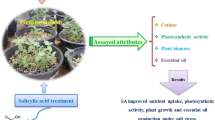
Abstract
High soil salinity is a major abiotic stress affecting the growth, nutrition, development, and productivity of crops. This study investigated the modulating effect of combined microalgae-cyanobacteria extract formulations (MEF1%, MEF5%, and MEF10%) prepared from the species Dunaliella salina, Chlorella ellipsoidea, Aphanothece sp., and Arthrospira maxima, on tomato plant growth and tolerance under four NaCl concentrations (0, 80, 120, and 150 mM). MEF5% enhanced the vegetative growth of tomato plants, characterized by higher shoot and root weight and larger leaf area. According to principal component analysis (PCA), improved plant growth was closely associated with leaf photosynthetic pigments, which was mainly due to improved osmotic adjustment and ion homeostasis. Proline accumulation was significantly enhanced by MEF5%-treatment in plants grown under 120 mM and 150 mM NaCl conditions. MEF5%-treatment also significantly improved nitrogen (N), phosphorus (P), and potassium (K+) absorption in plants grown at 80 mM and 120 mM NaCl levels. Leaf lipid peroxidation through ROS oxidative stress significantly decreased with enhanced CAT and SOD activities in MEF5%-treated plants. MEF5% triggered a significant decline in fatty acid content, indicating fatty acid transformation into other lipid forms such as alkanes, which are essential in the cuticular wax synthesis of hydric stressed plants. Enhanced K+ uptake and reduced Na+/K+ ratio in the leaves of treated plants indicate MEF’s active role in reestablishing ion homeostasis. Nutrient uptake can be improved by enhanced root biomass, which subsequently increases the roots’ surface for nutrient absorption. These results indicate that MEF stimulated plant growth and tolerance responses through (i) enhanced antioxidant enzyme activities and (ii) improved root growth and nutrient uptake. Therefore, combined microalgae-cyanobacteria formulations could be another sustainable alternative to boost nutrient uptake, growth, and crop adaptability under normal and saline conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the global population increases and negative effects of climatic change escalate, modern agriculture is challenged to search for efficient and eco-friendly methods of increasing crop productivity and tolerance against the harsh environmental conditions. This means that producers are compelled to cut-down the excessive applications of chemical fertilizers and pesticides which pose both short- and long-term threats to human health and the entire ecosystem (Carvalho 2017; Rahman and Zhang 2018).
To address this challenge, innovative sustainable agricultural products including plant growth biostimulants have been largely described (Calvo et al. 2014; Elzaawely et al. 2017; Van Oosten et al. 2017; Yakhin et al. 2017; Desoky et al. 2018). According to the European Union Fertilizing Products Regulation 2019/1009, biostimulants are defined as “fertilizing products the function of which is to stimulate plant nutrition processes independently of the product’s nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: i) nutrient use efficiency, ii) tolerance to abiotic stress, iii) quality traits, or iv) availability of confined nutrients in the soil or rhizosphere” (Ricci 2020). Among the many categories of plant biostimulants, seaweeds, more than microalgae, have been exploited extensively and represent an important category of organic biostimulants (Khan et al. 2011; Battacharyya et al. 2015; Colla et al. 2017). Microalgae and cyanobacteria are now gaining popularity as renewable bioactive resources that can be exploited in agriculture for the development of plant biostimulants. Microalgae (eukaryotic) and cyanobacteria (prokaryotic) are unicellular microscopic photosynthetic organisms that grow in diverse aquatic habitats and even humid soils (Khan et al. 2018). They have their capacity to produce a diversity of biologically active molecules such as sulfated polysaccharides, osmolytes, phytohormones, amino acids, and phenolics ( Cuellar-Bermudez et al. 2015; De Morais et al. 2015; Renuka et al. 2018). An increasing number of studies have been conducted to highlight biostimulant properties of extracts from different microalgae and cyanobacteria species (Chiaiese et al. 2018; Ronga et al. 2019; Carillo et al. 2020; Colla and Rouphael 2020). Such extracts have been tested on a broad range of biological activities in higher plants, including nutrient uptake, crop performance, and tolerance to biotic and abiotic stress (Garcia-Gonzalez and Sommerfeld 2016; Barone et al. 2018; El Arroussi et al. 2018; Chanda et al. 2019; Farid et al. 2019; Rachidi et al. 2020).
Soil salinization is one of the major abiotic stressors in agriculture, affecting about 20% of the world irrigated surfaces, particularly in Mediterranean zones (Libutti et al. 2018; Shahid et al. 2018). Salt stress leads to ionic imbalance in plants due to excessive accumulation of Na+ and Cl–, which reduces the uptake of other mineral nutrients such as K+, Ca2+, and Mn2+. Excess Na+ accumulation leads to nutritional imbalance, membrane permeability and instability (resulting from Ca+ displacement by Na+), and an overproduction of reactive oxygen species (ROS), which cause oxidative damage on cellular macromolecules (Arif et al. 2020; Zhao et al. 2020). These biochemical alterations limit plant growth and performance, leading to crop yield reduction. To counteract salinity stress, plants induce a cascade of specific adaptative responses including K+ transport, phospholipid modifications, activation of ROS-scavenging enzymes, and production of compatible solutes to compensate for the osmotic pressure of Na+ (Acosta-Motos et al. 2017; Yang and Guo 2018; Arif et al. 2020; Van Zelm et al. 2020). Such adequate natural stress responses play an important role in inducing plant tolerance on saline soils.
The present study aimed to investigate the combined effect of microalgae-cyanobacteria extracts as stimulators of salt stress tolerance responses, nutrient uptake, and vegetative growth of tomato plants under saline conditions. The study also investigates possible action mechanisms of MEF formulations as enhancers of salt stress tolerance and crop growth under saline conditions.
Material and methods
Culture of microalgae
Two microalgae (Dunaliela salina MSD 002 and Chlorella ellipsoidea BEA 0337) and two cyanobacteria species (Aphanothece sp. BEA O935B and Arthrospira maxima MSS001) were selected from the AlgoBioTech collection of the Moroccan Foundation for Advanced Science, Innovation and Research (MAScIR). They were cultivated in 3 replicates using 250 mL Erlenmeyer flasks containing using the following media: D. salina was cultured in F2 medium at pH = 7.8; Arthospira maxima in Zarrouk’s medium at pH = 9.5; Aphanothece sp. and C. ellipsoidea were cultured in BG11 medium at pH = 7.2. The culture medium for D. salina was made with sea-salt solution. The initial optical density for all cultures was 2 ± 0.2. All cultures were placed in a photo-incubator at 25 °C under constant orbital agitation, and 135 µmol photons m−2 s−1 of continuous white fluorescent light. After 30 days, the biomass was harvested by centrifugation at 7000 xg for 5 min at 4 °C. The collected biomasses were dried at 50 °C for 7 days.
Preparation of the microalgae extract formulation
Based on the optimal extract concentrations previously established when screening microalgae extracts (Chanda et al. 2020), the dry biomasses of the two microalgae and cyanobacteria species were ground in liquid nitrogen. Then, 300 mg of the mixture was hydrolysed in 20 mL 2% sulfuric acid. The resultant slurry was heated for 3 h at 95 °C with constant stirring (interrupted every 30 min by 1 min vortexing and 15 min sonification). The mixture was then autoclaved at 121 °C and 106 kPa for 30 min. The total crude extract was cooled to room temperature, and stored at − 20 °C. The extract was tested at three concentrations: 1%, 5%, and 10% (v/v of MEF formulation in distilled water).
The pH of the extract formulations was adjusted to 5.8 using NaOH before application to plants by soil drenching.
Experimental design and plant growth conditions
The plant model used in this study was Solanum lycopersicum L. JANA F1 obtained from BAYER Nunhems Netherlands BV. Tomato seeds were sown on 24 cell seed trays (6 cm2) filled with peat moss (Gebr. Brill Substrate, Germany) and were irrigated with deionized water. After germination (5 days after sowing), the trays were placed in a Phytotron chamber set at 26 °C, 16:8 h photoperiod, 240 μmol photons m−2 s−1, and 60–70% relative humidity.
The experimental design consisted of four culture groups defined by NaCl concentrations. Each culture group consisted of treated and untreated plants:
Group0mM—Control 0 mM NaCl (Untreated plants under normal condition) + treated plants under normal conditions.
Group80mM—Control 80 mM NaCl (Untreated plants under 80 mM conditions) + treated plants at 80 mM conditions.
Group120mM—Control 120 mM NaCl (Untreated plants under 120 mM conditions) + treated plants at 120 mM conditions.
Group150mM—Control 150 mM NaCl (Untreated plants under 150 mM conditions) + treated plants at 150 mM conditions.
The seedling trays (one plant per pot) were arranged in a completely randomized block design in the growth chamber. Five replications were sampled in each treatment. Three most homogenous replicates were selected for biochemical studies. Plants were grown for 35 days (after germination) under controlled environmental conditions at the Moroccan Foundation for Advanced Science Research and Innovation (MAScIR). The growth chamber conditions were set at 25 °C, 16:8 day/night photoperiod, 240 μmol photons m−2 s−1, and 60–70% relative humidity.
At 40 days old, the plants were harvested. The roots were washed under running tap water and the root and shoot lengths were measured manually with a ruler.
Salinity application
To prevent salt-exciting reactions, 50 mM NaCl was initially applied to the peat moss and the NaCl concentration was gradually increased by a unit of 50 until the predetermined application concentration for each treatment was reached. MEF were applied to plants every week by irrigation (10 mL per pot). Two weeks after sowing, all plants were irrigated every 2 days with 10 mL of a plant nutrient solution prepared according to the table presented in Supplementary information (S2) . All plant groups (treated and non-treated control plants) were supplied with equal recommended doses of nutrients according to Smith et al. (1983) and Khan et al. (2012)
The nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and sodium (Na) concentrations in the leaf biomass were analyzed using a skalar nutrient auto analyser at MAScIR; the resulting differences observed in the nutrient concentrations were therefore an indicator of the nutrient absorption by roots.
Measuring NaCl retained in the peat moss soil
The peat moss salinity was measured by determining the electrical conductivity (EC) of the peat moss at 24 °C from 1:5 peatmoss/distilled water suspension, according to the method described by Hardie and Doyle (2012) with a few modifications. The peat moss was dried at 70 °C for 24 h. Then, 1:5 peat moss/water suspension was prepared by adding 10 g of the dried peat moss to 50 mL of deionized water in a 250-mL glass beaker. The beaker was thoroughly closed using aluminum foil and agitated on an orbital shaker at 145 rpm and 25 °C for 30 min to dissolve soluble salts. The peat moss mixture was filtered through a sterile hydrophilic cotton gauze due to the texture of the soil (peat moss) which is not easily sedimented at the ratio of 1:5. Then, the EC, soil salinity, and total dissolved solutes (TDS) were measured using an EC meter—EC300VWR—by dipping the electrode into the supernatant. The reference solutions were prepared by dissolving 3 g dried sodium chloride (NaCl) in 1 L of distilled water for 50 mM NaCl, 6 g for 100 mM, 9 g for 150 mM, and 12 g for 200 mM. The electrode was rinsed with distilled water between samples.
Determination of photosynthetic pigments content
For each replicate, 100 mg leaf biomass ground in liquid nitrogen was homogenized in 5 mL 95% ethanol containing 0.1% (w/v) CaCO3 and left overnight at 4 °C. The homogenate was then vortexed for 30 s and centrifuged at 7000 xg, 4 °C for 5 min. The optic densities of all the samples were measured using a UV/visible spectrophotometer (Ultropec 3100 pro_ Amersham Biosciences) and calculated using the method described by Lichtenthaler (1987) and Lichtenthaler and Buschmann (2001): 95% ethanol and 0.1% (w/v) of CaCO3 served as blank.
Determination of antioxidant enzyme activities
Superoxide dismutase, peroxidase, and catalase .
Determination of superoxide dismutase (SOD) antioxidant enzyme activities was performed using the method of Meloni et al. (2003). All specific activities of enzyme fractions were calculated based on the amount of protein in the fraction and the protein content was determined according to the Bradford method (Bradford 1976) using bovine serum albumin (BSA) as standard. For the extraction method, 500 mg leaf biomass was homogenized in 5 mL potassium phosphate buffer 10 mM (pH 7.0) containing 4% (w/v) polyvinylpyrrolidone. The homogenate was centrifuged at 3000 g for 30 min and the supernatant was used as the enzyme extract. All experiments on enzyme activities were carried out on ice.
For peroxidase (POD) antioxidant enzyme activity, the following reaction mixture was used: 3 mL containing 10 mM potassium phosphate buffer (pH 7), 600 µL guaiacol 1% (w/v), and 60 µL enzyme extract. The reaction was initiated with the addition of 150 µL 100 mM H2O2. The increase in absorbance due to the formation of tetraguaiacol was recorded at 470 nm; an identical mixture with no H2O2 was used as blank. The linear initial reaction rate was used to estimate the activity, expressed in mM of the guaiacol dehydrogenation product (GDHP) formed per milligram of protein per minute, using the extinction coefficient of 26.6 mM−1 cm−1.57 (U mg−1 min−1) (Velikova et al. 2000). The activity of catalase (CAT) was assayed by measuring the initial rate of the disappearance of H2O2. The CAT assay reaction mixture (3 mL) contained 10 mM potassium phosphate buffer (pH 7.0), 100 µL enzyme extract, and 0.035 mL H2O2 3%. The decrease in H2O2 was followed as decline in optic density at 240 nm, and the activity was calculated using the extinction coefficient (40 mM−1 cm−1) for H2O2 (Velikova et al. 2000).
Determination of malonyldialdehyde
Lipid peroxidation in the leaf biomass was determined according to the method described by Velikova et al. (2000), using thiobarbituric acid (TBA). TBA determines malonyldialdehyde (MDA) concentration (the final product of lipid peroxidation). The leaf biomass (500 mg FW) ground in liquid nitrogen was homogenized in 5 mL 0.1% trichloroacetic acid (TCA) solution (w/v) and the homogenates were centrifuged at 10000 g for 20 min at 4 °C. Then, 500 µL supernatant was added to 1 mL containing 0.5% (w/v) TBA and 20% TCA. The mixture was incubated in boiling water for 30 min, and the reaction was stopped by placing the reaction tubes in an ice bath. The samples were then centrifuged at 10000 g for 5 min, and the supernatant absorbance was read at 532 nm. The value of the non-specific absorption at 600 nm was subtracted. The amount of complex MDA–TBA (red pigment) was calculated from the extinction coefficient 155 mM−1 cm−1.572.
Determination of proline
In this method according to Carillo and Gibon (2011), 20 mg ground leaf biomass was homogenized in 1 mL ethanol:water (v / v) (70:30). For each sample, 500 µL of the ethanolic extract was added to 1 mL of the reaction mixture containing 1% (w/v) ninhydrin, 60% (v/v) acetic acid, and 20% (v/v) ethanol. The mixture was heated at 95 °C on a heat block for 20 min. After centrifugation at 10000 g for 1 min, the absorbance was measured at 520 nm and the proline content was calculated from the standard curve as µg mg−1 of protein. A standard curve for proline was prepared using L-proline.
Determination of polyphenols
Phenolics were assayed by homogenizing 20 mg leaf biomass in 2 mL 95% (v/v) methanol, according to Ainsworth and Gillespie (2007). Samples were then incubated at room temperature for 48 h in the dark and centrifuged at 13000 g for 5 min at room temperature; the supernatant was recovered. For each sample, 100 µL of the recovered supernatant was placed in 2 mL tubes, to which 200 µL 10% (v/v) Folin–Ciocalteu reagent was added. The reaction mixture was vortexed, and 800 µL Na2CO3 (700 mM) was added to each tube and incubated for 2 h at room temperature. The sample absorbance and gallic acid standard range were measured at 765 nm.
GC–MS lipidomic analysis of tomato plant
Extraction.
The extraction and transesterification of lipophilic metabolites were carried out according to Kamthan et al. (2012) with modifications optimized by MAScIR. Leaf biomass (400 mg FW) ground in liquid nitrogen was added to a glass vial. Then, 10 µL internal standard dodecane and 4 mL chloroform (pre-cooled at − 20 °C) were added to the vial. The vials were tightly covered with caps, thoroughly vortexed for 1 min and heated at 85 °C for 30 min on a heat block. The vials were then vortexed for 1 min and sonicated for 15 min at 60 °C in an ultrasound bath (Branson ultrasonic Sonifier 450, USA). The heating and sonication process were repeated four times. Then, 2 mL methanol was added to the vials, thoroughly vortexed and transferred back into the ultrasound bath for 2 h at 60 °C. The 2 h was interrupted by 1 min vortexing every 30 min. For the separation phase, 1 mL distilled water was added to the vials, and the bottom organic phase was transferred to clean vials with the help of a separating funnel. The CHCl3 solvent was then evaporated completely under nitrogen flow.
Transesterification.
For transesterification, 500 µL 6% methanolic HCl (v/v) was added to the dried residue. The mixture was heated for 30 min at 85 °C, then vortexed and sonicated for 15 min at 60 °C. The heating and sonication process was repeated four times. The mixture was dried under nitrogen flow, and 250 µL distilled H2O and 750 µL CHCl3 were added to the dried residue and vortexed for 1 min. The bottom organic phase was transferred to clean vials with the help of a separating funnel and stored at − 20 for GC–MS analysis.
Metabolomics analysis was carried out using gas chromatography (GC) (Agilent 7890A Series GC, USA) coupled to mass spectrometry (MS) equipped with multimode injector and BD-ASTMD6584 column (15 m × 0.320 mm × 0.1 µm) and electron impact ionization. The soluble extract (4 µL) was injected into the column by 1:5 split mode using helium as the carrier gas at 3 mL min−1. The detection was done using full scan mode between 30 and 1000 m/z, with gain factor of 5. The temperatures of the ion source and the quadrupoles were 230 and 150 °C, respectively. The oven temperature was maintained at 30 °C for 1 min and then increased at 10 °C min−1 to 250 °C then at 20 °C min−1 until 340 °C. The identification was carried out using NIST 2017 MS Library. The amount of each compound was estimated by comparing the peak area with that of the internal standard (dodecane).
Statistical analysis
Statistical analyses were performed by IBM SPSS statistics 22 and RStudio software. Results represent the descriptive statistics and statistically significant differences between the mean values of the control and treated plant samples. Data was analyzed with two-way ANOVA. The statistically significant differences between the mean values were determined using Tukey’s post hoc test. Results are expressed as the mean ± SE of three replicates. Significance levels (p < 0.05) are represented by different letters. The PCA and heatmap were generated using RStudio, visualization of corrplot, and ggplot packages, integrated into the R software. In order to perform the analysis, the data (mean values) was normalized into a standard range of − 1 to + 1 using the equation x′ = (xmean − xi) / (x max − x min). The first two components explained the maximum variance in the datasets.
Results
Plant morphological responses to MEF treatment across different NaCl concentrations
Shoot and root lengths
One of the first observable responses in plants subjected to salinity stress is the reduction in the shoot length (SL). Non-stressed control plants exhibited the highest shoot length, which decreased with increasing NaCl concentrations (Fig. 1a). No significant root length changes were recorded across all culture groups after treatment with MEF. Treatment with MEF 1%, 5%, and 10% exhibited significant effects (22.00%, 18.22%, and 21.62% SL increase, respectively) on the shoot length of plant cultures grown at 80 mM NaCl (Fig. 1b). Unstressed control plants treated with MEF1% showed very significant increase in shoot length (14.73%). There was also significant SL increase in plants treated with MEF 1%, 10%, and 1%, grown at 120 mM, 120 mM, and 150 mM, respectively (Fig. 1b).
Shoot and root weights
There were significant improvements on shoot and root weights (Fig. 1c and d). Treatment with MEF5% significantly enhanced root weight, notably in unstressed control plants and plants grown at 80 mM NaCl, where the highest percentage improvements were 68.60% and 87.50%, respectively (Fig. 1c). The results also show extremely significant increase in shoot weight (70.19%, 54.44%, and 53.44%) for plants grown at 80 mM NaCl and treated with MEF1%, MEF5%, and MEF10%, respectively (Fig. 2d). The effects were independent of the MEF concentration for each treatment group but MEF5% exhibited the most consistent significant effects on all morphological parameters. Thus, only samples treated with MEF5% were retained for biochemical studies.
Leaf chlorophyll content in tomato plants groups under different salt levels. Different small letters indicate significant differences between the MEF treatments across different NaCl levels. b The effect of MEF on the vegetative growth of tomato plant cultures, characterized by high shoot biomass and large total leaf area
Biochemical analysis of the effects of MEF on tomato plants
The effects of MEF treatment on tomato plants subjected to salt stress were independent of the MEF concentration for each treatment group. However, MEF5% exhibited the most consistent effects on all morphological parameters. Therefore, only plants treated with MEF5% were selected for the determination of photosynthetic pigment content and the study of certain enzymatic and biochemical processes affected by salt stress.
Effect on photosynthetic pigments content
Salt stress causes chlorophyll degradation, which is one of the major limitations of photosynthesis. Photosynthetic pigments’ content reduced with increasing salinity concentration. Treatment with MEF5% significantly increased pigment content in plant cultures subjected to lower salinity concentrations (80 mM NaCl) (Fig. 2a). The highest percentage increase in chlorophyll a, chlorophyll b, and β-carotene contents were 16.88%, 13.55%, and 16.62%, respectively, in plants grown at 80 mM NaCl. Application of MEF5% on plant cultures grown at 120 and 150 mM NaCl did not exhibit any effects on the leaf concentration of photosynthetic pigments. Treatment of plants with MEF5% at the lower saline levels (non-stressed and 80 mM NaCl) proved to improve the vegetative growth of tomato plants, characterized by increased shoot size and large total leaf area (Fig. 2b). See Supplementary Information S4 for statistically significant differences between the mean values (Tukey’s post hoc test).
Effect of MEF5% treatment on the accumulation of proline and polyphenols
Treatment of plants with MEF5% exhibited significant effects on the accumulation of proline under normal and high saline conditions (120 and 150 mM NaCl). Significant percentage increases were up to 140.5% and 87.89%, respectively, in plants grown at 120 and 150 mM NaCl levels (Fig. 3b). Contrarily, polyphenols (Fig. 3b) were only significantly enhanced in unstressed plant cultures after MEF5% treatment. The accumulation of proline was influenced NaCl concentration, whereas polyphenols remained unaffected by salinity increase.
Effect of MEF on lipid peroxidation and the activity of ROS scavenging enzymes
Lipid peroxidation
MEF5% exhibited very significant effects on the MDA content of plants grown at 80, 120, and 150 mM NaCl. There were no significant effects on unstressed control plants. All tomato plants treated with MEF5% except non-stressed control plants showed a significant decrease in MDA content, with the lowest decrease (− 27.49% and − 33.76%) in plants grown at 80 and 120 mM NaCl, respectively (Fig. 3c).
ROS scavenging enzyme activity
SOD and CAT enzyme activities were enhanced with increasing NaCl concentration compared to unstressed control plants. SOD activity exhibited the highest percentage increase (24.19% and 28.85%) in plant cultures subjected to 80 and 120 mM NaCl, respectively (Fig. 3d). Plant cultures grown at 80 mM exhibited the highest percentage increase in CAT activity, which increased by 144.38% after treatment with MEF5% (Fig. 3e). The highest percentage increase in POD activity was 97.59%, at 150 mM NaCl (Fig. 3f). Out of the three enzymes, SOD and CAT activities were significantly enhanced by MEF5% supplementation, notably in plants subjected to 80 and 120 mM NaCl.
Leaf concentration of nitrogen, phosphorus, and potassium
Nitrogen.
The concentration of N in the leaves decreased progressively with increasing NaCl concentrations. Supplementation of plants with MEF5% significantly enhanced N uptake by roots, with the highest N content improvement (182.95% and 21.87%) in plants grown at 80 and at 120 mM NaCl, respectively (See Supplementary Information (S3) for percentage increase values). N uptake positively correlated with MEF5% treatment in tomato culture groups grown at 80 and 120 mM NaCl (Fig. 4a).
Phosphorus.
MEF5% treatment exhibited similar effects on P uptake. The highest amelioration effects (78.35% and 50%) were recorded in plants grown under 80 and 120 mM NaCl conditions (S3). P uptake was also significantly enhanced (36.49%) in unstressed control plants after treatment with MEF5%. There was a positive correlation between P uptake and MEF5% treatment in plants grown at 80 and 120 mM NaCl (Fig. 4a ).
Potassium.
The highest K uptake amelioration was 327.04% and 165.13% in unstressed control plants and plants grown at 80 mM NaCl, respectively. The MEF5% effect on K uptake decreased with increasing salinity (58.63% at 120 mM NaCl) and exhibited negative effects (− 26.76%) in plants grown at 150 mM NaCl (S3).
Calcium, sodium ion levels, and Na + /K + ratios
MEF5% supplementation to salt-stressed plants showed no effect on Ca + uptake expect for plants grown at 80 mM NaCl. MEF5% exhibited the least effect on Na + leaf concentration with 9.19 and 22.74% percentage increase in unstressed control plants and plants grown at 80 mM NaCl. Sodium ion levels reduced by − 41.93% and − 14.08% upon MEF5% application in plants grown at 120 and 150 mM NaCl. The enhanced K uptake in unstressed control plants and plants grown at 80 mM NaCl lowered their Na+/K+ ratios (Fig. 4b). There was no significant effect on Na+/K+ ratios in plants grown at higher salinity concentrations (120 and 150 mM) (Fig. 4b).
Treatment-variable interactions through matrix correlation and PCA
The influence of salinity and MEF treatment could be distinguished into three major groups: treated and untreated control plant groups were closely associated, followed by treated plant groups grown at 80 and 120 mM NaCl. Treatments were distinguished into three major groups (Fig. 5b). Untreated plant groups grown at 80 and 120 mM NaCl and treated and untreated groups grown at 150 mM were all closely associated and exhibited the least effect on all the studied parameters (Fig. 5b). Improved shoot length and shoot and root weights were closely associated with improved leaf content of photosynthetic pigments, which were positively correlated with unstressed control tomato plant group (Fig. 5c). Treated plants grown at 80 and 120 mM NaCl were positively correlated with NPK nutrient uptake which was closely associated with SOD and CAT activities but negatively correlated with MDA content, implying that improved SOD and CAT activities reduced MDA content. This correlation indicates that significant increase of SOD and CAT alleviated lipid peroxidation in plants subjected to 80 mM and 120 mM salinity levels (refer to Figure S5 for plot cumulative variance and scores).
Correlation matrix (Fig. 5a) and principal component analysis (PCA) to understand treatment-variable associations (Fig. 5b and c). The entire data was analyzed using PCA biplot. The variables included RW and SW (root and shoot weights), RL and SL (root and shoot lengths), Chl a (chlorophyll a), Chl b (chlorophyll b), Car (carotenoids), nitrogen, phosphorus, and potassium as N, P and K, Na+ (sodium), Na+ /K+ ratio, MDA (malondialdehyde), CAT (catalase), SOD (superoxide dismutase), and POD (peroxidases). The lines originating from the central point of PCA biplot indicate positive or negative correlations of different variables (Fig. 5c), where their closeness indicates correlation strength with particular treatment. Figures were generated using RStudio software
Fatty acid profile analysis of MEF5%-treated plants under salt stress
These results suggest that the application of MEF5% to tomato plants induces changes in the profile of saturated fatty acids (SFA) and unsaturated fatty acids (UFA) (Table 1). Total leaf SFA content decreased by fourfold after MEF5% application in unstressed plants, by 1.8-fold in plants grown at 80 mM NaCl, and by sevenfold in plants grown at 120 mM. The were no effects on SFA in plants subjected to 150 mM. Unsaturated fatty acids exhibited a similar profile, where UFA decreased by 3.5-fold in unstressed control plants and by 1.6-fold and 1.4-fold in plants grown at 80 and 120 mM NaCl, respectively, with no effects recorded in plants grown 150 mM NaCl. There were significant effects on the levels of very long chain fatty acids (VLCFA) in all plants, including plants grown at 150 mM, where the total VLCFA content was lowered by 1.4-fold.
MEF5% effect on the profile of sterols in tomato plants at different NaCl concentrations
Table 2 shows the sterol levels in treated and non-treated plants grown at different NaCl concentrations. Apart from plant cultures grown at 150 mM, the sterol profile was highly variable and independent of the MEF5% treatment or NaCl concentration. No sterols were detected in MEF5%-treated plants subjected to 80 and 120 mM NaCl. Moreover, besides stigmasta-3,5-diene, no sterols were detected in MEF5%-treated plants of both non-stressed control plants or plants subjected to 120 mM NaCl. However, plants grown at 150 mM NaCl exhibited higher levels of sterols. Supplementation of MEF5% to plants grown at 150 mM NaCl conditions reduced total leaf sterol concentrations by 1.2-fold. The leaf content of stigmasta-3,5-diene; stigmasta-5,22-dien-3-ol, acetate, (3β); stigmasterol, and β cholesterol also reduced upon MEF5% application.
Effect of MEF5% on the profile of some alkanes detected in the leaf biomass
Certain alkane molecules were detected in high concentrations (S5). These results indicated that the application of MEF5% to tomato plants reduced the levels of nexacloroethane in the leaves of all plant groups, notably, in plants grown at 120 mM NaCl, which decreased by sixfold. Hentriacontane (n-C33) content in leaves exhibited a similar pattern, where hentriacontane levels reduced by 3.6-fold, 1.6-fold, 5.4-fold, and 2.2-fold upon MEF5% application in non-stressed control plants and plants grown 80 mM, 120 mM, and 150 mM respectively. The most common cyclic siloxanes were cyclohexasiloxane, dodecamethyl-, cycloheptasiloxane, tetradecamethy, cyclooctasiloxane, hexadecamethyl, cyclononasiloxane, and octadecamethyl-. The was a significant increase (1.4-fold and threefold) in the total leaf content of these cyclic siloxanes in non-stressed control plants and plants grown at 150 mM NaCl respectively. In contrast, total content of the four cyclic siloxanes decreased (by 9.6-fold and 4.8-fold) upon MEF5% application (see data in Supplementary information (S5).
Discussion
Salinity stress exerts negative effects on the growth, development, and metabolic machinery of plants. In this study, both plant size and leaf concentrations of photosynthetic pigments significantly reduced with increasing NaCl concentrations (Fig. 2a). High NaCl concentrations lead to chlorophyll degradation through photooxidative reactions (Mitsuya et al. 2003) and low osmotic potential. Low osmotic potential triggers stomatal cloture in plants, leading to reduced CO2 fixation, increased photorespiration, and H2O2 production in peroxisomes (Noctor 2002; Huang et al. 2015; Zhao et al. 2020). In the present study, treatment with MEF5% improved chlorophyll contents in unstressed plants and plants grown at 80 mM NaCl. According to PCA analysis, treatment with MEF5% was closely associated with improved nutrient uptake in plants grown at 80 and 120 mM NaCl. Improved nutrient uptake, notably K+, in salt-stressed plants can play a major role in improving salt tolerance (Acosta-Motos et al. 2017). These results show that MEF5% can be a plant growth promoter and stress tolerance enhancer under non-saline or moderate saline conditions. The significant increase of growth and chlorophyll content in MEF5%-treated plants subjected to no stress and plants grown at 80 mM NaCl can be explained by improved osmotic adjustment.
In normal conditions, osmotic adjustment or cell turgidity is achieved by vacuolar K+ pools. However, excessive Na+ influx induces membrane depolarization, resulting into cytosolic K+ efflux and subsequent liberation of vacuolar K+ leading to decrease in cell turgor (Barragán et al. 2012; Latz et al. 2013). In salt-stress conditions, plants maintain osmotic adjustment via two major ways: (i) through de novo synthesis of compatible osmolytes and (ii) by increased uptake of inorganic ions (Na+, Cl−, and K+) (Zhao et al. 2020). Compatible osmolytes including glycine-betaine and proline play an important role in osmotic adjustment by compensating for the osmotic pressure of Na+ (Yan et al. 2013; Acosta-Motos et al. 2017; Chun et al. 2018). Osmotic adjustment via inorganic ion uptake such as Na+ and K+ require very low carbon cost compared to the production of organic osmolytes (Munns et al. 2020). Na+ accumulation is generally preferred in halophytes that overcome Na+ toxicity through vacuolar Na+ sequestration in specialized leaf cells (Zhao et al. 2020). Induced K+ intracellular retention and cytosolic accumulation of proline and sugars also improved salt tolerance in transgenic tomato (Leidi et al. 2010). In the present study, proline accumulation was significantly triggered by MEF5% in plants subjected to high NaCl conditions (120 and 150 Mm NaCl). Slightly significant affects were also recorded in unstressed control plants (Fig. 3a). These results indicated that improved salt tolerance in MEF5%-treated plants resulted from both enhanced proline accumulation and K+ uptake. Improved root biomass in MEF5%-treated can lead to improved nutrient uptake and subsequent retention in leaves, leading to reduced Na+ toxicity, notably in plants grown at 80 mM.
Glycophytes, such as tomato, are plants adapted to low-Na+ environments and do not tolerate salinity greater than 100 mM NaCl (Assaha et al. 2017). The main contributing factor to ionic stress and salt stress sensitivity is almost exclusively attributable to excess Na+ accumulation and K+ deficiency, especially in the aerial parts of plants. K+ activate more than 50 enzymes in the plant, and cannot be substituted by Na+ (Tester 2003). Therefore, mechanisms of Na+ and K+ uptake and translocation are key to the survival of glycophytes in saline environments (Wu 2018). This ion homeostasis is mainly mediated by HKT and non-selective cation channels, and salt overly sensitive pathway (SOS1), a Na+/H+ antiporter (Ji et al. 2013; Assaha et al. 2017; Zhao et al. 2020). In this study, the investigation of improved ion homeostasis was based on the leaf Na+ to K+ ratios. The results showed that decreasing Na+/K+ ratios in the leaf biomass of treated plants was due to enhanced K+ uptake in plants grown at 80 mM NaCl. Lower Na+/K+ ratio in MEF-treated plants grown at 120 mM was due to both K+ accumulation and Na+ decrease (Fig. 4a and Supplementary information S3). Enhanced K+ and reduced Na+ content in the leaf biomass of MEF5%-treated plants imply that MEF5% improved both salt tolerance and growth through improved ion homeostasis, in plants subjected to 80 mM and 120 mM NaCl. Partial characterization of the microalgae and cyanobacteria species indicate that MEF extracts were composed of neutral sugars, polysaccharides, proteins, and nutrients (Table 3).
It can be suggested that this organic content of MEF may have similar effects as root exudates. Root exudates are a mix of a wide variety of compounds including carbohydrates, amino acids, and organic acids and intervene in salt stress tolerance (Vives-Peris et al. 2020). For example, salt-stressed Arabidopsis seedlings inoculated with Trichoderma spp. showed enhanced elimination of Na+ through root exudates (Contreras-Cornejo et al. 2014). Thus, the organic composition of MEF5% could have exhibited positive effects on Na+ toxicity in the soil and on nutrient uptake by mimicking root exudates.
NPK uptake parallels vegetative plant growth and is crucial for plant growth and development (Alcantara and Gonzaga 2020). Many crops take up and store the majority of the nutrients during the vegetative growth. These stored nutrients are translocated to developing fruit during reproductive growth (Mengel 1995). In this study, uptake of NPK significantly increased in MEF5%-treated, with the exception of plants grown at 150 mM NaCl conditions. Improved N absorption in salt-stressed plants progressively increases the accumulation of soluble sugar, soluble protein, and free amino acids and activity of the antioxidant defense system (Sikder et al. 2020). On the other hand, improved P absorption and use efficiency in salt stressed plants improve tolerance through increased chlorophyll, carotenoid, proline, soluble sugar, and free amino acid content (Bargaz et al. 2016). It can be suggested that improved root biomass in MEF5% increased the roots’ contact surface with the root substrate and favored nutrient absorption. However, PCA analysis indicated that root growth was not closely associated with NPK uptake. This indicates that other factors such as the organic composition of MEF may have improved nutrient uptake in plants under saline conditions.
MEF5% extracts also enhanced ROS scavenging enzyme activities of SOD, POD, and CAT (Fig. 3d, e,and f). ROS act as signaling molecules that regulate biological processes and plant responses to a variety of biotic and abiotic stresses (Turkan 2018). Excessive ROS accumulation under salt stress conditions is damaging to cellular structures (Huang et al. 2019; Zhao et al. 2020; Hasanuzzaman et al. 2020). To reduce the oxidative stress caused by excessive ROS accumulation, plants activate ROS-scavenging systems (Hanin et al. 2016). Enhancement of these antioxidant systems increases salt stress tolerance in plants due to their capacity to maintain ROS homeostasis (Bose et al. 2014). Enhancing SOD, POD, and CAT activities in salt-stressed plants alleviate lipid peroxidation by neutralizing ROS effects. SOD catalyzes the dismutation of O2− to H2O2 and O2 (Ahanger et al. 2018). H2O2, produced by SOD activity, is then decomposed into H2O and O2 by CAT in the cytoplasm or scavenged by ascorbate peroxidase in the chloroplast and the cytosol (Ahanger et al. 2018; Hasanuzzaman et al. 2020). In the present study, SOD and CAT activities in MEF-treated plants negatively correlated with MDA accumulation (Fig. 4c), indicating that enhanced CAT and SOD activities lowered MDA accumulation, a product of lipid peroxidation. Previous studies have demonstrated that antioxidant enzyme activities including CAT, SOD, and POD attenuate oxidative damage via the detoxification of ROS (Caverzan et al. 2019; Tahjib-UI-Arif et al. 2019; Khan et al. 2020), and alleviate salt stress in the wild salt-tolerant tomato species Lycopersicon pennellii (Mittova et al. 2003). The present study indicated that MEF5% can promote salt stress tolerance by reducing lipid peroxidation through enhanced ROS scavenging enzyme activities but have no effect on plants subjected to excessive saline conditions (150 mM). MEF5% can also improve plant growth under normal conditions, and reduce ROS damage resulting from natural cellular processes (photosynthesis and respiration).
Other parameters investigated in this study include comparative lipid profiling of MEF-treated and non-treated tomato plants. Soil supplementation with MEF5% induced a significant decline in leaf SFA, UFA, and VLCFA contents in all plant groups, with the exception of plants grown at 150 mM NaCl (Table 1). Modifications in membrane phospholipids act as signaling components during salt stress. Exposure of plants to both salt and osmotic stress induce several phospholipid signals such as polyphosphoinositides and phosphatidic acid (PA) within 5 min (Van Zelm et al. 2020). The formation of PA is another putative downstream response of Ca+ signaling during salt stress (Galvan-Ampudia et al. 2013).
Future studies should investigate the differential phytohormone expression profiles and accumulation of Na+ and K+ in the leaf, stem, and root biomass of treated and untreated tomato plants (salt-sensitive and salt-tolerant variants) subjected to salt stress. Comparative analysis of gene expression profiles of high affinity N, P, and K+ transporter proteins is also needed to elucidate MEF mode of action and effect on nutrient uptake. Studying the effects of microalgae and cyanobacteria extracts on tomato yield will be a major contribution to understanding biostimulant effects on salt stress tolerance. Figure 6 highlights the major mechanisms attributed to improved salinity tolerance in this study.
The possible biostimulant effects of MEF5% on tomato plants under saline conditions. Bioactive organic compounds in extracts may directly stimulate the antioxidative enzyme system, thereby mitigating lipid peroxidation in plant cells. Additionally, MEF5% favorizes root growth and consequently improve the plants’ nutrient uptake, ion homeostasis, and, subsequently, photosynthetic activity and plant growth under saline conditions
Conclusion
Combined microalgae-cyanobacteria formulation can be a stimulator of salt tolerance responses, nutrient uptake, and plant growth of Solanum lycopersicum, notably in plants grown at lower salinity levels. In the present study, salt tolerance in treated tomato plants was mainly due to improved ion homeostasis through enhanced NPK uptake and leaf accumulation, which resulted in lower Na+/K+ ratios. Based on the partial characterization, the organic composition of MEF5% could have exhibited positive effects on Na+ toxicity in the soil and nutrient uptake by mimicking root exudates. In addition, MEF5% treatment induced ROS scavenging enzyme activities which lowered lipid peroxidation in plants grown under 80 mM and 120 mM NaCl, with the exception of plants subjected to excessive saline conditions (150 mM). MEF5%-supplementation also lowered the total fatty acid content of tomato plants across all NaCl levels. Enhanced root mass subsequently increases the roots’ surface for nutrient absorption. These results indicate that MEF5% could be a plant growth promoter under normal conditions by improving plant chlorophyll content, biomass production, and lowering ROS damage resulting from natural cellular processes (photosynthesis and respiration).
Supplementation of crops with MEF5% in saline conditions could also be another important research tool to boost tolerance, nutrient uptake, and vegetative growth in tomato crops under saline soils. Such product innovation will contribute to the development of sustainable agriculture products.
Data availability statement
Besides the data provided in supplementary information, the data generated during and/or analyzed during the current study is available from the corresponding author on reasonable request.
References
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18.
Ahanger MA, Alyemeni MN, Wijaya L, Alamri SA, Alam P, Ashraf M, Ahmad P (2018) Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 13:e0202175.
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877
Alcantara CG, Gonzaga NR (2020) Nutrient uptake and yield of tomato (Solanum lycopersicum) in response to vermicast and vermi-foliar application. Org Agric 10:301–307
Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S (2020) Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physio Biochem 156:64–77
Arroussi HE, Benhima R, Elbaouchi A, Sijilmassi B, Mernissi NE, Aafsar A, Meftah-Kadmiri I, Bendaou N, Smouni A (2018) Dunaliella salina exopolysaccharides: a promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J Appl Phycol 30:2929–2941
Assaha DV, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509
Bargaz A, Nassar RM, Rady MM, Gaballah MS, Thompson SM, Brestic M, Schmidhalter U, Abdelhamid MT (2016) Improved salinity tolerance by phosphorus fertilizer in two Phaseolus vulgaris recombinant inbred lines contrasting in their P-efficiency. J Agron Crop Sci 202:497–507
Barone V, Baglieri A, Stevanato P, Broccanello C, Bertoldo G, Bertaggia M, Cagnin M, Pizzeghello D, Moliterni VM, Mandolino G, Fornasier F (2018) Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J Appl Phycol 30:1061–1071
Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernández JA, Cubero B, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24:1127–1142
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic (Amsterdam) 196:39–48
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Calvo P, Nelson L, Kloepper JW (2014) Agricultural uses of plant biostimulants. Plant Soil 383:3–41
Carillo P, Ciarmiello LF, Woodrow P, Corrado G, Chiaiese P, Rouphael Y (2020) Enhancing sustainability by improving plant salt tolerance through macro-and micro-algal biostimulants. Biology (Basel) 9:1–21
Carillo P, Gibon Y (2011) PROTOCOL: Extraction and determination of glycine betaine. https://www.researchgate.net/publication/215448078_PROTOCOL_Extraction_and_determination_of_glycine_betaine/link/00c433cb4efddc3c26cab80d/download (retrieved on 20 Feb 2021)
Carvalho FP (2017) Pesticides, environment, and food safety. Food Energy Secur 6:48–60
Caverzan A, Piasecki C, Chavarria G, Stewart CN, Vargas L (2019) Defenses against ROS in crops and weeds: the effects of interference and herbicides. Int J Mol Sci 20:1086
Chanda M joan, Merghoub N, EL Arroussi H (2019) Microalgae polysaccharides: the new sustainable bioactive products for the development of plant bio-stimulants? World J Microbio. Biotechno. 35
Chanda MJ, Benhima R, Elmernissi N, Kasmi Y, Karim L, Sbabou L, Youssef Z, El Arroussi H (2020) Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci Rep 10(1):2820
Chiaiese P, Corrado G, Colla G, Kyriacou MC, Rouphael Y (2018) Renewable sources of plant biostimulation: microalgae as a sustainable means to improve crop performance. Front Plant Sci 9:1782
Chun SC, Paramasivan M, Chandrasekaran M (2018) Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front Microbiol 9:2525
Colla G, Hoagland L, Ruzzi M, Cardarelli M, Bonini P, Canaguier R, Rouphael Y (2017) Biostimulant action of protein hydrolysates: unraveling their effects on plant physiology and microbiome. Front Plant Sci 8:2202
Colla G, Rouphael Y (2020) Microalgae: new source of plant biostimulants. Agronomy 10:1240
Contreras-Cornejo HA, Macías-Rodríguez L, Alfaro-Cuevas R, López-Bucio J (2014) Trichoderma spp. Improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol Plant-Microbe Interact 27:503–514
Cuellar-Bermudez SP, Aguilar-Hernandez I, Cardenas-Chavez DL, Ornelas-Soto N, Romero-Ogawa MA, Parra-Saldivar R (2015) Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb Biotechnol 8:190–209
De Morais MG, Vaz BDS, De Morais EG, Costa JAV (2015) Biologically active metabolites synthesized by microalgae. Biomed Res. Int. 2015:835761
Desoky ESM, Merwad ARM, Rady MM (2018) Natural biostimulants improve saline soil characteristics and salt stressed-Sorghum performance. Commun Soil Sci Plant Anal 49:967–983
Elzaawely AA, Ahmed ME, Maswada HF, Xuan TD (2017) Enhancing growth, yield, biochemical, and hormonal contents of snap bean (Phaseolus vulgaris L.) sprayed with moringa leaf extract. Arch Agron Soil Sci 63:687–699
Farid R, Mutale-Joan C, Redouane B, Najib EM, Abderahime A, Laila S, Hicham EA (2019) Effect of microalgae polysaccharides on biochemical and metabolomics pathways related to plant defense in Solanum lycopersicum. Appl Biochem Biotechnol 188:225–240
Galvan-Ampudia CS, Julkowska MM, Darwish E, Gandullo J, Korver RA, Brunoud G, Haring MA, Munnik T, Vernoux T, Testerink C (2013) Halotropism is a response of plant roots to avoid a saline environment. Curr Biol 23:2044–2050
Garcia-Gonzalez J, Sommerfeld M (2016) Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J Appl Phycol 28:1051–1061
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1787
Hardie M, Doyle R (2012) Measuring soil salinity. Meth Mol Biol 913:415–425
Hasanuzzaman M, Bhuyan MH, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator
Huang H, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800
Huang W, Hu H, Zhang S-B (2015) Photorespiration plays an important role in the regulation of photosynthetic electron flow under fluctuating light in tobacco plants grown under full sunlight. Front Plant Sci 6:621
Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X (2013) The Salt Overly Sensitive (SOS) pathway: established and emerging roles. Mol Plant 6:275–286
Kamthan A, Kamthan M, Chakraborty N, Chakraborty S, Datta A (2012) A simple protocol for extraction, derivatization, and analysis of tomato leaf and fruit lipophilic metabolites using GC-MS. Protocol Exchchange. https://doi.org/10.1038/protex.2012.061
Khan A, Numan M, Khan AL, Lee IJ, Imran M, Asaf S, Al-Harrasi A (2020) Melatonin: Awakening the defense mechanisms during plant oxidative stress. Plants 9:407
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17:36
Khan MN, Siddiqui MH, Mohammad F, Naeem M (2012) Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide - Biol Chem 27:210–218
Khan W, Hiltz D, Critchley AT, Prithiviraj B (2011) Bioassay to detect Ascophyllum nodosum extract-induced cytokinin-like activity in Arabidopsis thaliana. J Appl Phycol 23:409–414
Latz A, Mehlmer N, Zapf S, Mueller TD, Wurzinger B, Pfister B, Csaszar E, Hedrich R, Teige M, Becker D (2013) Salt stress triggers phosphorylation of the Arabidopsis vacuolar K+ channel TPK1 by calcium-dependent protein kinases (CDPKs) Mol Plant 6:1274–1289
Leidi EO, Barragán V, Rubio L, El-Hamdaoui A, Ruiz MT, Cubero B, Fernández JA, Bressan RA, Hasegawa PM, Quintero FJ, Pardo JM (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61:495–506
Libutti A, Cammerino A, Monteleone M (2018) Risk Assessment of soil salinization due to tomato cultivation in Mediterranean climate conditions. Water 10:1503
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1:F4.3.1–F4.3.8
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Mengel D (1995) Roots, growth and nutrient uptake. Department of Agronomy Publication # AGRY-95–08. Perdue University, West Lafayette p 8
Mitsuya S, Kawasaki M, Taniguchi M, Miyake H (2003) Light dependency of salinity-induced chloroplast degradation. Plant Prod Sci 6:219–223
Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 26:845–856
Munns R, Passioura JB, Colmer TD, Byrt CS (2020) Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol 225:1091–1096
Noctor G (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot 89:841–850
Rachidi F, Benhima R, Sbabou L, El Arroussi H (2020) Microalgae polysaccharides bio-stimulating effect on tomato plants: growth and metabolic distribution. Biotechnol Reports 25:e00426
Rahman KMA, Zhang D (2018) Effects of fertilizer broadcasting on the excessive use of inorganic fertilizers and environmental sustainability. Sustainability 10:759
Renuka N, Guldhe A, Prasanna R, Singh P, Bux F (2018) Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnol Adv 36:1255–1273
Ricci M (2020) Justifying plant biostimulants claims for European Regulation Chair of EBIC Agriculture Committee and Agronomy Manager at Lallemand Plant Care
Ronga D, Biazzi E, Parati K, Carminati D, Carminati E, Tava A (2019) Microalgal biostimulants and biofertilisers in crop productions. Agronomy 9:40192
Shahid SA, Zaman M, Heng L (2018) Soil salinity: historical perspectives and a world overview of the problem. In: Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques. Springer, Cham, pp 43–53
Sikder RK, Wang X, Zhang H, Gui H, Dong Q, Jin D, Song M (2020) Nitrogen enhances salt tolerance by modulating the antioxidant defense system and osmoregulation substance content in Gossypium hirsutum. Plants 9:40450
Smith GS, Johnston CM, Cornforth IS (1983) Comparison of nutrient solutions for growth of plants in sand culture. New Phytol 94:537–548
Tahjib-UI-Arif M, Sohag AA, Afrin S, Bashar KK, Afrin T, Mahamud AG, Polash MA, Hossain M, Sohel M, Taher A, Brestic M (2019) Differential response of sugar beet to long-term mild to severe salinity in a soil–pot culture. Agriculture 9:223
Tester M (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Turkan I (2018) ROS and RNS: key signalling molecules in plants. J Exp Bot 69:3313–3315
Van Oosten MJ, Pepe O, De Pascale S, Silletti S, Maggio A (2017) The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Technol Agric 4:1–12
Van Zelm E, Zhang Y, Testerink C (2020) Salt Tolerance Mechanisms of Plants. Annu Rev Plant Biol 71:403–433
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci 151:59–66
Vives-Peris V, de Ollas C, Gómez-Cadenas A, Pérez-Clemente RM (2020) Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep 39:3–17
Wu H (2018) Plant salt tolerance and Na+ sensing and transport. Crop J 6:215–225
Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH (2017) Biostimulants in plant science: a global perspective. Front Plant Sci 7:2049
Yan K, Shao H, Shao C, Chen P, Zhao S, Brestic M, Chen X (2013) Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol Plant 35:2867–2878
Yang Y, Guo Y (2018) Unraveling salt stress signaling in plants. J Integr Plant Biol 60:796–804
Zhao C, Zhang H, Song C, Zhu JK, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. Innov 1:100017
Funding
Special thanks for the financial support of OCP-Agribiotech BU for the realization of this project under the best conditions.
Author information
Authors and Affiliations
Contributions
HA = project conception, funding acquisition, project supervision, design of methodology and revision of the manuscript. CMJ = writing (original draft preparation, review and editing), the study of physiological parameters, analysis of results. FR = experimental work, the study of physiological and biochemical parameters and manuscript revision. HAM = experimental work and analysis of results. NM = Study of nutrient uptake using Skalar nutrient auto analyser. AA = study of metabolomics using GC–MS and manuscript revision. MB = project supervision. DM = statistical analysis, visualization of results and generation of graphs using GraphPad Prism software. LS = thesis director.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mutale-joan, C., Rachidi, F., Mohamed, H.A. et al. Microalgae-cyanobacteria–based biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J Appl Phycol 33, 3779–3795 (2021). https://doi.org/10.1007/s10811-021-02559-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02559-0