appl Phycol 15:21–27
Richardson AD, Aikens M, Berlyn GP, Marshall P (2004) Drought stress and paper birch (Betula papyrifera) seedlings: effects of an organic biostimulant on plant health and stress tolerance, and detection of stress effects with instrument-based, noninvasive methods. J Arb 30:52–60
Schmidt RE, Ervin EH, Zhang X (2003) Questions and answers about biostimulants. Golf Course Manage 71:91–94
Shabala S, Shabala L, Gradmann D, Chen Z, Newman I, Mancuso S (2006) Oscillations in plant membrane-transport activity: model predictions, experimental validation, and physiological implications. J Exp Bot 57:171–184
Slàvik M (2005) Production of Norway spruce (Picea abies) seedlings on substrate mixes using growth stimulants. J For Sci 51:15–23
Stirk WA, Novàk O, Strnad M, van Staden J (2003) Cytokinins in macroalgae. Plant Growth Regul 41:13–24
Tanimoto E (2005) Regulation of root growth by plant hormones. Roles for auxin and gibberellin. Crit Rev Plant Sci 24:249–265
Thompson B (2004) Five years of Irish trials on biostimulants: the conversion of a skeptic. USDA Forest Serv Proc 33:72–79
Turan M, Köse C (2004) Seaweed extracts improve copper uptake of grapevine. Acta Agric Scand 54:213–220
Vernieri P, Borghesi E, Ferrante A, Magnani G (2005) Application of biostimulants in floating system for improving rocket quality. J Food Agric Environ 3:86–88
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mugnai, S., Azzarello, E., Pandolfi, C. et al. Enhancement of ammonium and potassium root influxes by the application of marine bioactive substances positively affects Vitis vinifera plant growth. J Appl Phycol 20, 177–182 (2008). https://doi.org/10.1007/s10811-007-9203-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-007-9203-6