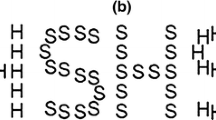Abstract
A large body of research has reported visual perception deficits in both people with dyslexia and autistic spectrum disorders. In this study, we compared form and motion coherence detection between a group of adults with high-functioning autism, a group with Asperger’s disorder, a group with dyslexia, and a matched control group. It was found that motion detection was intact in dyslexia and Asperger. Individuals with high-functioning autism showed a general impaired ability to detect coherent form and motion. Participants with Asperger’s syndrome showed lower form coherence thresholds than the dyslexic and normally developing adults. The results are discussed with respect to the involvement of the dorsal and ventral pathways in developmental disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Autistic spectrum disorders (ASD) and dyslexia are complex developmental disorders involving male predominance, neurophysiological and neuroanatomical abnormalities and strong genetic components. Despite the qualitatively different clinical symptomatology between specific reading disability and ASD, both conditions show a higher prevalence of cognitive and sensorimotor impairments in comparison to typically developing populations. In fact, a significant degree of overlap in the neuropsychological and sensorimotor profiles of individuals with these disorders has been found (Rumsey and Hamburger 1990; Shea and Mesibov 1985; White et al. 2006); sensory-perceptual impairments are rather more prevalent in the autistic than in the dyslexic population (White et al. 2006). Although the identification of some common characteristics between ASD and dyslexia has been reported, little research has focused on the relationship between these conditions. Comparisons of the performances of these groups on tasks where both have been found to be impaired is relevant to understanding their underlying pathology and might have potential diagnostic utility.
Recently, there has been a revival of interest in the low-level perceptual processing in ASD and dyslexia. A relatively popular but controversial approach (Atkinson 2000; Braddick et al. 2003) suggests that several developmental disorders, including ASD and dyslexia, share a common deficit in global motion processing caused by a specific dysfunction of the visual dorsal pathway. Beyond the primary visual cortex, the visual system segregates into two specialized processing pathways, namely the dorsal and ventral streams (Milner and Goodale 1995). The dorsal stream receives input preferentially from the magnocellular layers of the LGN and includes areas of posterior parietal cortex such as MT/V5, while the ventral stream receives input from both the magnocellular and parvocellular layers of the LGN and includes anterior regions of the inferior temporal lobe (Livingstone and Hubel 1988; Merigan and Maunsell 1993; Underleider and Mishkin 1982). Functionally, dorsal stream cells are highly responsive to global moving information, whilst responses in the ventral stream are comparatively sustained and dedicated to fine analysis of the visual scene in terms of texture, colour, fine pattern and form (Livingstone and Hubel 1988; Merigan 1989; Tootell et al. 1995).
Therefore, form and motion coherence thresholds are thought to provide comparable psychophysical measures of global processing by pattern- and motion-sensitive mechanisms in the two visual streams, respectively. Indeed, an important line of evidence in support of the “dorsal stream vulnerability” hypothesis comes from experiments where ASDs (Milne et al. 2002; Spencer et al. 2000) and dyslexics (Hansen et al. 2001; Slaghuis and Ryan 1999; Talcott et al. 2000, 1998) have been found to show high motion coherence thresholds, but intact performance on form coherence tasks. However, findings of a dorsal stream impairment are not unchallenged; a considerable amount of studies have failed to find a general motion deficit in dyslexia with only a fraction of dyslexics seeming to be affected (Ramus 2003; Skottun 2000). In addition, the hypothesis of a specific dorsal stream vulnerability has been questioned by a recent finding of an additional ventral stream deficit in autism (Spencer and O’Brien 2006), where none was previously reported. Most importantly, a deficit in motion processing cannot offer a lucid explanation of how an impaired dorsal stream can play a causal role in ASD or dyslexia since a similar impairment has been demonstrated in many other neurological disorders, such as schizophrenia (Wertheim et al. 1985; O’Donnell et al. 1996), multiple sclerosis (Regan and Maxner 1986), amblyopia (Giaschi et al. 1992), fragile X syndrome (Kogan et al. 2004), migraine (McKendrick et al. 2006), Alzheimer’s disease (Rizzo and Nawrot 1998), and William’s syndrome (Atkinson et al. 2003).
Despite the fact there have been several separate experiments investigating form and motion processing in ASD and dyslexia; as yet there has only been one direct comparison of these populations within the same study. In this recent study, White et al. (2006) failed to find any significant differences between children with autism, dyslexia and controls on either task. In their study the autism sample was comprised by children from the whole autistic spectrum; however, research has shown that there are behavioural (Macintosh and Dissanayake 2004; Rinehart et al. 2002) and neuroanatomical differences (Lotspeich et al. 2004) between high-functioning autism (HFA) and Asperger’s syndrome (AS). Indeed, recent findings reveal that children with autism exhibit both a form and motion deficit, with no evidence of either a motion or form deficit in the Asperger’s syndrome (Spencer and O’Brien 2006). Here we attempt to replicate these findings in an adult population, as we believe this is a more efficient approach of controlling for developmental discrepancies.
In addition, we sought to address some methodological issues involving task selection by modifying a paradigm that has been widely employed in psychophysical studies in developmental disorders. Bertone et al. (2005) argued that form and motion dissociations reported in previous studies may be stimulus dependent, since static stimuli were not equivalent to their complex dynamic counterparts in terms of processing requirements and therefore did not assess dorsal and ventral functioning at the same level of neural complexity. Indeed, although previous reports argued they used identical task demands, this was not the case as the form tasks used non-noisy circular stimuli whilst motion coherence was measured by stimuli that moved horizontally against a noisy field, leading to the possibility that participants could use different strategies to respond on form and motion. In the present study, we employed conceptually identical stimuli allowing for a better matching of the task processing requirements. In addition, stimuli were displayed for only a short duration, in order to prevent the use of serial search strategies. A secondary interest was to examine whether performance of form and motion tasks are correlated; previous experiments reported statistical independence between those tasks in dyslexia (Hansen et al. 2001) and autism (Spencer et al. 2000), but this could have been due to the tasks not being identical rather than a direct segregation amongst the visual pathways in these populations.
The present study therefore employs the same stimuli used by Spencer and O’Brien (2006) with the aim to replicate their findings in an adult sample. We have also included a group with dyslexic to compare performances on form and motion coherence between ASDs and dyslexia. Comparison of multiple developmental disorders’ populations could provide some empirical evidence on the role of visual integration in such conditions and might help identify disorder-specific visual perceptual signatures.
Methods
Participants
In total, 20 adults with dyslexia were compared to 21 adults with ASD and 20 controls. The groups were matched for chronological age F (3, 57) = .944, p = .429 and full-scale IQ F (3, 57) = 2.07, p = .119, as measured by two sub-tests (vocabulary and block design) from the Weschler Abbreviated Scale of Intelligence (WASI, Weschler 1999). The participants were aged between 17 and 40 years old and had full-scale IQs of at least 85, with the exception of one high functioning autistic individual with full-scale IQ of 73.
The dyslexic participants were recruited from the Educational Support Office at Royal Holloway University of London and the Disability and Dyslexia Service at Brunel University, UK. They were all university students with a childhood history of specific reading difficulties and had all received a formal diagnosis of dyslexia within the previous 2 years. A diagnosis of dyslexia was confirmed by a licensed educational psychologist, according to a discrepancy between reading/spelling performance and general cognitive abilities, based on a battery of standardized tests. We did not assess the literacy skills of our participants, as it is quite common with adult dyslexics to show a pattern of remediated symptoms (Hansen et al. 2001). Individuals in the ASD groups were referred by two specialized colleges for adults with ASDs, accredited by the National Autistic Society, UK. They had been diagnosed as having either high functioning autism (n = 10) or Asperger’s syndrome (n = 11) by qualified clinicians using DSM-IV criteria (American Psychiatric Association 1994). We inspected clinical records and developmental histories to confirm that children with HFA had a history of significant impairments in language functioning as outlined in DSM-IV. Exclusion of clinically significant delays in language development was necessary for the diagnosis of Asperger’s Disorder. Separate research diagnosis with the use of a diagnostic instrument (i.e. ADI & ADOS, Lord et al. 1999) was not carried out as it has been found that there is high degree of agreement between clinical and research diagnosis, with research instruments sometimes leading to over-diagnosis of ASDs (Mazefski and Oswald 2006). Typically developing adults were recruited from the student population of Brunel University and according to self-report had no history of learning difficulties diagnosis. Demographics are displayed in Table 1.
Participants were excluded if they had major physical disability (e.g. deafness, paralysis) or any co-morbid psychiatric or developmental disorders (e.g. schizophrenia, ADHD), or if they were taking any medication known to affect sensorimotor and cognitive performance. Subjects volunteered without pay, all gave informed consent, and all had normal or corrected to normal visual acuity. The experimental protocol was approved by the National Autistic Society, UK and Brunel University’s Ethics Committee.
Stimuli and Procedure
Stimuli were running on a Pentium IV (1,200 MHz) computer and were displayed on a 17-in 8bit luminance scale monitor. Participants sat 50 cm from the screen in an otherwise darkened room. We employed circular stimuli for both form and motion tasks to ensure that there was as little difference in the spatial information presented as possible. The form stimuli were Glass patterns (Glass 1969) consisting of randomly orientated dots (white dots on a black background, 4 dots/deg2) containing a target area on one side of the display where dots were orientated tangentially to form circular patches defined by correlated dot triplets. The ratio of correlated amongst the randomly displaced dot triplets in the target area defined the coherence value for each trial. Coherence was initially set at 1.0 while in the remainder of the display coherence was set at 0.0. Coherence was lowered by reducing the proportion of coherent to incoherent dot triplets. The motion coherence random-dot stimulus was matched in visual angle, size and luminance to the form task, except that a dot moved from the position of the first to the position of the last dot in a triplet. Dots moved at 5.8 deg/s and had a limited lifetime of three frames (frame rate: 60 Hz) to limit subjects’ use of tracking strategies. Figure 1 illustrates the form and motion displays.
Perceptual thresholds were obtained using two-alternative forced-choice paradigms whereby participants were required to locate the circular patch (radius 6.6 deg), which was centred 7.7 deg either on the left or the right hand of the display centre. In either task, the initial coherence level was set to 100% and progressively reduced until an error was made, in the manner of a 2-up, 1-down staircase. Threshold was defined as the mean coherence level from the mean of six reversals, ignoring two initial reversals. For both tasks stimulus duration was 250 ms and an observer’s task was to locate the circular patch (radius 6.6) centred 7.7 deg either side of the display centre.
Prior to data collection, participants were given a practice session with 5,000 ms duration set at 100% coherence to familiarize with the task. Participants were asked to fixate on a white cross at the centre of the screen and identify the target stimulus. The experiment began once participants identified the coherent patch five times over five consecutive trails. No feedback for accuracy was provided except in the practice sessions. The order of the two tasks was randomized between participants.
Responses can be influenced by fatigue, boredom, lack of directed attention, preservation or intermittent confusion over left/right response. In order to ensure that any differences found were perceptual ones, we excluded all data in which there was any possibility of an unwanted factor influence performance. Specifically, we excluded from analysis all those data in which at any point an observer made an incorrect response to stimulus coherence larger than 75%. This can happen by chance or due to a momentary lapse in concentration, but it ensures that all data collected are representative of an observer’s optimum performance. Subsequently one HFA participant who showed coherence levels >75% was excluded from analyses.
Data Analysis
Responses to a wide range of visual stimuli are non-linear, and are best measured and compared on non-linear (e.g., log) scales. However, there is clear evidence that neural responses to motion coherence are linear. Parker and Newsome (1998) have shown that individual neurons in V5 have sufficiently precise direction signals to account for macaque’s measured psychophysical sensitivity to motion (see also Britten et al. 1992). Removal or excision of V5 significantly reduces task performance on motion direction discrimination (Newsome and Pare 1988), and stimulation of V5 changes macaque’s choices in such tasks (Salzman et al. 1990). Importantly, individual neurons show monotonically increasing responses to motion coherence (Britten et al. 1993). This finding of a linear relationship between motion coherence and response in V5 has been replicated in humans using fMRI (Rees et al. 2000), who argue that their finding shows a simple relationship between BOLD contrast and average neuronal firing rate.
It is important not to characterise the perception of motion coherence as a process involving the summation of dots to give a directional signal proportional to coherence. Any translating pattern can be described in terms of its spatial and temporal frequency components in three dimensions (Adelson and Bergen 1985; Britten et al. 1993). For a random dot display of the type we used, at 100% coherence, the spectral power is concentrated on a plane (the spatial orientation of the plane depends on the translational velocity alone). At a coherence of 0%, the spectral power is uniformly distributed in spatiotemporal frequency space. Between these two extremes, the percentage of spectral power in the plane is proportional to coherence (Britten et al. 1993). The same researchers found that in macaque V5, for most neurons measured, a linear fit for the relationship between firing rate and stimulus coherence was optimal. For those cells for which a quadratic term improved model fit, the quadratic term was very small, and was as likely to be negative as positive.
In summary, the neuronal, BOLD fMRI, and psychophysical responses to motion coherence are all linear, and computational modelling of motion responses also shows a monotonic fit. We therefore conclude that a linear analysis of participant responses is most appropriate. We argue that the response to form coherence is analogous, although there is less physiological and fMRI data to support this. However, both types of stimulus must be analysed in the same way.
Results
Non-parametric tests were used as the data were not normally distributed. Comparison of the average coherence thresholds between the controls, the dyslexics and the ASDs (including both HFA and AS participants) showed no significant differences between the groups in motion (Kruskall–Wallis, χ2 = 4.125, p = .127) and form (Kruskall–Wallis, χ2 = 2.077, p = .355). Because differences in form and motion processing were found between participants with HFA and AS by Spencer and O’Brien (2006), we separated the HFA participants from the AS participants here for the purposes of analysis. The results showed a significant difference between the three clinical groups and controls for motion coherence (Kruskall–Wallis, χ2 = 10.42, p = .015) and form coherence (Kruskall–Wallis, χ2 = 20.26, p = .001).
Further Mann–Whitney tests using the Bonferroni adjustment were used to explore the differences between the groups. The results from the form coherence task indicated that the median scores for the Asperger’s group (median: 17.6, range: 10.9–22.6) was significantly lower than the median scores for the controls (median: 23.9, range: 14.8–45.2, U = 32.00, Z = −3.22, p = .004) but not for the dyslexia group (median: 19.9, range: 10.5–37.3, U = 67.00, Z = −1.77, p = 3.16). In addition, form coherence thresholds were found to be significantly higher for the HFA group (median: 41.5, range: 13.6–49.2) in comparison to controls (U = 32.00, Z = −2.73, p = .020), the dyslexia group (U = 23.00, Z = −3.15, p = .004) and the AS group (U = 7.00, Z = −3.22, p = .004). No significant differences were found between controls and dyslexics (U = 142.5, Z = −1.556, p = .121).
For the motion coherence task the results again indicated that the median score for the HFA group was significantly higher (median: 31.8, range: 9.58–73.9) than the median score for the controls (median: 14.9, range: 5.0–24,1, U = 27.00, Z = −2.97, p = .008), the dyslexia group (median: 16.6, range: 7.0–31.6, U = 3 4.5, Z = −2.61, p = .028) and the AS group (median: 14.9, range: 11.3–14.9, U = 18.50, Z = −2.35, p = .048). No significant differences were found between typical development and dyslexia (U = 160.50, Z = −1.06, p = 1.15), typical development and AS (U = 104.00, Z = −.248, p = 3.292), or AS and dyslexia (U = 101.00.5, Z = −.372, p = 2.92).Group performance on each of the tasks is shown in Fig. 2.
An important issue that arises from more recent research is that of group-based versus individual analysis of data. Statistically significant differences in mean values between groups are usually demonstrated using parametric tests. This approach is valid if the data values in the groups are normally distributed (Stuart et al. 2006). However, if the distribution of the data is longer tailed in some groups, as in our case, the presentation of individual data is crucial. Individual data are shown in Fig. 3, with sensitivity on the motion task plotted against sensitivity on the form task. Inspection of this graph shows that there is a considerable overlap between the sensitivities of the AS, the dyslexic and the control groups. However, eight out of the HFA participants fell outside the main distribution of sensitivities on both tasks, with more obvious impairments on the motion task. Interestingly, the participant with IQ = 73 did not fall outside the mean control subject range. The higher variability in the responses among the HFA individuals compared to the other groups is also evident at the box plots in Fig. 2, suggesting a high degree of heterogeneity within this group. In addition, three controls and one dyslexic participant showed form coherence thresholds outside the range of 30%, and one participant with AS showed motion coherence thresholds outside the range of 35%.
Spearman’s correlation coefficients were calculated to evaluate the contributions of age and IQ to task performance. It was found that full scale IQ is not significantly correlated to form (Spearman’s rho = −.143, p = .386) or motion coherence detection (Spearman’s rho = −.282, p = .077). The relationships between chronological age and form coherence (Spearman’s rho = .146, p = .366) and between chronological age and motion coherence (Spearman’s rho = −.117, p = .448) were also found to be non-significant. These findings rule out the possibility that differences on task performance could be explained by chronological or mental abilities.
Form and motion coherence tasks are considered statistically independent as they tap different aspects of visual processing. However, in the present study, using the modified motion task, it was revealed that form and motion are significantly correlated (Spearman’s rho = .414, p = .001). Specifically, it was found that form and motion are not correlated for the control group (Spearman’s rho = .341, p = .141) or the HFA group (Spearman’s rho = .000, p = 1.000). However, there was a significant relationship between the two tasks on the AS group (Spearman’s rho = −.618, p = .043) and the dyslexic group (Spearman’s rho = .844, p = .000).
Discussion
In this study, a group diagnosed with dyslexia, two groups of adults diagnosed on the autism spectrum (high-functioning autism and Asperger’s disorder), and a control group of typically developing adults, matched for full-scale IQ and chronological age were tested on tasks which measure coherent form and motion detection. In agreement with White et al. (2006), when the two groups of ASD were integrated into one group, we were unable to find any significant differences between ASD, dyslexia and typical development. However, when the individuals with high functioning autism and Asperger’s disorder were treated as separate groups the results were fundamentally transformed. Once again, there was no evidence for motion deficit in adults with a diagnosis of specific reading disability or Asperger’s disorder. The results, however, confirmed that high functioning autistic individuals suffer from both a form and motion deficit (Spencer and O’Brien 2006). Interestingly, this is the first study to report superior form processing in adults with Asperger’s disorder.
Despite several investigations that suggest abnormal processing of motion coherence in dyslexia (Hansen et al. 2001; Talcott et al. 1998, 2000) and ASD (Milne et al. 2002; Spencer et al. 2000), we were unable to find any direct evidence of such a deficit in the dyslexic and AS participants. The only group that did show high motion coherence thresholds was that of high-functioning autistic adults, however the same group also showed impairment in form processing as well. Given that proponents of the specific dorsal stream vulnerability hypothesis have consistently used form and motion coherence tasks as measures of ventral and dorsal functioning, it must be acknowledged that the weight of evidence does not support the hypothesis of a common impairment of the sensitivity of the dorsal stream at threshold in developmental disorders. One possible explanation for failing to find a motion deficit in dyslexia and Asperger’s could be due to the modified psychophysical task used. However it is quite unlikely that the two groups’ normal performance in the motion task was due to the task being too easy. Using the rotational motion paradigm Spencer and O’Brien (2006) found that children with ASD showed 51% higher motion coherence thresholds compared to the thresholds derived by the translational motion stimuli used in an earlier study (Spencer et al. 2000).
Impairments in visual attention have been reported both in autism (van der Geest et al. 2001; Mann and Walker 2003) and dyslexia (Facoetti et al. 2001; Vidyasagar 2004); therefore, poor attentional capacities may have adversely affected individuals’ performance on the motion tasks in previous studies, leaving the implications of “dorsal stream deficit” findings somewhat uncertain. Attentional processes could potentially confound performance on tasks assessing global motion, as in long motion direction stimuli subjects might use feature tracking to discern motion direction. Indeed, Davis et al. (2006) found that children with ASD exhibit a motion coherence deficit only in a long duration (1,000 ms) condition, while there is no difference when the same motion stimuli are presented for a shorter duration (220 ms). Attention could not therefore account for the results in the present study as the stimuli used here assessed motion coherence without direction and were only presented for a short duration (i.e. 250 ms).
In addition, Chen et al. (1998) found that tasks of discriminating motion direction, commonly used in earlier experiments, initiated smooth pursuit tracking. Eye movements might produce unwanted retinal motion which the visual system interprets as image motion (Legge and Campbell 1981; Nakayama and Tyler 1981; Bedell and Johnson 1995). This is because some dorsal/motion areas, such as V5 are sensitive to both real and retinal motion (Newsome et al. 1988). Unwanted eye movements could therefore impair performance on motion tasks that rely on these areas. It is possible that motion deficits reported in developmental disorders are not due to a dorsal stream deficit per se but might actually be the result of inaccurate information derived from the eye-movement system. Indeed a number of studies have reported abnormalities of eye movements in autism (Rosenhall et al. 1988; Scharre and Creedon 1992; Takarae et al. 2004) and dyslexia (Biscaldi et al. 2000; Eden et al. 1994; Pavlidis 1981). The motion coherence task used in this study might be a more suitable in isolating motion perception as it did not initiate eye movements.
The results also replicated recent findings of a form deficit in high-functioning autism (Spencer and O’Brien 2006). However this is the first study to report low form coherence thresholds in Asperger’s disorder. In diagnostic terms, the main difference between high functioning autism and Asperger disorder is the absence of language delay in the latter syndrome. What is more interesting is a recent finding that high-noise task performance is correlated with language and literacy skills (Sperling et al. 2005). More specifically, it has been found that children with dyslexia and co-morbid language impairment show a deficit in dorsal and ventral stream tasks only in high noise conditions. It is quite possible that participants’ language abilities could have affected their performance as both our tasks used a noisy display. Unfortunately, we did not measure language ability in any of our groups, but as most of the AS and dyslexic participants had no history of specific language problems, it is quite possible that this could have been a factor. Certainly, more research is needed to uncover the relationship between the function of the visual system with other modalities, such as speech and reading.
The most fascinating implication of low coherence thresholds on the form task for AS and dyslexia (even though not statistically significant in the latter group) is that developmental disorders should not only be characterized by deficits but also by talent. This is in agreement with the finding of von Karolyi et al. (2003) that dyslexics show a specific superior global visuo-spatial ability. Our findings are also consistent with the evidence that children with Asperger’s syndrome are known to demonstrate superior performance on visuo-spatial tasks (Jolliffe and Baron-Cohen 1997). This is interesting as it suggests that increased performance on visual perceptual tasks in Asperger syndrome which previously has been explained by terms of “weak central coherence” could actually be a result of high levels of activity to high spatial channels in the visual pathways (Plaisted et al. 1999).
The results of this experiment also raise another important issue; the majority of studies that have investigated visual perception in autism have recruited participants from a wide range of the autistic spectrum treating them as a unique disorder. The few studies that have compared visual processing in autism and Asperger showed ambiguous results. Early studies examining general sensory-motor impairments relied on parental reports and reported no difference between children with autism and AS, although they were both more delayed in their achievements compared to non-autistic children (Szatmari et al. 1995). In a more recent study, Rinehart et al. (2000) showed that individuals with autism make more global errors than the controls on a shifting attention task of global and local stimuli, while the Asperger group made a similar number of errors as the control group leading to the conclusion that individuals with autism have a greater functional impairment. This is in consistency with our finding where the group of adults with autism showed both impaired form and motion detection whilst the group with Asperger showed intact motion and superior form performance in comparison to the normal controls.
Comparisons of the cognitive and neuropsychological profiles have also reported some differences amongst different syndromes within the autistic spectrum. For example, Gillberg and Gillberg (1989) found that individuals with AS demonstrate a higher frequency of gross motor clumsiness. It has also been suggested that persons with Asperger’s possess a distinct profile on intelligence tests characterised by a high verbal IQ and a relatively lower performance IQ in contrast to high functioning autism individuals who showed similar scores (Klin et al. 1995). Rinehart et al. (2001) also reported differences in movement preparation between autism and AS in a motor reprogramming task. The afore-mentioned findings invite speculation about how these disorders may differ neuropathologically. Imaging research has confirmed neuroanatomical differences between autism and Asperger syndrome (Lotspeich et al. 2004). In this study, a HFA group had the atypical pattern of decreasing IQ associated with increasing grey matter volume, whereas an AS group had the typical pattern of increasing IQ associated with associated with increasing grey matter. Taking these findings together, we can propose that results of studies treating ASD as a single disorder might be misleading, as we can still not rule out the possibility that these disorders may be clinically and neurobiologically separate (Rinehart et al. 2002).
Another interesting finding in our study was the fact that form and motion tasks were correlated in the dyslexics’ and the Asperger’s group. This is not the first time that form and motion tasks have been correlated in the dyslexic population; Hansen et al. (2001) have also reported a moderate correlation between the two tasks. However, traditionally form and motion processing have been attributed to anatomically and functionally separable neural pathways (Ungerleider and Mishkin 1982). Although significant progress has been made in uncovering the neural mechanisms that mediate motion and form perception in human primates, little is known about the possible interactions of these mechanisms that may underlie the unified perception in humans. Braddick et al. (2000) showed that coherent motion and form patterns activate distinct but neighbouring regions in both the ventral and dorsal streams. It is possible that unusual responses to visual stimuli in dyslexia and Asperger’s could be partially due to a more tightly integrated network among the dorsal and ventral streams in their visual system (Hansen et al. 2001). Abnormalities in form and motion processing, previously reported in dyslexia and ASD, could be due to an abnormality in the interaction between the dorsal and ventral systems in those populations. Imaging research investigating the functional connectivity between the two visual systems in developmental disorders could help unravel their complex relationship.
To conclude, we have found motion coherence detection is not impaired in dyslexia and AS. In terms of underlying neural mechanisms, our results suggest that the “dorsal stream vulnerability” hypothesis is not adequate to explain visual perceptual abnormalities in either Asperger’s syndrome or dyslexia. Moreover, our study demonstrated that detection of coherent form might play a more important role in visual processing in developmental disorders than previously thought. Most research on visual perception in ASD and dyslexia has been conducted on motion processing, however further research is needed to investigate whether individuals with these disorders differ on other static visuo-spatial tasks besides global form detection. Finally, the visual processing differences between high functioning autism and Asperger syndrome raise the issue of potential neurobiological dissociation between these conditions. Further study of the visual pathways, using more advanced techniques such as fMRI, may provide some evidence to explain how visual processing is related to autism, AS and dyslexia. This might give us some insight into the etiology of these disorders beyond the cognitive and language systems that could have potential use for early prognosis.
References
Adelson E. H., & Bergen, J. R. (1985). Spatiotemporal energy models for the perception of motion. Journal of the Optical Society of America, [A], 2, 284–299.
American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders (4th edn.). Washington: Author.
Atkinson, J. (2000). The developing visual brain. Oxford: Oxford University Press.
Atkinson, J., Braddick, O., Anker, S., Curran, W., Andrew, R., & Braddick, F. (2003). Neurobiological models of visuo-spatial cognition in young Williams’s syndrome children: Measures of dorsal stream and frontal function. Developmental Neuropsychology, 23, 139–172.
Bedell, H. E., & Johnson, C. A. (1995). The effect of flicker on foveal and peripheral thresholds for oscillatory motion. Vision Research, 35, 2179–2189.
Bertone, A., Mottron, L., Jelenic, P., & Faubert, J. (2005). Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain, 128, 2430–2441.
Biscaldi, M., Fischer, B., & Hartnegg, K. (2000). Voluntary saccadic control in dyslexia. Perception, 29, 509–521.
Braddick, O., Atkinson, J., & Wattam-Bell, J. (2003). Normal and anomalous development of visual motion processing: Motion coherence and “dorsal stream vulnerability”. Neuropsychologia, 13, 1769–1784.
Braddick, O. J., O’Brien, J. M. D., Wattam-Bell, J., Atkinson, J., & Turner, R. (2000). Form and motion coherence activate independent, but not dorsal/ventral segregated networks in the human brain. Current Biology, 10, 731–734.
Britten, K. H., Shadlen, M. N., Newsome, W. T., & Movshon, J. A. (1992). The analysis of visual motion: A comparison of neuronal and psychophysical performance. Journal of Neuroscience, 12, 4745–4765.
Britten, K. H., Shadlen, M. N., Newsome, W. T., & Movshon, J. A. (1993). Responses of neurons in macaque MT to stochastic motion signals. Visual Neuroscience, 10, 1157–1169.
Chen, Y., McPeek, R. M., Intriligator, J., Holzman, P. S., & Nakayama, K. (1998). Smooth pursuit to a movement flow and associated perceptual judgments. In W. Becker et al. (Eds.), Current oculomotor research: Physiological and psychological aspects. New York: Plenum.
Davis, R. A., Bockbrader, M. A., Murphy, R. R., Hetrick, W. P., & O’Donnell, B. F. (2006). Subjective perceptual distortions and visual dysfunction in children with autism. Journal of Autism and Developmental Disorders, 36, 199–210.
Eden, G. F., Stein, J. F, Wood, H. M., & Wood, F. B. (1994). Differences in eye movements and reading problems in dyslexic and normal children. Vision Research, 34, 1345–1358.
Facoetti, A., Turatto, M., Lorusso, M. L., & Mascetti, G. G. (2001). Orienting of visual attention in dyslexia: Evidence for asymmetric hemispheric control of attention. Experimental Brain Research, 138, 46–53.
Giaschi, D., Regan, D., Kraft, S., & Hong, X. H. (1992). Defective processing of motion in the fellow eye of unilateral amblyopes. Investigative Ophthalmology and Visual Science, 33, 2483–2489.
Gillberg C., & Gillberg, C. (1989). Asperger syndrome: Some epidemiological considerations: A research note. Journal of Child Psychology and Psychiatry, 30, 631–638.
Glass, L. (1969). Moire effect from random dots. Nature, 223, 578–580.
Hansen, P. C., Stein, J. F., Orde, S. R., Winter, J. L., & Talcott, J. B. (2001). Are dyslexics’ visual deficits limited to measures of dorsal stream function? NeuroReport 12, 1527–1530.
Jolliffe, T., & Baron-Cohen, S. (1997). Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? Journal of Child Psychology and Psychiatry, 38, 527–534.
Klin, A., Volkmar, F. R., Sparrow, S. S., Cicchetti, D. V., & Rourke, B. P. (1995). Validity and neuropsychological characterization of Asperger syndrome: Convergence with Nonverbal Learning Disabilities syndrome. Journal of Child Psychology and Psychiatry, 36, 1127–1140.
Kogan, C. S., Bertone, A., Cornish, K., Boutet, I., Der Kaloustian, V. M., Andermann, E., Faubert, J., & Chaudhuri A. (2004). Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology, 63, 1634–1639.
Legge, G. E, & Campbell, F. W. (1981). Displacement detection in human vision. Vision Research, 21, 205–213.
Livingstone, M. S., &. Hubel, D. H. (1988). Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science, 240, 740–749.
Lord, C., Rutter, M., DiLavore, P., & Risi, S. (1999). Autism diagnostic observation schedule. Los Angeles: Western Psychological Services.
Lotspeich, L. J., Kwon, H., Schumann, C. M., Fryer, S. L., Goodlin-Jones, B. L., Buonocore, M. H., Lammers, C. R., Amaral, D. G., & Reiss, A. L. (2004). Investigation of neuroanatomical differences between autism and Asperger syndrome. Archives of General Psychiatry, 61, 291–298.
Macintosh, K. E., & Dissanayake, C. (2004). Annotation: The similarities and differences between autistic disorder and Asperger’s disorder: A review of the empirical evidence. Journal of Child Psychology and Psychiatry, 45, 421–434.
Mann, T. A., & Walker, P. (2003). Autism and a deficit in broadening the spread of visual attention. Journal of Child Psychology and Psychiatry, 44, 274–284.
Mazefsky, C. A., & Oswald, D. P. (2006). The discriminative ability and diagnostic utility of the ADOS-G, ADI-R, and GARS for children in a clinical setting. Autism, 11, 553–549.
McKendrick, A. M., Badcock, D. R., & Gurgone, M. (2006). Vernier acuity is normal in migraine, whereas global form and global motion perception are not. Investigative Ophthalmology and Visual Science, 47, 3213–3219.
Merigan, W. H. (1989). Chromatic and achromatic vision of macaques: Role of the P pathway. Journal of Neuroscience, 9, 776–783.
Merigan, W. H., & Maunsell, J. H. R. (1993). How parallel are the primate visual pathways? In W. M. Cowan, E. M. Shooter, C. F. Stevens, & R. F. Thompson (Eds.), Ann. Rev. Neuroscience. Annual Reviews, Palo Alto, CA, pp. 369–402.
Milne, E., Swettenham, J., Hansen, P. C., Campbell, R., Jeffries, H., & Plaisted, K. (2002). High motion coherence thresholds in children with autism. Journal of Child Psychology and Psychiatry, 43, 255–263.
Milner, A. D., & Goodale, M. A. (1995). The visual brain in action. Oxford Psychology Series.
Nakayama, K., & Tyler, C. W. (1981). Psychophysical isolation of movement sensitivity by removal of familiar position cues. Vision Research, 21, 427–433.
Newsome, W. T., & Pare, E. B. (1988). A selective impairment of motion perception following lesions of the middle temporal visual area (MT). Journal of Neuroscience, 8, 2201–2211.
Newsome, W. T., Wurtz, R. H., & Komatsu, H. (1988). Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. Journal of Neurophysiology, 60, 604–620.
O’Donell, B. F., Swearer, J. M., Smith, L. T., Nestor, P. G., Shenton, M. E., & McCarley, R. W. (1996). Selective deficits in visual perception and recognition in schizophrenia. American Journal of Psychiatry, 153, 687–692.
Parker, A. J., & Newsome, W. T. (1998). Sense and the single neuron: Probing the physiology of perception. Annual Review of Neuroscience, 21, 227–277.
Pavlidis, G. T. (1981). Do eye movements hold the key to dyslexia? Neuropsychologia, 19, 57–64.
Plaisted, K. C., Swettenham, J., & Rees, L. (1999). Children with autism show local precedence in a divided attention task and global precedence in a selective attention task. Journal of Child Psychology and Psychiatry, 40, 733–742.
Ramus, F. (2003) Developmental dyslexia: Specific phonological deficit or general sensorimotor dysfunction? Current Opinion in Neurobiology, 13, 212–218.
Rees, G., Friston, K., & Koch, C. (2000). A direct quantitative relationship between the functional properties of human and macaque V5. Nature Neuroscience, 3, 716–723.
Regan, D., & Maxner, C. (1986). Orientation-dependent loss of contrast sensitivity for pattern and flicker sensitivity in multiple sclerosis. Clinical Vision Sciences, 1, 1–23.
Rinehart, N., Bradshaw, J., Moss, S., Brereton, A., & Tonge, B. (2000). Atypical interference of local detail on global processing in high functioning autism and Asperger’s disorder. Journal of Child Psychology and Psychiatry, 41, 769–778.
Rinehart, N. J., Bradshaw, J. L., Brereton, A. V., & Tonge, B. J. (2001). Movement preparation in high-functioning autism and Asperger’s disorder: A serial choice-reaction time task involving motor reprogramming. Journal of Autism and Developmental Disorders, 31, 79–88.
Rinehart, N. J., Bradshaw, J. L., Brereton, A. V., & Tonge, B. J. (2002). A clinical and neurobehavioural comparison of high-functioning autism and Asperger’s disorder. Australian and New Zealand Journal of Psychiatry, 36, 762–770.
Rizzo, M., & Nawrot, M. (1998). Perception of movement and shape in Alzheimer’s disease. Brain, 121, 2259–2270.
Rosenhall, U., Johansson, E., & Gillberg, C. (1988). Oculomotor findings in autistic children. Journal of Laryngology and Otology, 102, 435–439.
Rumsey, J. M., & Hamburger, D. (1990). Neuropsychological divergence of high-level autism and severe dyslexia. Journal of Autism and Developmental Disorders, 20, 155–168.
Salzman, C. D., Britten, K. H., & Newsome, W. T. (1990). Cortical microstimulation influences perceptual judgements of motion direction. Nature, 346, 174–177.
Scharre, J. E., & Creedon, M. P. (1992). Assessment of visual function in autistic children. Optometry and Vision Science, 69, 433–439.
Shea, V., & Mesibov, G. B. (1985). Brief report: The relationship of learning disabilities and higher-level autism. Journal of Autism and Developmental Disorders, 15, 425–435.
Skottun, B. C. (2000). The magnocellular deficit theory of dyslexia: The evidence from contrast sensitivity. Vision research, 40, 111–127.
Slaghuis, W. L., & Ryan, J. F. (1999). Spatio-temporal contrast sensitivity, coherent motion, and visible persistence in developmental dyslexia. Vision Research, 39, 651–668.
Spencer, J., & O’Brien, J. M. D. (2006). Visual form processing deficits in autism. Perception, 35, 1047–1055.
Spencer, J., O’Brien, J. M. D., Riggs, K., Braddick, O., Atkinson, J., & Wattam-Bell, J. (2000). Form and motion Coherence in autism: Is there a specific dorsal stream deficit in people with autism? NeuroReport, 11, 2765–2767.
Sperling, A. J., Zhong-Lin, L., Manis, F. R., & Seidenberg, M. S. (2005). Deficits in perceptual noise exclusion in developmental dyslexia. Nature Neuroscience, 8, 862–863.
Stuart, G. W., McAnally, K. I., McKay, A., Johnston, M., & Castles, A. (2006). A test of the magnocellular deficit theory of dyslexia in an adult sample. Cognitive Neuropsychology, 23, 1215–1229.
Szatmari, P., Archer, L., Fisman, S. M., Streiner, D. L., & Wilson, F. (1995). Asperger’s Syndrome and autism: Differences in behavior, cognition, and adaptive functioning. Journal of the American Academy of Child and Adolescent Psychiatry, 34, 1662–1671.
Takarae, Y., Minshew, N. J., Luna, B., Krisky, C. M., & Sweeney, J. A. (2004). Pursuit eye movement deficits in autism. Brain, 127, 2584–2594.
Talcott, J. B., Hansen, P. C., Assoku, E., & Stein, J. F. (2000). Visual motion sensitivity in dyslexia: Evidence for temporal and energy integration deficits. Neuropsychologia, 38, 935–943.
Talcott, J. B., Hansen, P. C., Willis-Owen, C., McKinnell, I. W., Richardson, A. J., & Stein, J. F. (1998). Visual magnocellular impairment in adult developmental dyslexics. Neuroophthalmology, 20, 187–201.
Tootell, R. G. H., Reppas, J. B., Kwong, K. K., Malach, R., Born, R. T.,Brady, T. J., Rosen, B. R., & Belliveau, J. W. (1995). Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. Journal of Neuroscience, 15, 3215–3230.
Ungeleider, L. G., & Mishkin, M. (1982). Two cortical visual systems. In D. J. Ingle, M. A. Goodale, & R. J. W. Mansfield (Eds.), Analysis of visual behaviour. Cambridge: MIT Press.
van der Geest, J. N., Kemner, C., Camfferman, G., Verbaten, M. N., & van Engeland, H. (2001). Eye movements, visual attention, and autism: A saccadic reaction time study using the gap and overlap paradigm. Biological Psychiatry, 50, 614–619.
Vidyasagar, T. R. (2004). Neural underpinnings of dyslexia as a disorder of visuo-spatial attention. Clinical and Experimental Optometry, 87, 4–10.
Von Karolyi, C., Winner, E., Gray, W., & Sherman, G. F. (2003). Dyslexia linked to talent: Global visual-spatial ability. Brain and Language, 85, 427–431.
Wechsler, D. (1999). WASI: Wechsler abbreviated scale of intelligence. San Antonio: The Psychological Corporation.
Wertheim, A. H., van Gelder, P., Lautin, A., Peselow, E., & Cohen, N. (1985). High thresholds for movement perception in schizophrenia may indicate abnormal extraneous noise levels of central vestibular activity. Biological Psychiatry, 20, 1197–1210.
White, S., Frith, U., Milne, E., Stuart, R., Swettenham, J., & Ramus, F. (2006). A double dissociation between sensorimotor impairments and reading disability: A comparison of autistic and dyslexic children. Cognitive Neuropsychology, 23, 748–761.
Acknowledgment
The authors are very thankful to the individuals and colleges who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsermentseli, S., O’Brien, J.M. & Spencer, J.V. Comparison of Form and Motion Coherence Processing in Autistic Spectrum Disorders and Dyslexia. J Autism Dev Disord 38, 1201–1210 (2008). https://doi.org/10.1007/s10803-007-0500-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-007-0500-3