Abstract
The prevalence of sleep disturbances in 52 children with Asperger syndrome (AS) as compared with 61 healthy controls (all subjects aged 5–17 years) was investigated. Problems with sleep onset and maintenance, sleep-related fears, negative attitudes toward sleeping, and daytime somnolence were more frequent among children with AS than among controls. Short sleep duration (<9 h) was almost twofold (59% vs. 32%), and the risk for sleep onset problems more than fivefold (53% vs. 10%) more common in the AS group than in the control group. Child-reported sleeping problems were also more prevalent in the AS group than in controls (58% vs. 7%). The results suggest that sleep disturbances should be routinely evaluated in children with AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sleep–wake cycle can easily be disrupted in any child, but children with neurological and psychiatric disabilities or disorders appear to be exceptionally prone to various sleep disturbances. For example, autistic disorder (AD), attention deficit and hyperactivity disorder (ADHD), and mental retardation are all associated with increased risk of sleeping difficulties (Gail Williams, Sears, & Allard, 2004; Stores, 2001; Stores & Wiggs, 2001). In children with autism, the sleeping difficulties are typically related to the sleep–wake rhythm and problems with sleep onset and maintenance (Gail Williams et al., 2004; Hoshino, Watanabe, Yashima, Kaneko, & Kumashiro, 1984; Patzold, Richdale, & Tonge, 1998; Richdale, 1999; Schreck & Mulick, 2000; Taira, Takase, & Sasaki, 1998). Both Taira et al. (1998) and Hoshino et al. (1984) reported that 65% of children with autism aged 3 years and under suffered from sleep onset problems, fragmented sleep, and early awakenings. Similarly, 54.5% of children with autism older than 3 years had sleeping problems, the most common being early night arousals and fragmented sleep (Hering, Epstein, Elroy, Iancu, & Zelnik, 1999). Sleeping patterns have also been reported to be more irregular in children with AD than in normal children (Hoshino et al., 1984).
Only few studies assessing sleep in individuals having other autistic spectrum disorders, such as Asperger syndrome (AS) have been published. Two studies reported that many sleep-related problems were more common in the AS group than in the control group and could be demonstrated using both objective actigraph measurements and parental reports (Allik, Larsson, & Smedje, 2006a; Wiggs & Stores, 2004). Three studies compared the prevalence of sleeping difficulties in children with AD and AS (Allik et al., 2006a; Patzold et al., 1998; Polimeni, Richdale, & Francis, 2005), and one in adults with AD and AS (Limoges, Mottron, Bolduc, Berthiaume, & Godbout, 2005). Allik et al. (2006a), Patzold et al. (1998), and Limoges et al. (2005) detected no consistent differences in sleeping difficulties between the two patient groups, but both AD and AS groups were reported to have more frequent insomnia complaints than the controls. However, Polimeni et al. (2005) reported that children with AS had significantly more total symptoms of sleep disturbances and more symptoms of disoriented waking than children with autism or typically developing children.
Current information on sleeping difficulties in children with AS is clearly limited. Many of the previous studies on children have used only parental reports to quantify sleeping difficulties, which can be a significant limitation as children’s and parents’ reports on sleep are often discrepant (Paavonen, 2004). In recent years, many studies have described the importance of sleep quality and quantity on children’s daytime functioning in both normative and clinical samples. Poor sleep affects children’s cognitive performance (Randazzo, Muehlbach, Schweitzer, & Walsh, 1998; Steenari et al., 2003) and behavior (Aronen, Paavonen, Fjallberg, Soininen, & Torronen, 2000; Paavonen, Solantaus, Almqvist, & Aronen, 2003b). As children with AS often have both cognitive and behavioral difficulties, the recognition and treatment of sleeping problems can be useful in preventing aggravation of behavioral symptoms or worsening of the child’s daytime performance.
The main objective of this study was to evaluate the prevalence of various sleep disturbances in children with AS as compared with normal controls using both children and parents as informants. Another objective was to describe sleep-related behaviors in children with AS.
Methods
Participants
Children for the control group were recruited as volunteers from two ordinary secondary schools in the city of Helsinki (n = 68). All children of the control group were screened for psychiatric and somatic problems using the Children’s Depression Inventory (CDI) (Kovacs, 1985) and the Child Behavior Checklist (Achenbach, 1991). One child was excluded because of dysphasia, one child because of lack of cooperation, three children because they scored above the clinical cut-off as defined by Achenbach (1991), and two children because they scored above the clinical cut-off in the CDI.
Asperger syndrome subjects were recruited from the Asperger patient series at the Unit of Child Neurology, Helsinki University Central Hospital, and at the Helsinki Asperger Centre, Medical Centre Dextra. The initial patient sample consisted of 68 cases aged 5–17 years, with a confirmed diagnosis of AS with no neuropsychiatric medication. The diagnosis was made by an experienced child neurologist, and the diagnostic procedure included the Asperger syndrome screening questionnaire, a thorough neuropsychological test battery, and a clinical interview of the children and the parents. A final diagnosis was set when DSM-IV and ICD-10 criteria were fulfilled.
The complete sample consisted of 61 controls (47.5% boys and 52.5% girls), and 52 children (76.9% boys and 23.1% girls) with AS. The mean age was 10.0 ± 1.9 years (range 76.9–13.1 years) in the control group and 10.1 ± 3.4 years (range 4.8–17.0 years) in the AS group. In the AS sample, the mean total IQ was 94.2 ± 15.2, the verbal IQ 91.8 ± 15.4, and the nonverbal IQ 98.2 ± 17.2.
Materials
The parents filled in a 26-item sleep questionnaire (Sleep Disturbance Scale for Children, SDSC), which has previously been validated (Bruni et al., 1996). Items are rated on a five-point scale, with response alternatives ranging from “never” to “always.” A total score and the following six subscale scores are calculated: disorders in initiating and maintaining sleep (DIMS), sleep breathing disorders (SBD), disorders of arousal (DA), sleep–wake transition disorders (SWTD), disorders of excessive somnolence (DES), and sleep hyperhydrosis (SHY). The categories reflect the DSM-IV subclasses for sleeping difficulties—insomnia (∼DIMS), sleep breathing disorders (∼SBD), hypersomnia (∼DES), and parasomnia (∼DA, SWTD). Subscale scores were calculated when less than 20% of the items belonging to the subscale score were missing. A set of background questions related to sleeping habits, sleeping rituals, seasonality of sleep, and medication was also included for the parents. The children filled in the Sleep Self-Report questionnaire, which contains 26 items on sleep (Owens, Spirito, McGuinn, & Nobile, 2000), including bedtime, sleep behavior, sleep-related fears, and daytime sleepiness. The items are rated on a three-point scale with response alternatives of “rarely = 0–1/week,” “sometimes = 2–4/week,” and “often = 5–7/week.” No subscale scores for this questionnaire have previously been reported. Parents were given written instructions on how to help if the child had difficulty completing the questionnaire on his/her own: “You can help the child to fill in the questionnaire whenever he/she has difficulties. The question can be read aloud or the child can be helped to understand the question. It is however important, that the explanations are not leading. The aim is that the child can choose the alternative he/she considers to be the most accurate one.”
Procedure
The study protocol was approved by the Ethics Committee for Pediatrics, Adolescent Medicine, and Psychiatry. Written informed consent was obtained from parents, and verbal assent from children. The parents of control group children received questionnaires through school teachers to be filled in at home. The parents of AS group children received the questionnaire by mail. One family could not be tracked down, but all other parents and children were sent a letter describing the study protocol, together with three sleep questionnaires. Two reminders were sent if no response was received in a month. The final response rate for the screening was 80.6% (54/67). Two children reported that they did not want to participate. Total IQ, verbal IQ, and nonverbal IQ were also determined for most of the AS children (n = 41).
Statistical Analysis
First, we performed a maximum likelihood factor analysis to reduce the number of items on the child’s questionnaire and to construct composite scales describing various dimensions of sleeping difficulties. All variables describing sleeping problems were included. Eight variables concerning sleeping habits, such as bedtime and sleeping arrangements, were excluded since this study evaluates sleeping difficulties not sleeping habits (items 1, 4–7, 12, 22, 25). Item 22 (“does pain wake you up at night?”) was excluded because it represents somatic problems, and item 2 was omitted for theoretical reasons. Fifteen items (3, 8–11, 13–16, 18–20, 23, 24, 26) were thus used in the factor analysis. A total of 108 missing values (6.4%) in the 113 observations of 15 sleep variables were replaced by regression imputation (Little & Rubin, 1987) using a simplified EM algorithm implemented as CORRMV operation of SURVO MM (Mustonen, 2001). Each variable in turn was iteratively explained by the other variables to find adequate estimates for the missing values and to avoid a list-wise deletion of a total of 18 observations. In addition to the sleep variables, sex and age were used in the imputation to enhance the estimates. Four descriptive factors with high reliabilities were finally extracted (Table 1). These factors explained 44.9% of the total variance in the data. Factor scores were calculated using the regression method and used in subsequent statistical analyses. Reliabilities of the factor scores were estimated by Tarkkonen’s ρ (Tarkkonen & Vehkalahti, 2005).
The data are presented as frequencies and percentages or means and standard deviations. Group differences in sleep quality were compared using nonparametric tests (χ 2-test for categorical variables and Mann–Whitney U-test for continuous variables). To estimate the risk of sleeping problems in the AS group, odds ratios with 95% confidence intervals were computed. The significance level was set at p < 0.05, and all statistical tests were two-sided.
Results
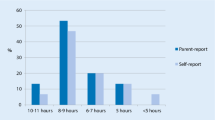
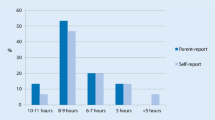
According to parental reports (SDSC), the average sleep duration was longer in the control group than in the AS group (χ 2 = 9.61, df = 3, p = 0.02). Most of the controls (68.5%, n = 37) slept at least 9 h a night, as opposed to less than half of AS children (41.2%, n = 21). None of the controls, but 5.9% (n = 3) of children with AS slept less than 7 h a night. Sleep onset difficulties also were significantly more common in the AS group than in the others (χ 2 = 26.31, df = 4, p < 0.01); falling asleep took more than 30 min in 10.9% (n = 6) of the controls, while the corresponding figure for the AS group was as high as 53.1% (n = 26).
Most sleep problems were more prevalent in the AS group than in the control group (Table 2) and the mean sleep subscale scores were correspondingly significantly higher in AS children than in control children. The only exception was in the variable of sleep breathing disturbance (Table 3).
Sleeping difficulties had begun at an average age of 3.7 ± 4.7 years in control group and 2.4 ± 2.7 years in the AS group, but the age was reported for only three control children; thus most children in the control group did not have sleeping difficulties. Children with AS had significantly more sleep-related fears than other children. Most of the controls (86.2%, n = 50) had no sleep-related fears, with the remainder having only occasional fears (13.8%, n = 8). Of the children with AS, only 57.4% (n = 27) were reported to never have had sleep-related fears while the rest had sleep-related fears at least occasionally. Fears occurred less than once a week in 23.1% (n = 11), once or twice a week in 6.4% (n = 3), three to six times a week in 4.3% (n = 2), and every night in 8.5% (n = 4) of children with AS (χ 2 = 15.36, df = 4, p < 0.01).
Bedtime rituals tended to be more common in the AS group (57.1%, n = 27) than in controls (40.7%, n = 24), but the difference was not significant (χ 2 = 3.36, df = 1, p = 0.07). Bed-sharing was also more common in the AS group than in the control group; 21.7% (n = 10) of AS children visited their parent’s beds at least weekly, whereas only 8.6% (n = 5) of controls did so (χ 2 = 3.58, df = 1, p = 0.06). Sharing a room with a parent tended to be less common in controls (5.1%, n = 3) than in AS children (10.6%, n = 5), but the difference was not significant (χ 2 = 3.21, df = 3, p = 0.26). Seasonal change in sleep quality (“is there seasonal change in sleep quality?”, response alternatives: yes/no) was reported significantly more often by parents of AS children (33.3%, n = 15) than by those of control children (13.8%, n = 8) (χ 2 = 5.58, df = 1, p = 0.02). In both groups, difficulties were most frequent during the spring or summer.
The children’s self-report questionnaire revealed many differences between the AS group and the controls (Fig. 1). First, only 7.0% (n = 4) of controls but 58.3% of children with AS felt that they had sleeping problems (χ 2 = 32.38, df = 1, p < 0.01). Insomnia was very typical in the AS group; half of the controls (50.9%) and a quarter of AS children (25.0%) reported often falling asleep in less than 20 min (χ 2 = 12.33, df = 2, p < 0.01). Only 6.8% of the control group and as many as 20.0% of children in the AS group reported frequent nocturnal awakenings (χ 2 = 9.42, df = 2, p = 0.01). When children did wake up at night, those in the control group had far less difficulties in falling asleep again than those with AS (6.8% vs. 28.9%) (χ 2 = 10.00, df = 2, p = 0.01). Children in the control group less frequently reported staying awake for a long time when their parents thought they had fallen asleep (8.5%) than children in the AS group (25.5%) (χ 2 = 7.39, df = 2, p = 0.03).
Second, too short sleep duration and tiredness were characteristic of AS children. Only 3.4% of controls reported sleeping too little, as opposed to 16.7% of children with AS (χ 2 = 5.65, df = 2, p = 0.06). One-fifth of controls (16.9%) found it difficult to wake up in the morning, while the same problem was reported more than twice as frequently by AS children (43.8%) (χ 2 = 11.34, df = 2, p < 0.01). Daytime tiredness was described by 5.4% of controls and 20.8% of children with AS (χ 2 = 12.34, df = 2, p < 0.01). More than two-thirds (71.2%) of controls reported often feeling rested while only 43.8% of the Asperger group children agreed (χ 2 = 10.71, df = 2, p = 0.01).
Third, the attitude toward sleeping was more negative among AS children than among controls. While most children in the control group (93.0%) liked to go to sleep, only 64.6% of children with AS had similar inclinations (χ 2 = 13.14, df = 1, p < 0.01). Moreover, most control group children were often willing to go to sleep at bedtime (59.6%), whereas only every fourth child with AS (25.0%) had the same opinion (χ 2 = 18.78, df = 2, p < 0.01). Likewise, only two control group children reported that they often found it hard to go to bed, in contrast to almost one-third of children with AS (27.1%, n = 13) (χ 2 = 18.78, df = 2, p < 0.01). Finally, controls had clearly fewer arguments with parents about bedtime than children with AS (10.2% vs. 22.9%) (χ 2 = 6.99, df = 2, p = 0.03).
Fourth, many AS children said that they suffered from sleep-related fears. Among the controls, one child (1.7%) reported often being afraid of the dark, whereas 18.8% of the AS children did so (χ 2 = 9.58, df = 2, p = 0.01). Almost all of the control group children were unafraid of sleeping alone (93.1%), while only 59.6% of AS children were rarely afraid of sleeping alone (χ 2 = 17.90, df = 2, p < 0.01). Occasional nightmares were quite common in both groups, 37.3% in controls and 57.4% in AS patients (χ 2 = 4.49, df = 2, p = 0.11).
These four dimensions also emerged in factor analysis. Factor scores significantly differed between the control group and the AS group (Fig. 2; Table 4).
No significant differences were observed in sleeping habits, such as regular bedtime (p = 0.30), sleeping every night in the same bed (p = 0.54), napping (p = 0.24), changing sleeping place at night (p = 0.32), falling asleep alone (p = 0.39), co-sleeping (p = 0.59), sleeping too much (p = 0.11), having nocturnal pains (p = 0.75), and using a sleep toy (p = 0.62), between AS and control group cases.
In the AS group, there were no correlations between total IQ, verbal IQ, nonverbal IQ, and any of the subscale scores on the parental sleep questionnaire (all r’s < 0.24). However, according to children’s reports, a positive attitude toward sleeping was strongly and positively correlated with total IQ (r = 0.49, p = 0.002), verbal IQ (r = 0.48, p = 0.002), and nonverbal IQ (r = 0.34, p = 0.03). Sleep-related fears, in contrast, were negatively correlated with verbal IQ (r = −0.30, p = 0.05).
Discussion
The frequency of sleep problems in children with AS as compared with normal children was high. According to parents, sleep onset problems, parasomnias, sleep hyperhydrosis, and daytime somnolence were more common in the AS group, whereas the risk for sleep-related breathing disorders was no greater than that of controls. Interestingly, parents reported sleep problems being more frequent during the spring and summertime, especially in children with AS. Similarly, children with AS themselves often reported sleep onset problems, too little sleep, sleep-related fears, nightmares, and a negative attitude toward sleeping. Finally, they reported often having arguments with parents about bedtime. Consistent with our findings, the few previous studies concerning sleep in children and adults with AS have systematically reported an increased prevalence of sleep problems in these individuals (Allik et al., 2006a; Allik, Larsson, & Smedje, 2006b; Godbout, Bergeron, Limoges, Stip, & Mottron, 2000; Limoges et al., 2005; Nieminen-von Wendt et al., 2005; Patzold et al., 1998; Polimeni et al., 2005; Richdale, 1999; Tani et al., 2003; Wiggs & Stores, 2004). Similar findings have been gathered concerning children with AD (Diomedi et al., 1999; Elia et al., 2000; Gail Williams et al., 2004; Hering et al., 1999; Richdale, 1999; Schreck & Mulick, 2000; Taira et al., 1998).
The rates of insomnia, in particular, seem to be increased in both AS and AD. For example, Patzold et al. (1998) reported that 63.1% of children with AD or AS have sleep disturbances according to parents, especially increased sleep latency, and prolonged nocturnal awakenings. The study by Patzold et al. (1998) was, however, limited by the small sample size (31 cases with AD and 7 cases with AS) and it did not present any detailed data about sleep disturbances in children with AS. In a later larger study, these findings were replicated using parental reports as the source of information; 73% of children with AS/AD had sleeping difficulties as compared with 50% of the control group children (Polimeni et al., 2005). Polimeni et al. (2005) found that even though parent-reported severity, and frequency of common problems like night waking were similar between AD and AS groups, the children with AS had significantly more total sleep disturbances and disoriented awakenings than those with autism or typical children. However, the study reported an exceptionally high frequency of sleeping problems in the control group (55%), which suggests the possibility of sampling bias.
Findings concerning sleep duration have been inconsistent. Neither Schreck and Mulick (2000) nor Polimeni et al. (2005) observed differences in sleep duration between children with AD and/or AS and controls, but Patzold et al. (1998) reported reduced sleep duration in children with AS which is consistent with our findings. In this study, the sleep duration reported by parents and children was less in the AS group than in the control group. Findings concerning irregular sleeping habits have also been inconsistent. We found no evidence that irregular sleeping habits are more common in AS subjects than in controls but Hoshino et al. (1984) and Tani et al. (2003) had contrasting findings in autism and adults with AS. Given the differences between these three samples, the discrepancy may reflect differences in diagnosis or age. For example, Richdale has speculated that changes in sleep quality may occur over time in autism (Richdale, 1999).
Sleep disturbances in AD have been speculated to be negatively correlated with developmental level, but the results have been contradictory. Hoshino et al. (1984) showed that both the duration of the sleep disturbance and the likelihood of sleep problems were greater in patients with a poor developmental level (Hoshino et al., 1984). Elia et al. (2000) found that the sleep impairment in AD was related to poorer coordination and inferior perceptual and verbal skills. Schreck, Mulick, and Smith (2004) reported that short sleep length was related to autism severity scores, stereotypic behavior and social skill deficits. However, neither Patzold et al. (1998) nor Diomedi et al. (1999) observed any correlation between IQ and sleep impairment in autistic children, even though Patzold et al. (1998) reported that difficult daytime behavior was correlated with present and past sleep problems. In our study, no correlation between developmental level and parent-reported sleep disturbances could be established, but child-reported sleep fears and negative attitudes toward sleeping were related to lower IQ. This is not surprising given the fact that the participants all had average intelligence without a large range in IQ and thus the variability in IQ was low as compared with autism.
Neuroendocrine function has been suggested to underlie sleep–wake rhythm alterations in individuals with autistic spectrum disorders (Tordjman, Anderson, Pichard, Charbuy, & Touitou, 2005). Melatonin, a central hormone regulating the sleep–wake cycle (Dahlitz et al., 1991), is of special interest in individuals with autistic spectrum disorders as suggested for example by Wiggs & Stores (2004) and Richdale (1999). Tordjman et al. (2005) noted that nocturnal 6-sulfatoxymelatonin excretion was significantly lower in children and adolescents with AD than in normal controls and as melatonin secretion depends upon such exogenous factors as light and season (Brzezinski, 1997), it was interesting that parents reported a higher frequency of sleep problems during the spring and summertime than during other seasons especially in children with AS. Thus it can be speculated that, alternations in the sleep–wake cycle of children with AS syndrome may be related to a melatonin secretion dysfunction, making these children particularly vulnerable to the disruptive effect of a seasonal increase in light on circadian rhythms. In Nordic countries day length (season) is related to nocturnal activity level and sleep in a normative sample of children (Aronen, Fjallberg, Paavonen, & Soininen, 2002).
The question of comorbidity may also be important in seeking explanations for the increased prevalence of sleeping difficulties in children with autistic spectrum disorders. The increased frequency of sleeping difficulties has been speculated to be related to an increased anxiety level in the AS group (Patzold et al., 1998; Richdale, 1999; Wiggs & Stores, 2004). Lending support to this hypothesis, Allik et al. (2006b) reported more emotional symptoms in children with AS and sleep problems as compared to those without sleep disturbances. Limoges et al. (2005) found that increased anxiety levels accompany sleeping difficulties in adults with AS/AD. Tani et al. (2003) reported a high frequency (13/20) of clinical anxiety disorders in individuals with AS. Our findings of a high prevalence of sleep fears and negative attitudes toward sleeping are consistent with the supposition that underlying anxiety constitutes a significant predisposing factor for sleeping difficulties. However, increased anxiety levels may also be caused by sleep deprivation, and not vice versa and therefore the question of causality requires further studies. The role of anxiety in the sleep problems of children with AS warrants special attention, as it may be decisive in the selection of the most effective treatment.
Given the low prevalence of AS in population and the high participation rate, our study examined a highly representative group of AS children. Furthermore, this study is, to our knowledge, the first to include self-report of sleep of children with autistic spectrum disorders. Earlier studies have shown that parents’ ability to notice children’s sleeping difficulties can be low (Paavonen et al., 2000, 2002). Our study of sleep in children with AS using a larger sample size and a complementary assessment of sleep quality and sleep quantity (including the child’s and parents’ reports) gives important new information concerning sleep in children with AS. However, some limitations of the study are worth noting. The control group was not matched by gender with the patient group. However, in the present study we found no gender differences. Moreover, we did not control for long-term medication, comorbidity, and chronic/temporary illnesses. Finally, we used the children as informants about their sleep. In the AS group, the parents were informed on how to help the children in case they had difficulties in filling in the form. Nevertheless, these children may have had more problems in understanding the questions than did the normal children, which may have affected their responses.
To summarize, consistent with previous studies on AD or AS, we found an increased prevalence of sleep problems in children with AS as compared with normal controls. Both parents and children in the AS group reported an increased amount of dyssomnias as compared with parents and children in the control group. The children’s report gave interesting additional information concerning sleep-related fears and attitudes toward sleeping. As sleep problems in autistic spectrum disorders are common and tend to begin at an early age and to persist for several years, their clinical impact is considerable (Hoshino et al., 1984; Taira et al., 1998). Children with severe disabilities are reported to display more difficult daytime behavior in association with sleep problems (Wiggs & Stores, 1999) and these problems may also interfere with educational efforts (Durand, 1998). Poor sleep can also aggravate other symptoms typical of autistic spectrum disorders (Schreck et al., 2004; Wiggs & Stores, 1996). Thus sleep difficulties among those with autism spectrum disorders is of considerable concern for families. There is evidence to suggest that treatment of sleep problems may ameliorate daytime behavior both in autism (Malow, McGrew, Harvey, Hendersson, Stone, 2006) and AS (Paavonen, Nieminen-von Wendt, Vanhala, Aronen, & von Wendt, 2003a). In addition to affecting the child’s behavior and cognitive functioning, sleeping difficulties can increase parental stress and may even compromise the child–parent relationship, potentially leading to severe problems for the whole family (Patzold et al., 1998; Richdale, 1999). Therefore, in clinical practice, all children with AS should be screened to identify different types of sleep problems. Sleep-related anxiety should be evaluated, as this is important in being able to choose the best treatment for the child’s sleep problem.
References
Achenbach, T. M. (1991). Child behavior checklist/4–18 and 1991 profile. Burlington, VT: Department of Psychiatry, University of Vermont.
Allik, H., Larsson, J.-O., & Smedje, H. (2006a). Sleep patterns of school-age children with Asperger syndrome or high-functioning autism. Journal of Autism and Developmental Disorders, 36, 585–595.
Allik, H., Larsson, J.-O., & Smedje, H. (2006b). Insomnia in school-age children with Asperger Syndrome or high-functioning autism. BMC Psychiatry, 6, 18.
Aronen, E. T., Fjallberg, M., Paavonen, E. J., & Soininen, M. (2002). Day length associates with activity level in children living at 60 degrees north. Child Psychiatry and Human Development, 32(3), 217–226.
Aronen, E. T., Paavonen, E. J., Fjallberg, M., Soininen, M., & Torronen, J. (2000). Sleep and psychiatric symptoms in school-age children. Journal of the American Academy of Child and Adolescent Psychiatry, 39(4), 502–508.
Bruni, O., Ottaviano, S., Guidetti, V., Romoli, M., Innocenzi, M., Cortesi, F. et al. (1996). The sleep disturbance scale for children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. Journal of Sleep Research, 5(4), 251–261.
Brzezinski, A. (1997). Melatonin in humans. The New England Journal of Medicine, 336(3), 186–195.
Dahlitz, M., Alvarez, B., Vignau, J., English, J., Arendt, J., & Parkes, J. D. (1991). Delayed sleep phase syndrome. Lancet, 337, 1121–1124.
Diomedi, M., Curatolo, P., Scalise, A., Placidi, F., Caretto, F., & Gigli, G. L. (1999). Sleep abnormalities in mentally retarded autistic subjects: Down’s syndrome with mental retardation and normal subjects. Brain and Development, 21(8), 548–553.
Durand, V. M. (1998). Sleep better!: A guide to improving sleep for children with special needs. Baltimore, MD: Paul H. Brookes.
Elia, M., Ferri, R., Musumeci, S. A., Del Gracco, S., Bottitta, M., Scuderi, C. et al. (2000). Sleep in subjects with autistic disorder: A neurophysiological and psychological study. Brain and Development, 22(2), 88–92.
Gail Williams, P., Sears, L. L., & Allard, A. (2004). Sleep problems in children with autism. Journal of Sleep Research, 13, 265–268.
Godbout, R., Bergeron, C., Limoges, E., Stip, E., & Mottron, L. (2000). A laboratory study of sleep in Asperger’s syndrome. Neuroreport, 11(1), 127–130.
Hering, E., Epstein, R., Elroy, S., Iancu, D. R., & Zelnik, N. (1999). Sleep patterns in autistic children. Journal of Autism and Developmental Disorders, 29(2), 143–147.
Hoshino, Y., Watanabe, H., Yashima, Y., Kaneko, M., & Kumashiro, H. (1984). An investigation on sleep disturbance of autistic children. Folia Psychiatrica et Neurologica Japonica, 38(1), 45–52.
Kovacs, M. (1985). The children’s depression inventory (CDI). Psychopharmacology Bulletin, 21(4), 995–998.
Limoges, E., Mottron, L., Bolduc, C., Berthiaume, C., & Godbout, R. (2005). Atypical sleep architecture and the autism phenotype. Brain, 128(Pt. 5), 1049–1061.
Little, R. J. A., & Rubin, D. B. (1987). Statistical analysis with missing data. New York: Wiley.
Malow, B. A., McGrew, S. G., Harvey, M., Hendersson, L. M., & Stone, W. L. (2006). Impact of treating sleep apnea in a child with autism spectrum disorder. Pediatric Neurology, 34, 325–328.
Mustonen, S. (2001). The new Windows version of Survo, 2.32 ed. Helsinki: Survo Systems.
Nieminen-von Wendt, T., Paavonen, E. J., Ylisaukko-Oja, T., Sarenius, S., Källman, T., & von Wendt, L. (2005). Subjective face recognition difficulties, aberrant sensibility, sleeping disturbances and aberrant eating habits in families with Asperger syndrome. BMC Psychiatry, 5(20), 1–8.
Owens, J. A., Spirito, A., McGuinn, M., & Nobile, C. (2000). Sleep habits and sleep disturbance in elementary school-aged children. Journal of Developmental and Behavioral Pediatrics, 21(1), 27–36.
Paavonen, E. J. (2004). Sleep disturbances and psychiatric symptoms in school-aged children. Thesis, University of Helsinki, Helsinki.
Paavonen, E. J., Almqvist, F., Tamminen, T., Moilanen, I., Piha, J., Rasanen, E. et al. (2002). Poor sleep and psychiatric symptoms at school: An epidemiological study. European Child and Adolescent Psychiatry, 11(1), 10–17.
Paavonen, E. J., Aronen, E. T., Moilanen, I., Piha, J., Rasanen, E., Tamminen, T. et al. (2000). Sleep problems of school-aged children: A complementary view. Acta Paediatrica, 89(2), 223–228.
Paavonen, E. J., Nieminen-von Wendt, T., Vanhala, R., Aronen, E. T., & von Wendt, L. (2003a). Effectiveness of melatonin in the treatment of sleep disturbances in children with Asperger disorder. Journal of Child and Adolescent Psychopharmacology, 13(1), 83–95.
Paavonen, E. J., Solantaus, T., Almqvist, F., & Aronen, E. T. (2003b). Four-year follow-up study of sleep and psychiatric symptoms in preadolescents: Relationship of persistent and temporary sleep problems to psychiatric symptoms. Journal of Developmental and Behavioral Pediatrics, 24(5), 307–314.
Patzold, L. M., Richdale, A. L., & Tonge, B. J. (1998). An investigation into sleep characteristics of children with autism and Asperger’s disorder. Journal of Paediatrics and Child Health, 34(6), 528–533.
Polimeni, M. A., Richdale, A. L., & Francis, A. J. (2005). A survey of sleep problems in autism, Asperger’s disorder and typically developing children. Journal of Intellectual Disability Research, 49(Pt. 4), 260–268.
Randazzo, A. C., Muehlbach, M. J., Schweitzer, P. K., & Walsh, J. K. (1998). Cognitive function following acute sleep restriction in children ages 10–14. Sleep, 21(8), 861–868.
Richdale, A. L. (1999). Sleep problems in autism: Prevalence, cause, and intervention. Developmental Medicine and Child Neurology, 41(1), 60–66.
Schreck, K. A., & Mulick, J. A. (2000). Parental report of sleep problems in children with autism. Journal of Autism and Developmental Disorders, 30(2), 127–135.
Schreck, K. A., Mulick, J. A., & Smith, A. F. (2004). Sleep problems as possible predictors of intensified symptoms of autism. Research in Developmental Disabilities, 25(1), 57–66.
Steenari, M. R., Vuontela, V., Paavonen, E. J., Carlson, S., Fjallberg, M., & Aronen, E. (2003). Working memory and sleep in 6- to 13-year-old schoolchildren. Journal of the American Academy of Child and Adolescent Psychiatry, 42(1), 85–92.
Stores, G. (2001). Sleep–wake function in children with neurodevelopmental and psychiatric disorders. Seminars in Pediatric Neurology, 8(4), 188–197.
Stores G., & Wiggs L. (eds.) (2001). Sleep disturbance in children and adolescents with disorders of development: Its significance and management. Series: Clinics in developmental medicine, n.o. 155. London: Mac Keith Press.
Taira, M., Takase, M., & Sasaki, H. (1998). Sleep disorder in children with autism. Psychiatry and Clinical Neurosciences, 52(2), 182–183.
Tani, P., Lindberg, N., Nieminen-von Wendt, T., von Wendt, L., Alanko, L., Appelberg, B. et al. (2003). Insomnia is a frequent finding in adults with Asperger syndrome. BMC Psychiatry, 3(12), doi: 10.1186/1471-244X-3-12.
Tarkkonen, L., & Vehkalahti, K. (2005). Measurement errors in multivariate measurement scales. Journal of Multivariate Analysis, 96(1), 172–189.
Tordjman, S., Anderson, G. M., Pichard, N., Charbuy, H., & Touitou, Y. (2005). Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biological Psychiatry, 57, 134–138.
Wiggs, L., & Stores, G. (1996). Severe sleep disturbance and daytime challenging behaviour in children with severe learning disabilities. Journal of Intellectual Disability Research, 40(Pt. 6), 518–528.
Wiggs, L., & Stores, G. (1999). Behavioural treatment for sleep problems in children with severe learning disabilities and challenging daytime behaviour: Effect on daytime behaviour. Journal of Child Psychology and Psychiatry and Allied Disciplines, 40(4), 627–635.
Wiggs, L., & Stores, G. (2004). Sleep patterns and sleep disorders in children with autistic spectrum disorders: Insights using parent report and actigraphy. Developmental Medicine and Child Neurology, 46, 372–380.
Acknowledgments
This study was financially supported by the Finnish Cultural Foundation, the Foundation for Child Psychiatric Research, the Finnish Sleep Research Society, the Foundation for Pediatric Research, the Finnish Medical Foundation, the Signe and Ane Gyllenberg Foundation, the Yrjö Jahnsson Foundation, the Helsinki University Central Hospital Funds (EVO TYH 4217), and the Helsinki University Research Funds (Project 2103035), Hospital for Children and Adolescents, Departments of Child Psychiatry and Neurology, Helsinki University Central Hospital, HUS, University of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paavonen, E.J., Vehkalahti, K., Vanhala, R. et al. Sleep in Children with Asperger Syndrome. J Autism Dev Disord 38, 41–51 (2008). https://doi.org/10.1007/s10803-007-0360-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-007-0360-x