Abstract
Altered motor behaviour is commonly reported in Autism Spectrum Disorder, but the aetiology remains unclear. Here, we have taken a computational approach in order to break down motor control into different components and review the functioning of each process. Our findings suggest abnormalities in two areas—poor integration of information for efficient motor planning, and increased variability in basic sensory inputs and motor outputs. In contrast, motor learning processes are relatively intact and there is inconsistent evidence for deficits in predictive control. We suggest future work on motor abilities in autism should focus on sensorimotor noise and on higher level motor planning, as these seem to have a significant role in causing motor difficulties for autistic individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although sensory and motor impairments in Autism Spectrum Disorder (ASD) are not considered to be core features of autism, there is increasing acknowledgment that they are nevertheless highly prevalent and can have a significant impact on quality of life and social development. The current review examines motor control in autism, within a framework derived from computational models of the motor system, and with a focus on whether specific stages of motor computation are abnormal.
Motor abnormalities in ASD can be observed in infancy (Brian et al. 2008; Provost et al. 2007; Teitelbaum et al. 1998; although see Ozonoff et al. 2008) and are apparent throughout childhood and into adulthood (Fournier et al. 2010; Ming et al. 2007; Van Waelvelde et al. 2010). A number of different motor deficits have been observed using standardized test batteries (Table 1) and the prevalence of such deficits has been reported to be between 21 and 100 % (Ghaziuddin et al. 1994; Green et al. 2002; Manjiviona and Prior 1995; Miyahara et al. 1997; Pan et al. 2009), highlighting that motor impairment is a significant but potentially variable aspect of ASD. As acquisition of good motor skills is important for a range of everyday abilities such as communication and language development (Gernsbacher et al. 2008), playing and interacting with others (Clearfield 2011), mental imagery (Williams et al. 2008) and perception (Blaesi and Wilson 2010; Eskenazi et al. 2009; Wilson and Knoblich 2005), it is likely that abnormal development of motor control can have far reaching consequences on development (Leary and Hill 1996). For example, it has been shown that motor ability is correlated with daily living skills in autistic children (Jasmin et al. 2009) and that better motor control is related to decreased severity of ASD in later life (Sutera et al. 2007). Therefore, increasing our understanding of the aetiology of motor deficits in ASD is a crucial step towards treating and preventing this potential developmental cascade.
Our current understanding of motor function in autism is limited in two ways. First, it is unclear if there are certain motor problems which are specific to autism (as opposed to other developmental disorders). For example, motor deficits are observed in Developmental Coordination Disorder (DCD) and Attention Deficit Hyperactivity Disorder (ADHD), but it is not known if these are similar to the motor deficits in autism (Dewey et al. 2007; Green et al. 2002; Ozonoff et al. 2008; Provost et al. 2007). It is also unclear whether there are differences in motor ability between individuals diagnosed with different forms of ASD such as Asperger’s or autism (Ghaziuddin et al. 1994; Jansiewicz et al. 2006; Manjiviona and Prior 1995; Rinehart et al. 2006a, b). Second, commonly used clinical and standardized measures of motor performance such as those in Table 1, do not always provide much information about the underlying motor processes. For example, test batteries may distinguish “fine” motor skills involving manual dexterity and visuomotor control from “gross” motor skills involving walking or throwing, and may consider posture or balance as separate categories. While these categories may be helpful in considering how an individual needs support in daily life, they do not relate closely to the underlying motor mechanisms. Fine motor skills encompass a number of different processes relating to sensory, planning and execution aspects of motor control whereas balance and posture may actually share some aspects such as integrating different senses and predicting sensory consequences of movement. Thus, it is often hard to know which specific motor processes are abnormal in autism. Understanding motor difficulties in autism in terms of specific computational mechanisms may allow clearer distinctions between different developmental disorders and has the potential to reveal if and how poor motor skills might be causally related to poor social skills. A better understanding of the origins and nature of motor difficulties in autism will also contribute to better training and intervention methods and is well suited to tackling the issues of heterogeneity within the autistic spectrum.
In recent years, studies of motor systems in typical adults have given rise to sophisticated computational models of the different control and feedback processes required for accurate everyday movement (Jordan and Wolpert 1999; Wolpert and Ghahramani 2000) (Fig. 1). However, these models have rarely been applied to developmental motor disorders (Sanger 2003). Our aim in this review is to determine how the component processes identified in computational models can be mapped onto the developmental dysfunction of motor systems seen in autism. We base our review on a model advanced by Wolpert and colleagues (Wolpert 1997), which incorporates Optimal Control Theory (Diedrichsen et al. 2010) and provides a flexible framework within which to examine a number of different motor processes. There are other models of motor control, for example, Dynamical Systems Theory (Kelso 1995) suggests that behavior arises from the dynamics of coupled oscillators, while theories of motor synergies examine how different muscle groups work together as functional units (d’Avella and Bizzi 2005). We have chosen to focus on the model from Wolpert and colleagues because it has a broad scope, covering many different motor functions and proposing distinct computational units which can potentially be linked to different cognitive tasks.
Different computational processes involved in motor control. Information from different senses is integrated to form a state estimate of the current location of the body and surrounding environment (1, 2). This state estimate is used by the inverse model to plan a movement to obtain the desired state (3). The resulting motor command is used by the forward model to predict the motor and sensory outcome of the intended movement and compared to the actual sensory state to check for errors (4). The motor plan is converted into muscle activity in order to execute the movement (5) and sensory feedback used to update the state estimate. Dashed line indicates processes involved in sensorimotor integration
In the current review we attempt to bring together those studies that focus on particular motor computations in ASD, with emphasis on quantitative experimental procedures. As we have specifically selected those articles that fall within the framework of the computation model in Fig. 1, our review is not intended to be a comprehensive summary of all previous motor studies in ASD. There are three main sections. Firstly, we briefly discuss the different processes identified in typical adult motor control. Then we review whether there is evidence for involvement of each of these processes in autistic motor ability. Finally, we draw together some conclusions and suggest approaches to move forward our understanding of motor control in ASD.
Brief Overview of Motor Control Processes
Consider the simple task of reaching your hand out to pick up a mug of tea. Figure 1 provides a summary of the basic motor control processes involved (Shadmehr and Krakauer 2008; Wolpert and Ghahramani 2000), and the numbers in this description refer to the components of the figure. First, sensory inputs from the visual system and the proprioceptive system provide essential information about the task, including an image of the mug on the table and a sense of your hand in space (1). These different sensory inputs must be integrated into a unified state estimate which specifies where the mug is, how big it is, where your hand is and other task-relevant information (2). The estimate of the current state of the world must next be compared to the desired state—‘my hand is by my side; I want the hand on the mug’—and the motor system must plan how to move your hand smoothly and efficiently from its current location to the mug. This planning process is also termed the inverse model because it solves the inverse problem of how to convert a goal (hand-on-mug) into a sequence of motor commands (3). This sequence of motor commands can then be executed by the body (5).
However, during execution, errors may arise due to planning failure, external perturbations or inherent noise within the motor system. Sensory feedback is too slow to allow efficient error correction in rapid hand movements, as it takes at least 165 ms to detect and correct errors (Young and Zelaznik 1992). To reduce this problem, the brain uses a forward model (4) which takes a copy of the outgoing motor command (efference copy) and generates a prediction of the expected sensory input. As the movement progresses, the actual sensory input is compared to the predicted input to allow rapid detection and correction of errors. In this way, it is possible for the hand to accurately move to the mug of tea and grasp it appropriately. A core process within this model is sensorimotor integration, which is defined as integration of forward model output with the state estimate as well as the use of the state estimate by the inverse model to create a motor plan (dashed lines in Fig. 1).
In everyday behaviour, this motor circuit functions in a highly integrated manner, as almost all tasks make use of all the different components. Similarly, the numbering of the circuit components does not necessarily reflect the order of the different processes: aspects of the inverse model may already be planned prior to the estimation of the current state. This means that it is challenging for the psychologist to separate the different motor computations. However, it is possible to find tasks which load more or less heavily on different components. For example, when preparing to pick up a knife and cut an apple, you must plan (3) how your hand should approach the knife to grip the handle ready for cutting, rather than gripping the blade. Here, the correct inverse model for planning how and where to grip the knife is an essential element. However, when picking a raspberry, it is important to grip with just enough strength to pull it from the plant without crushing the delicate berry (Wolpert and Ghahramani 2000). In this case, an inverse model (3) would initially plan the reach and grip of the raspberry, but a forward model (4) of the predicted grip strength is essential to fine tune the grip and prevent the berry falling or being crushed. Thus, studies of participant’s performance on different functional tasks can inform us about the relative integrity of the different components of the motor circuit.
Two further critical processes are not illustrated in Fig. 1. First, motor control systems are not fixed at birth, but rapidly and continually learn new information and adapt to the environment. This motor learning takes place on all timescales and in all components of the motor circuit. For example, a lady who puts on a heavy bracelet must adapt to the additional weight on her arm over a few minutes. This learning involves updating the forward model (4) because with a heavy bracelet on, the same muscle activity results in less arm movement. The lady must also plan her actions to take into account the extra weight (3), and pay more attention to proprioceptive information (2) from her arm until she has grown accustomed to the bracelet. Similar processes allow the growing child to learn how his limbs grow in length and mass over the lifespan. The term motor learning covers all these changes, as well as more abstract learning about using tools and sequencing actions. In the present review, we focus mainly on adaptation of the motor model in response to changes within the environment or body dynamics. We leave aside the literature on learning to use tools or learning to sequence actions although we do examine how well autistic people are able to perform these aspects of planning. Second, Fig. 1 does not show how motor processes are organized hierarchically. For example, the goal of baking a cake is accomplished by breaking it down into sub-goals (crack the eggs, sieve the flour, …) which each involve a sequence of motor steps (lift the egg, move to bowl, …) and each step is implemented by a sequence of kinematic movements (close fingers, lift hand …) (Berstein 1967; Botvinick 2008; Grafton and Hamilton 2007; Jordan and Wolpert 1999). The simple computational framework outlined in Fig. 1 can be subsumed within a broader hierarchical framework, in which the forward and inverse models which implement basic movements are themselves controlled by higher lever forward-inverse models responsible for sequencing of movements (Haruno et al. 2001). The detail of this approach is beyond the scope of the current paper, but we will consider if potential motor difficulties in autism arise from lower levels of the motor system (e.g., control of individual finger movements) or higher levels (e.g., sequencing of actions).
Finally, tentative efforts have been made to localize different components of motor processing to different brain regions, but we will not consider these localisations here. Similarly, space constraints preclude the discussion of motor related processes such as observational learning and imitation, which have been considered extensively elsewhere (Hamilton 2009: Gallese et al. 2009; Williams et al. 2004b). Almost all of the studies include high functioning autistic individuals so we are not able to comment specifically on motor functioning in lower functioning groups. However, the model serves as a useful starting point for focusing on those processes that are considered the most basic and fundamental to motor control. A more detailed knowledge of how these essential motor components function in autism will provide good grounding for further understanding higher level skills such as imitation. We consider here the evidence for the integrity of each of the 5 components of Fig. 1 in order and Table 2 gives a summary of this evidence.
(1) Sensory Systems
Precise motor performance requires accurate sensory inputs concerning the body and the world. We focus here on vision, touch and proprioception because these are most important for movement. Difficulties in sensory systems could arise at different stages. First, the brain must receive the raw input signals from the eye and from skin and joint receptors without excess noise or error. Second, these signals must be interpreted, for example, transforming the retinal image into a representation of hand location. Integration across different senses is considered in the next section.
Data on basic sensory processing in autism presents a mixed picture. Questionnaires and individual reports often describe altered sensory experiences such as hyper- and hypo-sensitivity across all modalities in autism (Baranek et al. 2006; Crane et al. 2009; Harrison and Hare 2004; Kern et al. 2006; Leekam et al. 2007). For example, both autistic children and adults are more likely to display or report greater discomfort in response to visual or tactile stimuli and avoid situations where they may encounter such stimuli. There is also much intra and inter-subject variability in terms of both the nature and degree of these sensory experiences (Crane et al. 2009). More quantitative visual studies indicate relatively intact low level functions such as flicker and static contrast sensitivity (Bertone et al. 2005; de Jonge et al. 2007; Pellicano and Gibson 2008; Pellicano et al. 2005). Detection of tactile stimuli and discrimination between different textures also appears not to differ between autistic and neurotypical participants (O’Riordan and Passetti 2006). Moreover, it has been reported that autistic individuals show better detection and localization of low level vibrotactile stimuli then neurotypical individuals (Blakemore et al. 2006; Cascio et al. 2008; Tommerdahl et al. 2007).
Fuentes et al. (2011) recently reported similar levels of proprioceptive ability in twelve adolescent autistic children and twelve matched controls. These children carried out three different tasks where they were asked to match a visual stimulus using their left hand to the felt position of their unseen right arm or finger or to actively move their unseen arm to match the position of a visual stimulus. Notably, these children did show motor difficulties despite their normal proprioception. In addition, indirect evidence for intact proprioception can be derived from a study performed by Glazebrook et al. (2009). They asked autistic adults to perform a simple pointing task where vision of their hand and visual environment was either available or removed and reported that without visual feedback, the autistic participants produced equally accurate end points to neurotypical controls. However, when visual feedback was present, the autistic group exhibited relatively longer movement durations and consistently overshot the target compared to the control group. These results suggest that autistic individuals are able to successfully use proprioception and/or efference copy to guide their movement but find it harder to use visual feedback to control movement.
Several studies suggest that higher level visual processing may be atypical in autism. Thresholds for detecting coherent motion and biological motion are higher in autistic participants than typical participants, indicating difficulties in integrating sensory signals (Bertone et al. 2003; Cook et al. 2009; Freitag et al. 2008; Koldewyn et al. 2010; Milne et al. 2002, 2006; Pellicano and Gibson 2008; Pellicano et al. 2005; Tsermentseli et al. 2008). Similarly, the recognition and discrimination of faces is frequently impaired (Boucher and Lewis 1992; Klin et al. 1999). However, autistic individuals also show superior performance on tasks that place greater emphasis on lower level local detail as opposed to a more global, contextual approach such as the Embedded Figures Task (Jolliffe and Baron-Cohen 1997; Shah and Frith 1993), the Wechsler Block Design subtest (Caron et al. 2006; Shah and Frith 1993) and in visual search (Joseph et al. 2009; Kemner et al. 1998; O’Riordan and Plaisted 2001; O’Riordan et al. 2001). Researchers have proposed that this perceptual style is a result of either a reduced drive to extract overall meaning, termed weak central coherence (Happe and Frith 2006) or an increased dependence on local detail, termed Enhanced Perceptual Functioning (Mottron et al. 2006; for reviews encompassing all levels of visual function see Behrmann et al. 2006; Dakin and Frith 2005; Kaiser and Shiffrar 2009; Simmons et al. 2009). It is not yet known if weak central coherence is also found for tactile and proprioceptive information processing.
In summary, the evidence suggests that low level visual, tactile and proprioceptive inputs are intact or enhanced in autism. On the other hand, evidence from the visual domain suggests that impairments arise at the level of interpretation and integration of these signals, although additional studies are required to test whether this suggestion holds for the other senses. Hypersensitivity and an enhanced ability to detect detail in a stimulus is combined with difficulties in integrating sensory information into a coherent whole. These differences in sensory systems could contribute to motor deficits. This link between sensory input and motor control is emphasized by findings that measures of motion coherence are correlated with motor skills in autistic and neurotypical individuals (Milne et al. 2006). Furthermore, Gowen and Miall (2005) observed that performance of participants with Asperger’s appeared to be worse on tasks that required greater sensory processing (e.g., pointing and timing compared to repetitive tapping and hand turning). Altered sensory input will have a direct impact on calculation of the state estimate, which is used to plan and modify movements and is discussed next.
(2) State Estimation
In order to create and update motor plans, the brain requires a state estimate of where the body is currently located as well as a sensory representation of the location, weight, speed or direction of a particular target. For example, to reach a mug of tea, you must estimate the location of the mug and the location of your own hand. Vision makes the major contribution to defining target locations, while both visual and proprioceptive/tactile information must be integrated in determining hand location. Predicted sensory inputs derived from forward models can also make an important contribution to state estimation, especially during rapid movements. The process of bringing together all these different signals is a form of multi-sensory integration (MSI).
Two core challenges can be identified in MSI. First, it is essential to determine which signals to integrate—should the cool metal of the teaspoon be integrated with the sound of the telephone or the gentle clink of stirring tea? This problem can be solved using both spatial and temporal windows, only integrating information from different senses that occurs close in space or time (Spence et al. 2004). Second, the different information sources must be weighted appropriately to make best use of the available data. For example, in daylight vision often provides the most reliable estimate of hand location, but when reaching for your alarm clock in the dark, it is better to use proprioception. Studies of typical adults demonstrate statistically optimal multisensory integration which takes into account the variability of each sensory signal (Alais and Burr 2004; Ernst and Banks 2002; Landy et al. 1995).
There are few quantitative studies examining MSI in autistic individuals at present, but it appears that integration of visual and auditory signals presented at a similar time point is comparable between ASD and neurotypical participants (Williams et al. 2004a; van der Smagt et al. 2007). More recent studies have tested whether the usual reduction in integration found with increasing temporal separation between the visual and temporal stimuli is also present for autistic groups. Interestingly, autistic children appear to integrate visual and auditory stimuli over a larger temporal window (Foss-Feig et al. 2010; Kwakye et al. 2011) and begin to integrate these two senses at a later age than neurotypical participants (Taylor et al. 2010). In addition, the rubber hand illusion has been used to examine integration between vision, touch and proprioception (Cascio et al. 2012). In this illusion, the participant watches a rubber hand on the table in front of them, while the rubber hand and their own unseen hand beneath the table are synchronously stroked. Integration between vision and touch transfers a sense of hand ownership to the rubber hand and participants think their own hand is positioned closer to the rubber hand. Cascio et al. (2012) observed that proprioceptive drift of the participants hand towards the rubber hand occurred later in the autistic children, suggesting that proprioception is less affected by visual inputs.
No studies to date have directly examined how the different senses are weighted in ASD. With evidence for higher level sensory impairments it will be important to investigate whether sensory input is optimally weighted according to these noisy inputs. Moreover, recent studies in neurotypical participants reveal the important impact that multi-sensory weighting can have on both uni-sensory perception and motor control (Binda et al. 2007; Shams et al. 2011; Wozny and Shams 2011). For example, Binda et al. (2007) used a spatial mislocalization task where targets presented near the onset of a saccade are mislocated in the direction of the saccade due to noisy visual signals. However, the authors showed that when participants were required to localize auditory-visual targets, spatial localization during the saccade was improved due to greater weighting of the less noisy auditory signal. These findings highlight the dynamic and interdependent nature of MSI and action control as well as how inappropriate sensory weighting could lead to inaccurate and slower motor control.
(3) Motor Planning
Motor planning is the process of converting a current state (my hand is by my side) and a desired state (my hand should be on the mug) into a sequence of motor commands (move the arm, close the fingers …). In computational terms, this is an inverse problem and is solved by an inverse model. Planning often begins before a movement is initiated, but the inverse model continues to control action and correct errors during execution. Motor planning is often considered to be hierarchical, for example, beginning with the abstract goal of making a cup of tea, it is necessary to plan the sequence of actions and then the detail of each individual movement to achieve the goal. The more abstract stages involve computing our intentions as well as using processes like memory, which help us to remember things such as which cupboard the teabags are located in. As autistic individuals are known to have impairments in executive functioning (Corbett et al. 2009; Hill 2004) we will focus on lower level aspects of planning which are more directly related to motor control.
The simplest way to assess motor planning is to study reaction times before a movement is performed, which provides a basic measure of the time taken to formulate a motor plan. Autistic participants typically show longer reaction times for reaching movements then their neurotypical counterparts (Glazebrook et al. 2006; Glazebrook et al. 2008, 2009; Mari et al. 2003; Nazarali et al. 2009; Rinehart et al. 2001; Rinehart et al. 2006a). In contrast, saccadic reaction times are similar to neurotypical comparison groups (D’Cruz et al. 2009; Goldberg et al. 2002; Luna et al. 2007; Mosconi et al. 2009; Stanley-Cary et al. 2011; Takarae et al. 2004), suggesting that planning difficulties may be more significant for limb than eye movements. In the following we discuss which aspects of the planning hierarchy appear problematic starting with the more complex and finishing with the lower level processes.
One challenge for motor planning in skilled action is the appropriate storage and deployment of motor knowledge, that is, the knowledge of how to hold and move a tool or shape the hand for a particular gesture. Impairments of skilled movements is termed dyspraxia and there is extensive evidence indicating that compared to neurotypical controls, autistic children perform worse when asked to execute a gesture (e.g., waving) and when asked to demonstrate a gesture using a tool (e.g., hammering a nail) (Dewey et al. 2007; Dowell et al. 2009; Dziuk et al. 2007; Green et al. 2002; Mostofsky et al. 2006). Even when basic motor impairments measured using test batteries are taken into account, dyspraxia is still present (Dewey et al. 2007; Dowell et al. 2009; Dziuk et al. 2007; Macneil and Mostofsky 2012). Such findings suggest the presence of specific deficits in the organization of motor knowledge involved in skilled movement performance. Those studies that report the nature of the dyspraxia reveal a number of different errors including delayed performance, altered amplitude, force or timing of the movement, incorrect limb orientation, using a body part as an object (e.g., combing hair with the hand rather than demonstrating the use of a comb) and performing an incorrect action (Dewey et al. 2007; Mostofsky et al. 2006). The example of using a body part as an object highlights that deficits may begin with higher level motor knowledge but also extend down to lower levels of control as with incorrect limb orientation. However, the finding that autistic children can recognize object based and symbolic gestures as well as neurotypical children (Hamilton et al. 2007; Dowell et al. 2009) suggests that it is the actual transfer of motor knowledge into action that is problematic.
A second challenge in motor planning involves considering the whole of an action sequence, not just the next step. When planning grasping and placing movements, typical individuals will often pick objects up using an awkward posture in order to end in a more comfortable position (Cohen and Rosenbaum 2004; Rosenbaum et al. 1990). For example, in a grip selection task, participants are asked to pick up a bar and rotate it into a final position using either supination or pronation of the wrist. By changing the starting angle of the bar, participants are forced to choose between an awkward start, but comfortable end posture or vice versa and tend to select the former (Cohen and Rosenbaum 2004; Rosenbaum et al. 1990; Fig. 2). However, Hughes (1996) observed that a group of thirty-six autistic children were more likely to end their movement in an awkward posture, suggesting that they did not take the end position into account when planning their movements. In contrast to the above findings, van Swieten et al. (2010) found that autistic children showed equivalent sensitivity to end state comfort as age matched control children. A younger group of children as well as those with DCD were also tested with the results indicating that these groups showed a bias towards selecting a more comfortable starting grip than end position. The authors argued that the task reflects motor experience rather than predictive planning, with participants replicating the most reliable movements according to their movement ability and experience. This would suggest that the autistic children in Hughes (1996) either had less motor experience or poorer motor skills, than those in the latter studies. Hamilton et al. (2007) also tested a group of twenty-five autistic children on the grip selection task and found no group differences, further suggesting that the performance of autistic and neurotypical children is equivalent when asked to select the appropriate sequence of task-related movements.
Example of a version of the grip selection task. The participant’s task is to put the pale end of the bar on the black target circle. This can either be accomplished by grasping the dark end in an awkward underhand grip and finishing with a more comfortable posture (shown in picture) or the participant could grasp the bar using a comfortable overhand grip, but finish in an uncomfortable posture. Adapted from Hamilton et al. (2007)
An alternative approach to understanding action sequencing is to consider how actions are linked together in overlapping segments, sometimes termed chunking or chaining (Berstein 1967; Gobet et al. 2001; Graybiel 1998). For example, in reaching for a piece of food, a child may begin to open his mouth to eat before even grasping the food. Thus two action components (hand movement and mouth opening) overlap in time. Some studies suggest that autistic children have difficulty in tasks involving action chaining, and are more likely to perform each action component individually. Cattaneo et al. (2007) employed electromyography (EMG) to record muscle activity related to mouth opening during a sequence of actions in eight autistic children. Participants were asked to lift an item of food and bring it to either their mouth or a container on their shoulder. During the eating task only, EMG activity in neurotypical children started before the hand even grasped the object. In contrast, EMG activity in the autistic children started much later, when the hand was bringing the food to the mouth (although Pascolo and Cattarinussi 2012 have recently failed to replicate this finding).
In a follow-up study, Fabbri-Destro et al. (2009) explored action chaining in twelve autistic children using a task where the children were required to pick up an object and place it inside either a small or large container. In the typically developing children, the initial reach to the object was slower when the final container was smaller, indicating that the difficulty of the final action goal was programmed into the entire movement sequence. In contrast, the autistic children showed no difference in movement duration between the container sizes. This could be due to a planning failure, if autistic children do not take the final goal into account when planning their actions. Alternatively, it could be a result of perceptual issues: the autistic children may have over-estimated the size of the second container so negating the requirement to adjust the speed of their initial movement.
There is also suggestion that some autistic individuals may show desynchronisation of sub-movements within a single action. Mari et al. (2003) used a reach-to-grasp task in twenty autistic children, where participants were instructed to pick up objects that varied according to size and distance. The authors found that the behaviour of their participants differed according to IQ. A lower functioning group (IQ 70–79) showed evidence of desynchonisation between the reach and grasp component, with delayed onset of the grasp component, while a higher functioning group (IQ between 80 and 109) demonstrated normal reach to grasp actions. However, this latter group produced faster movements than the control and low functioning groups suggesting that use of any visual feedback would have been minimal. These findings raise the important issue of heterogeneity within the autism spectrum, although they contrast with the findings of Glazebrook et al. (2009) discussed earlier who found that visual feedback increased movement duration. Due to the smaller participant number (n = 13) and absence of individual IQ scores in the work by Glazebrook and colleagues, it is not possible to compare the two studies, but both highlight that the use of visual information in motor control may be problematic for autistic individuals.
Once a movement sequence has been planned, the kinematics of the actions must be determined. Fitt’s law describes how movement duration is larger for smaller and more distant targets (Fitts 1954). Similarly, the timing and kinematics of reach and grasp components varies according to the size and distance of the object to be grasped: Typically, smaller and further objects result in longer movement durations, prolonged deceleration, lower amplitudes of peak velocity and decreased time of peak grip aperture. As target characteristics are taken into consideration when planning a movement (Rosenbaum et al. 2006), examining the effect of target size and distance in autistic individuals can provide information on their planning ability. Appropriate adjustments to target size and distance have been observed during pointing or grasping movements (Glazebrook et al. 2006; Fabbri-Destro et al. 2009; Mari et al. 2003), suggesting that target properties are appropriately programmed (although more slowly) for at least the immediate if not the final goal (Fabbri-Destro et al. 2009).
Prior to movement execution, movement sub-goals are planned in a hierarchical order, such as effector first, then direction and finally amplitude. Evidence for such a hierarchy comes from studies using precues where participants are given advance warning about different aspects of the upcoming movement (e.g., effector, direction, amplitude). As the effector is selected first, advance warning about which hand to use results in the largest reduction of reaction time (Rosenbaum 1980). Glazebrook et al. 2008 used a modified version of the precue technique and reported that although adult autistic participants took longer to plan their movements, they showed a similar reduction in reaction times as the control group to the different combination of precues (see also experiment 1 of Nazarali et al. 2009). Therefore, autistic individuals appear to program movement kinematics using a similar order to neurotypicals.
Overall, results on motor planning studies seem somewhat contradictory. Dyspraxia is commonly reported in autism (Dewey et al. 2007; Dowell et al. 2009; Dziuk et al. 2007; Green et al. 2002; Mostofsky et al. 2006), but knowledge of action postures is not always impaired (Hamilton et al. 2007; Dowell et al. 2009). Individuals with autism show good performance on some versions of the grip selection task (Hamilton et al. 2007; van Swieten et al. 2010) and in planning the appropriate kinematics for particular targets (Glazebrook et al. 2006; Fabbri-Destro et al. 2009; Mari et al. 2003), but poor performance on chaining tasks and a similar grip selection task (Cattaneo et al. 2007; Fabbri-Destro et al. 2009; Hughes 1996). One possibility is that task complexity and participant experience influence results across different studies. For example, dyspraxia studies use actions that are composed of several steps and have a purpose whereas studies investigating planning of movement kinematics use relatively simple and meaningless pointing tasks. An alternative possibility is that autistic individuals are able to plan individual aspects of their actions (how to grasp the bar) but are less good at organizing the temporal detail of the action in the chaining tasks. Thus, autistic individuals may plan and execute each component of the action separately and the degree to which they separate action sub-goals may depend on whether they are low or high functioning. Such a strategy is reminiscent of the autistic perceptual style described by the weak central coherence (Happe and Frith 2006) and Enhanced Perceptual Functioning theories (Mottron et al. 2006), which both emphasis that individuals with autism are good at processing details and small components but less good at integrating these into a global percept. Further study of the relationship between perceptual integration and motor integration could be used to test this possibility.
(4) Feedforward Control and Prediction
During movement execution it is essential to check if the executed action is proceeding as planned, and to correct for errors if needed. One option is to compare the sensory feedback resulting from the movement with the intended goal, termed feedback control. However, delays in sensory and motor systems make feedback control slow and unstable, especially for rapid hand movements (Miall and Wolpert 1996). To deal with these delays, the motor system uses forward models or predictors. A copy of the motor command (efference copy) is sent to a forward model, which rapidly generates a prediction of the sensory consequences of the action (Wolpert and Flanagan 2001). This sensory prediction is compared with the incoming sensory signals, so that errors can be detected rapidly.
Predictive motor control can be studied by examining rapid movement corrections before feedback would normally be available (Wolpert and Flanagan 2001). For example, when drinking your mug of tea it is critical to both grip the mug tightly (grip force) and lift upwards (load force). In a natural action, the grip force exerted by the fingers on the mug is closely synchronized with the load force of the lifting arm (Fig. 3a). However, if a passerby knocked the mug, the knock would exert a load force on the mug and you would respond around 100 ms later with a stronger grip (Fig. 3b). Thus, synchronization of grip force and load force indicates predictive control, while desynchronisation indicates reactive control (Flanagan and Wing 1997). Unloading paradigms measure the same predictive process—if you hold a heavy object on the palm of your left hand and then lift it with your right hand, predictive control allows you to keep the left hand steady (Fig. 3c). Finally, the sensory consequences of forward models can be measured in tickling and force cancellation paradigms, in which a stimulus is perceived as weaker (less tickly) when it is self-generated and thus predictable, than when it is externally generated (Blakemore et al. 2000).
In order to pick up the cup without it slipping, sufficient grip force needs to be exerted in excess of load force. When the load is self generated (lifting the cup), an efference copy of the motor command is used to predict the load force and generate enough grip force that parallels the load force (a). However, when the load is externally generated (another person knocks the cup), there is no prediction so grip force follows load force in a reactive manner (b) In the unloading paradigm, when lifting an object off your own hand (c), predictive control determines the time at which unloading will occur. Signals are sent to the unloading arm so that it relaxes and remains steady at the time of unloading. When another person takes an object from your hand (d) sensory signals are used to determine that the object has been moved. This results in reactive control with your arm relaxing following object removal and becoming less steady (adapted from Wolpert and Flanagan 2001)
Schmitz et al. (2003) used a bimanual load-lifting task where eight autistic children and neurotypical children were asked to lift a load off one hand, using their other hand while activity of their loaded arm was measured using EMG. As expected for the control children, changes in muscle activity occurred prior to unloading. However, the autistic children displayed a longer duration of voluntary unloading and reactive, rather than predictive changes in muscle activity. A potential issue with using a bimanual task is that autistic infants have been reported to display difficulties with coordinating the two sides of the body exhibiting postural and crawling asymmetry (Esposito et al. 2009; Teitelbaum et al. 1998). Consequently, the observed reactive changes may have resulted from general deficits in bimanual coordination rather than specific problems of prediction.
As part of a wider range of tests, Gowen and Miall (2005) examined grip force control in twelve Asperger and matched control participants. Participants lifted a manipulandum up and down for ten cycles between their thumb and index finger. In the case of reduced predictive mechanisms one would expect increased latency of peak grip force in relation to peak load forced and compensatory higher grip forces. However, no significant differences were found between the groups. In contrast, David et al. (2009) observed increased latency of grip to load force in 13 autistic children and adolescents compared to a matched control sample. Blakemore et al. (2006) examined whether the sensation of tickly stimuli were attenuated in a self generated compared to externally generated condition in 16 adult ASD and 16 control participants. They observed similar attenuation of self generated tickly stimuli in both the ASD and control groups, suggesting that these participants were able to use a sensory prediction generated by a forward model to compare with actual sensory consequences in order to attenuate the stimulation. Finally, forward models need to be adaptive in order to provide accurate predictions when changes occur to our bodies or the external environment (Shadmehr et al. 2010). Evidence reviewed later under “Motor learning” indicates that forward models involved in motor control are calibrated relatively effectively in autistic people, suggesting that the overall organization of predictive control is intact.
An alternative way to study forward models is by analogy with cerebellar patients, because the implementation of forward models is linked to the cerebellum (Blakemore et al. 2001; Kawato et al. 2003). Patients with cerebellar damage show jerky and dysmetric movements (Haggard et al. 1995), with a particular impairment in grip force-load force tasks (Hermsdorfer et al. 1994; Muller and Dichgans 1994). Individuals with autism also show slower, dysmetric movements (Glazebrook et al. 2006, 2008; Gowen and Miall 2005; Nazarali et al. 2009). However, close examination suggests these difficulties could be attributed to other motor impairments. When examining velocity and acceleration profiles of single pointing movements performed by autistic individuals, Glazebrook et al. (2006) could find no evidence of the typical discontinuities seen in cerebellar patients. However, as the autistic group performed slower movements than the control group, this could have hidden any discontinuities by optimizing the use of feedback (Beppu et al. 1984; Bastian 2006). Consequently, further data is needed on arm movement kinematics when autistic participants are required to make faster movements and where visual feedback is important.
To date, the evidence regarding the functioning of predictive control in autistic individuals appears to be mixed. Two studies using grip force and load-lifting have reported impairments in autistic children (David et al. 2009; Schmitz et al. 2003), whereas another grip force study (Gowen and Miall 2005) and one using the tickling cancellation paradigm observed no differences between adult autistic and neurotypical participants (Blakemore et al. 2006). This ambiguity could be due to heterogeneity in the groups tested, in terms of age or level of function as well as the intrinsic heterogeneity of autism. Studies with a larger sample size would help here. It is also possible that different paradigms test different aspects of predictive control. For example, in contrast to single arm grip force experiments, bimanual unloading involves inter-limb communication, which as mentioned earlier may be impaired in autism. Moreover, forward models might independently be involved in cancellation of expected sensory feedback and in online control of grasp force (Miall and Wolpert 1996). Thus, it would be informative to perform a variety of predictive experiments that have differing complexity levels and involve different movement systems within one individual.
(5) Motor Execution
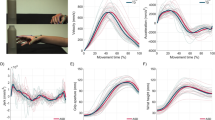
Once a movement plan has been formulated, motor commands are sent to the motor cortex and on to the peripheral nerves and musculature to be executed. Errors could then arise in either the amplitude or timing of the motor signals. Variability in the amplitude of the motor signal is ubiquitous in all movement (Harris and Wolpert 1998; Jones et al. 2002), but a substantial increase in output variability would have important consequences for motor performance. For example, increased output variability could lead to errors in movement endpoint and substantial time spent on corrective movements or even task failure. Motor execution also relies on precise timing of agonist and antagonist muscle groups on a millisecond scale so that the position, orientation and speed of limbs can produce accurate movement outcomes (Hore et al. 1991, 1996). In the case of increased motor signal noise or imprecise timing one might expect dysmetric movements and increased spatial and temporal variability in certain movement parameters such as peak velocity, acceleration and duration. As highlighted earlier, dysmetria and increased end point variability is frequently reported for autistic individuals during reaching (Glazebrook et al. 2006, 2008; Gowen and Miall 2005; Nazarali et al. 2009) and saccadic movements (Luna et al. 2007; Stanley-Cary et al. 2011; Takarae et al. 2004). Increased variability of movement kinematics has also been reported. Examining pointing movements in eight autistic adults, Glazebrook et al. (2006) observed greater variability in time to peak velocity and greater spatial variability of peak acceleration which was replicated in a later study of thirteen autistic participants (Glazebrook et al. 2009). In addition, the autistic group displayed less scaling of peak velocity and acceleration when the movement amplitude increased. The authors suggested that the results indicate a problem with the generation and timing of muscular forces leading to increased variability and a strategic slowing of the movement to reduce this variability and use visual feedback (see Elliott et al. 2010 for a discussion of this study). Increased variability of stride length has also been observed in autistic but not Asperger children (Rinehart et al. 2006b; see Stanley-Cary et al. 2011 for a similar group dissociation with saccade variability) as well as increased variability of head shoulder and trunk position during walking (Vernazza-Martin et al. 2005).
Timing has been directly examined in autistic individuals using reproduction or perception tasks. In the former, participants reproduce the interval between two tones and in the latter they judge the length of one interval compared to another so that motor demands are higher for the reproduction task. Compared to neurotypical groups, autistic performance on reproduction tasks in the range of 0.4–5.5 s consistently results in poorer accuracy and higher variability of timed responses (Gowen and Miall 2005; Martin et al. 2010; Szelag et al. 2004). These timing errors could arise from imprecision of a central clock timer or errors in a peripheral implementation system that executes the command from the clock (Wing and Kristofferson 1973). Although no previous study has differentiated between a central and peripheral timing deficit, the one study that used a perception task in children and adolescents indicated no differences between control and ASD groups (Mostofsky et al. 2000), suggesting that alterations to a peripheral implementation system as opposed to a central timekeeper may be more significant in ASD.
In summary, there is good evidence to suggest the presence of increased motor noise and timing deficits in autistic individuals and that these may lead to increased variability in temporal and spatial aspects of execution. Consequently, it may be more cognitively challenging for autistic individuals to produce accurate movements. Alternatively, increased variability could indicate deficits earlier in the model relating to planning or feedforward control, although evidence in the next section on motor learning suggests that autistic individuals can adapt and improve their movement accuracy. It will also be important for future work to determine whether timing deficits are a result of a central timekeeper or more peripheral mechanisms and how they impact upon daily motor skills.
Motor Learning
Motor adaptation is essential for enabling us to achieve motor goals when the environment and our own body dynamics are constantly changing. For example, adaptation is important in making adjustments to external properties of the environment such as the weight and location of objects to be manipulated. It also allows the motor system to make adjustments in response to changes in body dynamics such as short-term changes (e.g., muscle fatigue) as well as changes that occur over a longer timescale (e.g., developmental growth) (Shadmehr and Krakauer 2008). During adaptation, the inverse and forward models are gradually updated by comparing the actual sensory state with predictions generated by the forward model, so improving planning accuracy with successive attempts (Shadmehr et al. 2010). Adaptation paradigms generally involve an adaptation phase where participants improve their accuracy on a task where there is imposed discrepancy between motor commands and sensory feedback. This discrepancy is then removed and participants perform the identical movement during a post adaptation phase, to test for after-effects that would indicate adaptation (or generalisation) has taken place.
Mostofsky et al. (2004) examined adaptation in eight high functioning autistic boys using a ball catching task. Participants were instructed to catch a ball dropped onto the palm of their hand and adaptation effects were measured according to impact displacement of the hand. A light ball was used in the first baseline block of trials, followed by a heavier ball during the adaptation trials with the experiment finishing with the light ball again to examine post adaptation effects. In the case of successful adaptation one would expect greater initial hand displacement with the heavier ball, reducing to a steady state displacement, followed by less hand displacement for the post-adaptation phase compared to the initial lighter ball trials. These adaptation and post adaptation effects were similar for both groups, suggesting that the autistic children in this small sample were able to adapt. Gidley Larson et al. (2008) asked autistic children to perform three tasks that required adapting to changes in the relationship between the sensory consequences of a motor output. In the prism adaptation task, children threw balls at a target before, during and after wearing prism goggles that created a shift in the visual environment. The final two tasks involved moving a cursor controlled by a robot arm to a target, where either forces were applied to the arm or the cursor was displaced in reference to the arm position. For all three tasks, the autistic children adapted their motor output to the applied perturbations and showed after effects of a similar level to the control children. Haswell et al. (2009) also observed that autistic children learnt to adapt to forces applied to a robotic arm while moving the arm to a particular location. The authors then went on to examine how well the adapted state generalised to a movement performed using intrinsic coordinates (identical joint rotations) or extrinsic coordinates (similar target location), that rely more on proprioceptive or visual signals respectively. Generalisation occurred during both conditions for the control children, but only using intrinsic coordinates for the autistic children. Moreover, generalisation was stronger for the autistic compared to control children during the intrinsic condition, suggesting that autistic children may place more weight on the proprioceptive sense when updating the forward model. These findings suggest that proprioceptive input may be more reliable than visual input and also tie in with evidence that autistic individuals are able to successfully combine proprioception with efference copy. For example, the improvement in proprioceptive precision that occurs using an active rather than passive movement task, appears similar in both adult autistic and neurotypical participants (Fuentes et al. 2011).
A different type of motor learning that appears intact in autistic people is implicit motor learning. Implicit motor learning occurs during practice within a structured environment but where the learning is unintentional, and cannot easily be verbally described by the participant. It is commonly examined using the serial reaction time task (SRT) where participants are asked to respond to stimuli that are presented in different locations (Nissen and Bullemer 1987). Unknown to the participant, the stimuli are presented in a repetitive sequence so that the reaction times become faster for the stimuli that form part of this sequence, but participants are unable to verbally describe the repeated sequences. There is consistent evidence that the reduction in reaction times of the repeated sequences is equivalent in both ASD and neurotypical groups (Barnes et al. 2008; Brown et al. 2010; Nemeth et al. 2010). Similarly, autistic adults show unimpaired rates of learning using a predictive saccade task, where participants saccade to a target appearing repeatedly to the left then right (D’Cruz et al. 2009). However, the autistic group did show faster rightward responses, leading the authors to suggest an alteration in timing of internally generated movements, fitting with our earlier discussion on timing. Returning to arm movements, autistic participants may benefit more from practice than neurotypical participants as their overall reaction times become similar to those of the neurotypical group following practice on the repeated sequences (Brown et al. 2010). Similarly, there is some suggestion that it is possible to reduce the variability present in autistic movements through practice. Using an imitation task where participants were required to imitate simple pointing movements, we have shown that following a period of practice, variability in peak velocity of imitated movements reduced for the autistic participants to similar levels to the neurotypical group (Wild et al., unpublished data). However, it is unclear whether the benefits from practice on one task generalize to other behaviours.
In summary, there is general consensus that autistic individuals are able to successfully modify the inverse model and update a forward model prediction of arm movement when the environment changes and with repeated practice. Whether they use similar mechanisms to neurotypical individuals or rely more on proprioception during motor learning deserves further exploration.
Conclusions and Future Directions
Using a computational framework, we have reviewed different aspects of motor control in ASD and Table 2 summarizes some of the main findings. Firstly, low level sensory input seems to be the same or in some cases better in autism, while higher order sensory processing is abnormal. Secondly, it is possible that integration of these different senses is abnormal which could lead to inaccuracies in state estimation. In particular, over-reliance on proprioception could reflect either difficulties in sensorimotor integration, or an optimal response to an abnormal visual input. This requires further investigation. Thirdly, motor planning appears more challenging for autistic individuals, with difficulties in organizing motor knowledge and longer reaction times when planning movements. The evidence so far suggests that movement kinematics are planned appropriately but more slowly and that actions may not be chained together. Fourthly, evidence for the integrity of feed-forward control is mixed, indicating that further investigation is required but that predictive ability is perhaps not a key element of impaired autistic motor control. Fifthly, consistent findings of dysmetric and more variable movements suggest that increased noise and/or mistimed muscular forces may hamper movement execution. Finally, the consensus that autistic individuals show relatively intact motor adaptation indicates that basic motor learning must be intact and that flexibility exists: autistic people may have different input and output signals but the underlying “motor machinery” is functioning.
An important aspect to highlight from our review is that not all motor processes are impaired in high functioning autistic individuals and they are capable of performing a range of motor skills, but perhaps using modified processes. In particular, there is consistent evidence that motor learning is intact but that the multi-sensory inputs may be different (e.g., proprioception weighted over vision). In addition, some of the lower level planning mechanisms also appear relatively spared. In contrast, the aspects which are problematic for autistic people include sensory input and motor execution as well as higher level planning involving coordination of motor knowledge into appropriate sequences. Two non-mutually exclusive explanations can be given for this pattern of data. We consider first an explanation in terms of input/output noise, and second an explanation in terms of poor integration of information and weak central coherence.
Increased variability in both sensory inputs and motor execution are noted above. This added noise generates an additional burden at all levels of motor processing, and might make it particularly difficult to perform smooth action sequences. Planning movements in a serial fashion may represent a strategy to deal with low level noise, rather than a deficit in planning itself. Similarly, individuals might adapt to excess variability in one sensory domain by relying more on other senses. Again, apparent abnormalities in sensory integration might be an appropriate response to noisy inputs. In regards to experience and learning, as reviewed earlier there is good evidence that autistic participants are able to adapt their motor system and benefit from repeated practice of movement sequences. Perhaps with more experience and practice, ASD individuals (particularly those who are high functioning and older) are able to overcome some of the detrimental effects of a noisy system. Such a possibility could be related to the tendency to perform repetitive behaviours: repeating an action leads to improved reaction times whereas a new action may result in a slower and less accurate outcome.
To test this input/output noise hypothesis, it would be helpful to examine sensory and motor variability in more detail in autism using methods which dissociate noise generated through sensory or execution processes (Osborne et al. 2005; van Beers 2007). Such paradigms could be used to assess whether practice reduces sensory or motor noise and how this affects motor ability. Furthermore, a useful future approach would be to explore the relationship between sensory noise and the weighting of vision, proprioception and touch and what impact this has on motor ability.
A second possible account of motor difficulties in autism focuses on the integration of motor information. Multisensory information must be brought together in state estimation, and motor knowledge must be integrated for effective planning over many timescales. A processing style focused on detail, as found for sensory systems (Happe and Frith 2006) might also have critical consequences for motor systems. In particular, impaired MSI might lead to noisier state estimates. Poor motor knowledge might lead to dyspraxia and difficulties in tool use and action knowledge tasks. Therefore, weak central coherence may extend across multiple systems in autistic individuals. To test this hypothesis, it would be helpful to know if weak central coherence in purely perceptual tasks correlates with performance on motor planning and multisensory integration tasks.
In conclusion, our review suggests that altered sensory input and variability in motor execution, together with deficits in organizing motor knowledge may play an important role in the motor abilities of autistic people. Future research should examine the precise role of sensorimotor noise in autistic motor performance and the link between weak central coherence and motor planning. An improved understanding of motor systems in autism also raises important questions for future research such as whether the underlying motor difficulties in autism and DCD overlap. Furthermore, it has been suggested that we can use our own motor processes to predict and understand the behaviour of others (Wolpert et al. 2003). This possibility raises the question of how motor difficulties relate to social difficulties—are they independent or do underlying motor issues cause the social characteristics? Using a computational approach to understanding autistic motor control may provide some insight into these questions.
References
Alais, D., & Burr, D. (2004). The ventriloquist effect results from near-optimal bimodal integration. Current Biology, 14, 257–262.
Baranek, G. T., David, F. J., Poe, M. D., Stone, W. L., & Watson, L. R. (2006). Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, 47, 591–601.
Barnes, K. A., Howard, J. H., Jr., Howard, D. V., Gilotty, L., Kenworthy, L., Gaillard, W. D., et al. (2008). Intact implicit learning of spatial context and temporal sequences in childhood autism spectrum disorder. Neuropsychology, 22, 563–570.
Bastian, A. J. (2006). Learning to predict the future: The cerebellum adapts feedforward movement control. Current Opinion in Neurobiology, 16, 645–649.
Behrmann, M., Thomas, C., & Humphreys, K. (2006). Seeing it differently: Visual processing in autism. Trends in Cognitive Sciences, 10, 258–264.
Beppu, H., Suda, M., & Tanaka, R. (1984). Analysis of cerebellar motor disorders by visually guided elbow tracking movement. Brain, 107(Pt 3), 787–809.
Berstein, N. A. (1967). The coordination and regulation of movements Oxford. New York: Pergamon Press.
Bertone, A., Mottron, L., Jelenic, P., & Faubert, J. (2003). Motion perception in autism: A “complex” issue. Journal of Cognitive Neuroscience, 15, 218–225.
Bertone, A., Mottron, L., Jelenic, P., & Faubert, J. (2005). Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain, 128, 2430–2441.
Binda, P., Bruno, A., Burr, D. C., & Morrone, M. C. (2007). Fusion of visual and auditory stimuli during saccades: A Bayesian explanation for perisaccadic distortions. Journal of Neuroscience, 27, 8525–8532.
Blaesi, S., & Wilson, M. (2010). The mirror reflects both ways: Action influences perception of others. Brain and Cognition, 72, 306–309.
Blakemore, S. J., Frith, C. D., & Wolpert, D. M. (2001). The cerebellum is involved in predicting the sensory consequences of action. NeuroReport, 12, 1879–1884.
Blakemore, S. J., Tavassoli, T., Calo, S., Thomas, R. M., Catmur, C., Frith, U., et al. (2006). Tactile sensitivity in Asperger syndrome. Brain and Cognition, 61, 5–13.
Blakemore, S. J., Wolpert, D., & Frith, C. (2000). Why can’t you tickle yourself? NeuroReport, 11, R11–R16.
Botvinick, M. M. (2008). Hierarchical models of behavior and prefrontal function. Trends in Cognitive Sciences, 12, 201–208.
Boucher, J., & Lewis, V. (1992). Unfamiliar face recognition in relatively able autistic children. Journal of Child Psychology and Psychiatry, 33, 843–859.
Brian, J., Bryson, S. E., Garon, N., Roberts, W., Smith, I. M., Szatmari, P., et al. (2008). Clinical assessment of autism in high-risk 18-month-olds. Autism, 12, 433–456.
Brown, J., Aczel, B., Jimenez, L., Kaufman, S. B., & Grant, K. P. (2010). Intact implicit learning in autism spectrum conditions. The Quarterly Journal of Experimental Psychology (Colchester.), 63, 1789–1812.
Caron, M. J., Mottron, L., Berthiaume, C., & Dawson, M. (2006). Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain, 129, 1789–1802.
Cascio, C. J., Foss-Feig, J. H., Burnette, C. P., Heacock. J. L., Cosby, A. A. (2012) The rubber hand illusion in children with autism spectrum disorders: Delayed influence of combined tactile and visual input on proprioception. Autism (in press).
Cascio, C., McGlone, F., Folger, S., Tannan, V., Baranek, G., Pelphrey, K. A., et al. (2008). Tactile perception in adults with autism: A multidimensional psychophysical study. Journal of Autism and Developmental Disorders, 38, 127–137.
Cattaneo, L., Fabbri-Destro, M., Boria, S., Pieraccini, C., Monti, A., Cossu, G., et al. (2007). Impairment of actions chains in autism and its possible role in intention understanding. Proceedings of National Academy of Science USA, 104, 17825–17830.
Clearfield, M. W. (2011). Learning to walk changes infants’ social interactions. Infant Behavior and Development, 34, 15–25.
Cohen, R. G., & Rosenbaum, D. A. (2004). Where grasps are made reveals how grasps are planned: Generation and recall of motor plans. Experimental Brain Research, 157, 486–495.
Cook, J., Saygin, A. P., Swain, R., & Blakemore, S. J. (2009). Reduced sensitivity to minimum-jerk biological motion in autism spectrum conditions. Neuropsychologia, 47, 3275–3278.
Corbett, B. A., Constantine, L. J., Hendren, R., Rocke, D., & Ozonoff, S. (2009). Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research, 166, 210–222.
Crane, L., Goddard, L., & Pring, L. (2009). Sensory processing in adults with autism spectrum disorders. Autism, 13, 215–228.
Dakin, S., & Frith, U. (2005). Vagaries of visual perception in autism. Neuron, 48, 497–507.
d’Avella, A., & Bizzi, E. (2005). Shared and specific muscle synergies in natural motor behaviors. Proceedings of National Academy of Science USA, 102, 3076–3081.
David, F. J., Baranek, G. T., Giuliani, C. A., Mercer, V. S., Poe, M. D., & Thorpe, D. E. (2009). A pilot study: Coordination of precision grip in children and adolescents with high functioning autism. Pediatric Physical Therapy, 21, 205–211.
D'Cruz, A. M., Mosconi, M. W., Steels, S., Rubin, L. H., Luna, B., Minshew, N., et al. (2009). Lateralized response timing deficits in autism. Biological Psychiatry, 66(4), 393–397.
de Jonge, M. V., Kemner, C., de Haan, E. H., Coppens, J. E., van den Berg, T. J., & van Engeland, H. (2007). Visual information processing in high-functioning individuals with autism spectrum disorders and their parents. Neuropsychology, 21, 65–73.
Denckla, M. B. (1985). Revised neurological examination for subtle signs. Psychopharmacology Bulletin, 21(4), 773–800.
Dewey, D., Cantell, M., & Crawford, S. G. (2007). Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of International Neuropsycholy Society, 13, 246–256.
Diedrichsen, J., Shadmehr, R., & Ivry, R. B. (2010). The coordination of movement: Optimal feedback control and beyond. Trends in Cognitive Sciences, 14, 31–39.
Dowell, L. R., Mahone, E. M., & Mostofsky, S. H. (2009). Associations of postural knowledge and basic motor skill with dyspraxia in autism: Implication for abnormalities in distributed connectivity and motor learning. Neuropsychology, 23(5), 563–570.
Dziuk, M. A., Gidley Larson, J. C., Apostu, A., Mahone, E. M., Denckla, M. B., & Mostofsky, S. H. (2007). Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental Medicine and Child Neurology, 49, 734–739.
Elliott, D., Hansen, S., Grierson, L. E., Lyons, J., Bennett, S. J., & Hayes, S. J. (2010). Goal-directed aiming: Two components but multiple processes. Psychological Bulletin, 136, 1023–1044.
Ernst, M. O., & Banks, M. S. (2002). Humans integrate visual and haptic information in a statistically optimal fashion. Nature, 415, 429–433.
Eskenazi, T., Grosjean, M., Humphreys, G. W., & Knoblich, G. (2009). The role of motor simulation in action perception: A neuropsychological case study. Psychological Research, 73, 477–485.
Esposito, G., Venuti, P., Maestro, S., & Muratori, F. (2009). An exploration of symmetry in early autism spectrum disorders: Analysis of lying. Brain and Development, 31, 131–138.
Fabbri-Destro, M., Cattaneo, L., Boria, S., & Rizzolatti, G. (2009). Planning actions in autism. Experimental Brain Research, 192, 521–525.
Fitts, P. M. (1954). The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology, 47, 381–391.
Flanagan, J. R., & Wing, A. M. (1997). The role of internal models in motion planning and control: Evidence from grip force adjustments during movements of hand-held loads. Journal of Neuroscience, 17, 1519–1528.
Foss-Feig, J. H., Kwakye, L. D., Cascio, C. J., Burnette, C. P., Kadivar, H., Stone, W. L., et al. (2010). An extended multisensory temporal binding window in autism spectrum disorders. Experimental Brain Research, 203, 381–389.
Fournier, K. A., Hass, C. J., Naik, S. K., Lodha, N., & Cauraugh, J. H. (2010). Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders, 40, 1227–1240.
Freitag, C. M., Kleser, C., Schneider, M., & von Gontard, A. (2007). Quantitative assessment of neuromotor function in adolescents with high functioning autism and asperger syndrome. Journal of Autism and Developmental Disorders, 37(5), 948–959.
Freitag, C. M., Konrad, C., Haberlen, M., Kleser, C., von Gontard, A., Reith, W., et al. (2008). Perception of biological motion in autism spectrum disorders. Neuropsychologia, 46(5), 1480–1496.
Fuentes, C. T., Mostofsky, S. H., & Bastian, A. J. (2011). No proprioceptive deficits in autism despite movement-related sensory and execution impairments. Journal of Autism and Developmental Disorders, 41(10), 1352–1361.
Gallese, V., Rochat, M., Cossu, G., & Sinigaglia, C. (2009). Motor cognition and its role in the phylogeny and ontogeny of action understanding. Developmental Psychology, 45(1), 103–313.
Gepner, B., Mestre, D., Masson, G., & de Schonen, S. (1995). Postural effects of motion vision in young autistic children. NeuroReport, 6, 1211–1214.
Gernsbacher, M. A., Sauer, E. A., Geye, H. M., Schweigert, E. K., & Hill, G. H. (2008). Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry, 49, 43–50.
Ghaziuddin, M., Butler, E., Tsai, L., & Ghaziuddin, N. (1994). Is clumsiness a marker for Asperger syndrome? Journal of Intellectual Disability Research, 38(Pt 5), 519–527.
Gidley Larson, J. C., Bastian, A. J., Donchin, O., Shadmehr, R., & Mostofsky, S. H. (2008). Acquisition of internal models of motor tasks in children with autism. Brain, 131, 2894–2903.
Glazebrook, C. M., Elliott, D., & Lyons, J. (2006). A kinematic analysis of how young adults with and without autism plan and control goal-directed movements. Motor Control, 10, 244–264.
Glazebrook, C. M., Elliott, D., & Szatmari, P. (2008). How do individuals with autism plan their movements? Journal of Autism and Developmental Disorders, 38, 114–126.
Glazebrook, C. M., Gonzalez, D., Hansen, S., & Elliott, D. (2009). The role of vision for online control of manual aiming movements in persons with autism spectrum disorders. Autism, 13(4), 411–433.
Gobet, F., Lane, P. C., Croker, S., Cheng, P. C., Jones, G., Oliver, I., et al. (2001). Chunking mechanisms in human learning. Trends in Cognitive Sciences, 5, 236–243.
Goldberg, M. C., Lasker, A. G., Zee, D. S., Garth, E., Tien, A., & Landa, R. J. (2002). Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia, 40(12), 2039–2049.
Gowen, E., & Miall, R. C. (2005). Behavioural aspects of cerebellar function in adults with Asperger syndrome. Cerebellum, 4, 279–289.
Grafton, S. T., & Hamilton, A. F. (2007). Evidence for a distributed hierarchy of action representation in the brain. Human Movement Science, 26(4), 590–616.
Graybiel, A. M. (1998). The basal ganglia and chunking of action repertoires. Neurobiology of Learning and Memory, 70, 119–136.
Green, D., Baird, G., Barnett, A. L., Henderson, L., Huber, J., & Henderson, S. E. (2002). The severity and nature of motor impairment in Asperger’s syndrome: A comparison with specific developmental disorder of motor function. Journal of Child Psychology and Psychiatry, 43, 655–668.
Haggard, P., Miall, R. C., Wade, D., Fowler, S., Richardson, A., Anslow, P., et al. (1995). Damage to cerebellocortical pathways after closed head injury: A behavioural and magnetic resonance imaging study. Journal of Neurology and Neurosurgery Psychiatry, 58, 433–438.
Hamilton, A. F. (2009). Research review: Goals, intentions and mental states: Challenges for theories of autism. Journal of Child Psychology and Psychiatry, 50(8), 881–892.
Hamilton, A. F., Brindley, R. M., & Frith, U. (2007). Imitation and action understanding in autistic spectrum disorders: How valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia, 45, 1859–1868.
Happe, F., & Frith, U. (2006). The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders, 36, 5–25.
Harris, C. M., & Wolpert, D. M. (1998). Signal-dependent noise determines motor planning. Nature, 394, 780–784.
Harrison, J., & Hare, D. J. (2004). Brief report: Assessment of sensory abnormalities in people with autistic spectrum disorders. Journal of Autism and Developmental Disorders, 34, 727–730.
Haruno, M., Wolpert, D. M., & Kawato, M. (2001). Mosaic model for sensorimotor learning and control. Neural Computation, 13, 2201–2220.
Haswell, C. C., Izawa, J., Dowell, L. R., Mostofsky, S. H., & Shadmehr, R. (2009). Representation of internal models of action in the autistic brain. Nature Neuroscience, 12, 970–972.
Henderson, S. E., & Sugden, D. A. (1992). Movement assessment battery for children. London: Psychological Corporation.
Hermsdorfer, J., Wessel, K., Mai, N., & Marquardt, C. (1994). Perturbation of precision grip in Friedreich’s ataxia and late-onset cerebellar ataxia. Movement Disorders, 9, 650–654.
Hill, E. L. (2004). Executive dysfunction in autism. Trends in Cognitive Sciences, 8, 26–32.
Hore, J., Watts, S., Tweed, D., & Miller, B. (1996). Overarm throws with the nondominant arm: Kinematics of accuracy. Journal of Neurophysiology, 76, 3693–3704.
Hore, J., Wild, B., & Diener, H. C. (1991). Cerebellar dysmetria at the elbow, wrist, and fingers. Journal of Neurophysiology, 65, 563–571.
Hughes, C. (1996). Brief report: Planning problems in autism at the level of motor control. Journal of Autism and Developmental Disorders, 26, 99–107.
Jansiewicz, E. M., Goldberg, M. C., Newschaffer, C. J., Denckla, M. B., Landa, R., & Mostofsky, S. H. (2006). Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of Autism and Developmental Disorders, 36, 613–621.
Jasmin, E., Couture, M., McKinley, P., Reid, G., Fombonne, E., & Gisel, E. (2009). Sensori-motor and daily living skills of preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 231–241.
Jolliffe, T., & Baron-Cohen, S. (1997). Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? Journal of Child Psychology and Psychiatry, 38, 527–534.
Jones, K. E., Hamilton, A. F., & Wolpert, D. M. (2002). Sources of signal-dependent noise during isometric force production. Journal of Neurophysiology, 88, 1533–1544.
Jordan, M. I., & Wolpert, D. (1999). Computational motor control. In M. Gazzaniga (Ed.), The cognitive neurosciences. Cambridge, MA: MIT Press.
Joseph, R. M., Keehn, B., Connolly, C., Wolfe, J. M., & Horowitz, T. S. (2009). Why is visual search superior in autism spectrum disorder? Developmental Science, 12, 1083–1096.
Kaiser, M. D., & Shiffrar, M. (2009). The visual perception of motion by observers with autism spectrum disorders: A review and synthesis. Psychonomic Bulletin and Review, 16, 761–777.
Kawato, M., Kuroda, T., & Imamizu, H. (2003). Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Progress in Brain Research, 142, 171–188.
Kelso, J. A. S. (1995). Dynamic patterns: The self-organization of brain and behaviour. Cambridge, MA: MIT Press.
Kemner, C., Verbaten, M. N., Cuperus, J. M., Camfferman, G., & van Engeland, H. (1998). Abnormal saccadic eye movements in autistic children. Journal of Autism and Developmental Disorders, 28, 61–67.
Kern, J. K., Trivedi, M. H., Garver, C. R., Grannemann, B. D., Andrews, A. A., Savla, J. S., et al. (2006). The pattern of sensory processing abnormalities in autism. Autism, 10, 480–494.
Klin, A., Sparrow, S. S., de Bildt, A., Cicchetti, D. V., Cohen, D. J., & Volkmar, F. R. (1999). A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders, 29, 499–508.
Kohen-Raz, R., Volkmar, F. R., & Cohen, D. J. (1992). Postural control in children with autism. Journal of Autism and Developmental Disorders, 22, 419–432.
Koldewyn, K., Whitney, D., & Rivera, S. M. (2010). The psychophysics of visual motion and global form processing in autism. Brain, 133, 599–610.
Kwakye, L. D., Foss-Feig, J. H., Cascio, C. J., Stone, W. L., & Wallace, M. T. (2011). Altered auditory and multisensory temporal processing in autism spectrum disorders. Frontiers in Integrative Neuroscience, 4, 129.
Landy, M. S., Maloney, L. T., Johnston, E. B., & Young, M. (1995). Measurement and modeling of depth cue combination: In defense of weak fusion. Vision Research, 35, 389–412.
Largo, R. H., Fischer, J., & Caflish, J. (2002). Zürcher neuromotorik. Zürich: AWE-Verlag.
Leary, M. R., & Hill, D. A. (1996). Moving on: Autism and movement disturbance. Mental Retardation, 34, 39–53.
Leekam, S. R., Nieto, C., Libby, S. J., Wing, L., & Gould, J. (2007). Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders, 37, 894–910.
Luna, B., Doll, S. K., Hegedus, S. J., Minshew, N. J., & Sweeney, J. A. (2007). Maturation of executive function in autism. Biological Psychiatry, 61, 474–481.
Macneil, L. K., & Mostofsky, S. H. (2012). Specificity of dyspraxia in children with autism. Neuropsychology, 26, 165–171.
Manjiviona, J., & Prior, M. (1995). Comparison of Asperger syndrome and high-functioning autistic children on a test of motor impairment. Journal of Autism and Developmental Disorders, 25, 23–39.
Mari, M., Castiello, U., Marks, D., Marraffa, C., & Prior, M. (2003). The reach-to-grasp movement in children with autism spectrum disorder. Philosophical Transactions of the Royal Society of London B Biological Science, 358, 393–403.
Martin, J. S., Poirier, M., & Bowler, D. M. (2010). Brief report: Impaired temporal reproduction performance in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 40, 640–646.
Miall, R. C., & Wolpert, D. M. (1996). Forward models for physiological motor control. Neural Network, 9(8), 1265–1279.
Milne, E., Swettenham, J., Hansen, P., Campbell, R., Jeffries, H., & Plaisted, K. (2002). High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry, 43, 255–263.
Milne, E., White, S., Campbell, R., Swettenham, J., Hansen, P., & Ramus, F. (2006). Motion and form coherence detection in autistic spectrum disorder: Relationship to motor control and 2:4 digit ratio. Journal of Autism and Developmental Disorders, 36, 225–237.
Ming, X., Brimacombe, M., & Wagner, G. C. (2007). Prevalence of motor impairment in autism spectrum disorders. Brain and Development, 29, 565–570.
Minshew, N. J., Sung, K., Jones, B. L., & Furman, J. M. (2004). Underdevelopment of the postural control system in autism. Neurology, 63, 2056–2061.
Miyahara, M., Tsujii, M., Hori, M., Nakanishi, K., Kageyama, H., & Sugiyama, T. (1997). Brief report: motor incoordination in children with Asperger syndrome and learning disabilities. Journal of Autism and Developmental Disorders, 27, 595–603.
Molloy, C. A., Dietrich, K. N., & Bhattacharya, A. (2003). Postural stability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 33, 643–652.
Mosconi, M. W., Kay, M., D'Cruz, A. M., Seidenfeld, A., Guter, S., Standford, L. D., et al. (2009). Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychological Medicine, 39(9), 1559–1566.
Mostofsky, S. H., Bunoski, R., Morton, S. M., Goldberg, M. C., & Bastian, A. J. (2004). Children with autism adapt normally during a catching task requiring the cerebellum. Neurocase, 10, 60–64.
Mostofsky, S. H., Dubey, P., Jerath, V. K., Jansiewicz, E. M., Goldberg, M. C., & Denckla, M. B. (2006). Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of International Neuropsycholy Society, 12(3), 314–326.
Mostofsky, S. H., Goldberg, M. C., Landa, R. J., & Denckla, M. B. (2000). Evidence for a deficit in procedural learning in children and adolescents with autism: implications for cerebellar contribution. Journal of International Neuropsycholy Society, 6, 752–759.
Mottron, L., Dawson, M., Soulieres, I., Hubert, B., & Burack, J. (2006). Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders, 36, 27–43.
Muller, F., & Dichgans, J. (1994). Dyscoordination of pinch and lift forces during grasp in patients with cerebellar lesions. Experimental Brain Research, 101, 485–492.
Nazarali, N., Glazebrook, C. M., & Elliott, D. (2009). Movement planning and reprogramming in individuals with autism. Journal of Autism and Developmental Disorders, 39, 1401–1411.
Nemeth, D., Janacsek, K., Balogh, V., Londe, Z., Mingesz, R., Fazekas, M., et al. (2010). Learning in autism: Implicitly superb. PLoSOne, 5, e11731.
Nissen, M. J., & Bullemer, P. (1987). Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology, 19(1), 1–32.
O’Riordan, M., & Passetti, F. (2006). Discrimination in autism within different sensory modalities. Journal of Autism and Developmental Disorders, 36, 665–675.
O’Riordan, M., & Plaisted, K. (2001). Enhanced discrimination in autism. The Quarterly Journal of Experimental Psychology A, 54, 961–979.
O’Riordan, M. A., Plaisted, K. C., Driver, J., & Baron-Cohen, S. (2001). Superior visual search in autism. Journal of Experimental Psychology: Human Perception and Performance, 27, 719–730.
Osborne, L. C., Lisberger, S. G., & Bialek, W. (2005). A sensory source for motor variation. Nature, 437(7057), 412–416.
Ozonoff, S., Young, G. S., Goldring, S., Greiss-Hess, L., Herrera, A. M., Steele, J., et al. (2008). Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders, 38, 644–656.
Pan, C. Y., Tsai, C. L., & Chu, C. H. (2009). Fundamental movement skills in children diagnosed with autism spectrum disorders and attention deficit hyperactivity disorder. Journal of Autism and Developmental Disorders, 39(12), 1694–1705.
Pascolo, P. B., & Cattarinussi, A. (2012). On the relationship between mouth opening and “broken mirror neurons” in autistic individuals. Journal of Electromyography and Kinesiology, 22, 98–102.
Pellicano, E., & Gibson, L. Y. (2008). Investigating the functional integrity of the dorsal visual pathway in autism and dyslexia. Neuropsychologia, 46, 2593–2596.
Pellicano, E., Gibson, L., Maybery, M., Durkin, K., & Badcock, D. R. (2005). Abnormal global processing along the dorsal visual pathway in autism: A possible mechanism for weak visuospatial coherence? Neuropsychologia, 43, 1044–1053.
Provost, B., Lopez, B. R., & Heimerl, S. (2007). A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders, 37, 321–328.
Rinehart, N. J., Bellgrove, M. A., Tonge, B. J., Brereton, A. V., Howells-Rankin, D., & Bradshaw, J. L. (2006a). An examination of movement kinematics in young people with high-functioning autism and Asperger’s disorder: Further evidence for a motor planning deficit. Journal of Autism and Developmental Disorders, 36, 757–767.
Rinehart, N. J., Bradshaw, J. L., Brereton, A. V., & Tonge, B. J. (2001). Movement preparation in high-functioning autism and Asperger disorder: A serial choice reaction time task involving motor reprogramming. Journal of Autism and Developmental Disorders, 31, 79–88.
Rinehart, N. J., Tonge, B. J., Bradshaw, J. L., Iansek, R., Enticott, P. G., & McGinley, J. (2006b). Gait function in high-functioning autism and Asperger’s disorder: Evidence for basal-ganglia and cerebellar involvement? European Child and Adolescent Psychiatry, 15, 256–264.
Rosenbaum, D. A. (1980). Human movement initiation: Specification of arm, direction, and extent. Journal of Experimental Psychology-General, 109(4), 444–474.
Rosenbaum, D. A., Halloran, E. S., & Cohen, R. G. (2006). Grasping movement plans. Psychonomic Bulletin and Review, 13, 918–922.
Rosenbaum, D. A., Marchak, F., Barnes, H. J., Vaughan, J., Slotta, J. D., & Jorgensen, M. J. (1990). Constraints for action selection: Overhand versus underhand grips. In M. Jeannerod (Ed.), Attention and performance XIII (pp. 321–342). Hillsdale, NJ: Erlbaum.
Sanger, T. D. (2003). Neural population codes. Current Opinion in Neurobiology, 13, 238–249.
Schmitz, C., Martineau, J., Barthelemy, C., & Assaiante, C. (2003). Motor control and children with autism: Deficit of anticipatory function? Neuroscience Letters, 348, 17–20.
Shadmehr, R., & Krakauer, J. W. (2008). A computational neuroanatomy for motor control. Experimental Brain Research, 185, 359–381.
Shadmehr, R., Smith, M. A., & Krakauer, J. W. (2010). Error correction, sensory prediction, and adaptation in motor control. Annual Review of Neuroscience, 33, 89–108.
Shah, A., & Frith, U. (1993). Why do autistic individuals show superior performance on the block design task? Journal of Child Psychology and Psychiatry, 34, 1351–1364.
Shams, L., Wozny, D. R., Kim, R., & Seitz, A. (2011). Influences of multisensory experience on subsequent unisensory processing. Frontiers in Psychology, 2, 264.
Simmons, D. R., Robertson, A. E., McKay, L. S., Toal, E., McAleer, P., & Pollick, F. E. (2009). Vision in autism spectrum disorders. Vision Research, 49, 2705–2739.
Spence, C., Pavani, F., Maravita, A., & Holmes, N. (2004). Multisensory contributions to the 3-D representation of visuotactile peripersonal space in humans: evidence from the crossmodal congruency task. Journal of Physiology, 98, 171–189.
Stanley-Cary, C., Rinehart, N., Tonge, B., White, O., & Fielding, J. (2011). Greater disruption to control of voluntary saccades in autistic disorder than Asperger’s disorder: Evidence for greater cerebellar involvement in autism? Cerebellum, 10, 70–80.
Staples, K. L., & Reid G. (2010). Fundamental movement skills and autism spectrum disorders. Journal of autism and Developmental Disorders, 40(2), 209–217.
Sutera, S., Pandey, J., Esser, E. L., Rosenthal, M. A., Wilson, L. B., Barton, M., et al. (2007). Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37, 98–107.
Szelag, E., Kowalska, J., Galkowski, T., & Poppel, E. (2004). Temporal processing deficits in high-functioning children with autism. British Journal of Psychology, 95, 269–282.
Takarae, Y., Minshew, N. J., Luna, B., & Sweeney, J. A. (2004). Oculomotor abnormalities parallel cerebellar histopathology in autism. Neurology, Neurosurgery, and Psychiatry, 75, 1359–1361.
Taylor, N., Isaac, C., & Milne, E. (2010). A comparison of the development of audiovisual integration in children with autism spectrum disorders and typically developing children. Journal of Autism and Developmental Disorders, 40, 1403–1411.
Teitelbaum, P., Teitelbaum, O., Nye, J., Fryman, J., & Maurer, R. G. (1998). Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of National Academy of Science USA, 95, 13982–13987.
Tommerdahl, M., Tannan, V., Cascio, C. J., Baranek, G. T., & Whitsel, B. L. (2007). Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Research, 1154, 116–123.
Tsermentseli, S., O’Brien, J. M., & Spencer, J. V. (2008). Comparison of form and motion coherence processing in autistic spectrum disorders and dyslexia. Journal of Autism and Developmental Disorders, 38(7), 1201–1210.
Ulrich, D. A. (2000). Test of gross motor development: Examiner’s manual (2nd ed.). Austin, TX: Pro-Ed.
van Beers, R. J. (2007) The sources of variability in saccadic eye movements. Journal of Neuroscience, 27(33), 8757–8770.
van der Smagt, M. J., van Engeland, H., & Kemner, C. (2007). Brief report: Can you see what is not there? Low-level auditory-visual integration in autism spectrum disorder. Journal of Autism and Developmental Disorders, 37(10), 2014–2019.
van Swieten, L. M., van Bergen, E., Williams, J. H., Wilson, A. D., Plumb, M. S., Kent, S. W., et al. (2010). A test of motor (not executive) planning in developmental coordination disorder and autism. Journal of Experimental Psychology: Human Perception and Performance, 36, 493–499.
Van Waelvelde, H., Oostra, A., Dewitte, G., Van Den Broeck, C., Jongmans, M. J. (2010). Stability of motor problems in young children with or at risk of autism spectrum disorders, ADHD, and or developmental coordination disorder. Developmental Medicine and Child Neurology, 52(8), e174–178.
Vernazza-Martin, S., Martin, N., Vernazza, A., Lepellec-Muller, A., Rufo, M., Massion, J., et al. (2005). Goal directed locomotion and balance control in autistic children. Journal of Autism and Developmental Disorders, 35, 91–102.
Williams, J. H., Massaro, D. W., Peel, N. J., Bosseler, A., & Suddendorf, T. (2004a). Visual-auditory integration during speech imitation in autism. Research in Developmental Disabilities, 25, 559–575.
Williams, J., Thomas, P. R., Maruff, P., & Wilson, P. H. (2008). The link between motor impairment level and motor imagery ability in children with developmental coordination disorder. Human Movement Science, 27, 270–285.
Williams, J. H., Whiten, A., Singh, T. (2004b). A systematic review of action imitation in autistic spectrum disorder. Journal of Autism and Developmental Disorder, 34(3), 285–299.
Wilson, M., & Knoblich, G. (2005). The case for motor involvement in perceiving conspecifics. Psychological Bulletin, 131, 460–473.
Wing, A. M., & Kristofferson, A. B. (1973). Response delays and the timing of discrete motor responses. Perception and Psychophysics, 14, 5–12.
Wolpert, D. M. (1997). Computational approaches to motor control. Trends in Cognitive Sciences, 1, 209–216.
Wolpert, D. M., Doya, K., & Kawato, M. (2003). A unifying computational framework for motor control and social interaction. Philosophical Transactions of the Royal Society of London series B-Biological Sciences, 358(1431), 593–602.
Wolpert, D. M., & Flanagan, J. R. (2001). Motor prediction. Current Biology, 11, R729–R732.
Wolpert, D. M. & Ghahramani, Z. (2000). Computational principles of movement neuroscience. Nautre Neuroscience, 3(Suppl), 1212–1217.
Wozny, D. R., & Shams, L. (2011). Computational characterization of visually induced auditory spatial adaptation. Frontiers in Integrative Neuroscience, 5, 75.
Young, R. P., & Zelaznik, H. N. (1992). The visual control of aimed hand movements to stationary and moving targets. Acta Psychologica (Amst), 79, 59–78.
Acknowledgments
We would like to thank Chris Miall, Ellen Poliakoff and Paul Warren for their useful comments during preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gowen, E., Hamilton, A. Motor Abilities in Autism: A Review Using a Computational Context. J Autism Dev Disord 43, 323–344 (2013). https://doi.org/10.1007/s10803-012-1574-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-012-1574-0