Abstract
A selective and sensitive electrochemical sensor has been prepared for the determination of 6-benzylaminopurine (BAP) in complex matrices. It was fabricated by thoroughly mixing multiwall carbon nanotube@SnS2 (MWNT@SnS2) with molecularly imprinted chitosan (CHIT), and then covering on the surface of glassy carbon electrode (GCE). The core/shell-structured MWNT@SnS2 dramatically improved the sensitivity of the developed sensor through providing increased binding and preconcentration onto the modified GCE, while CHIT imprinted with BAP served as the selective recognition sites. Several experimental parameters such as pH, amount of modifier, extraction time, and incubation time were optimized. Under the optimal conditions, selective detection of BAP in a linear concentration range of 0.1 nM–10 mM was performed with the detection limit of 50 pM (3S b/S). The relative standard deviation of repeatability and reproducibility of the sensor was 1.14 and 2.42 %, respectively. Furthermore, the sensor was successfully applied to the determination of BAP in vegetable and fruit samples, indicating the molecularly imprinted polymer-based electrochemical sensing platform might provide a rapid, sensitive, and cost-effective strategy for BAP determination and related food safety analysis.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pesticides are inevitably essential in modern agriculture, but many experiments and surveys indicate that the abuse of pesticides results in adverse effects not only on the human health but also on the environmental risks [1]. Therefore, the widespread application of pesticides has become a global issue. Among the pesticides, 6-benzylaminopurine (BAP) is identified as the first-generation man-made plant growth regulator, which can be used for the stimulation of plant cell growth and division, basal shoot formation, lateral bud emergence, flowering, and fruit set [2]. Based on these functions, some market traders use excessive BAP in agriculture, horticulture, and fruits growing to improve their organoleptic quality, post-harvest life, and production to achieve high profit. However, an excessive intake of BAP may cause irritation and damage to mucous membranes, eyes, skin, and upper respiratory tract, and some symptoms such as emesia and sicchasia may occur consequently [3]. In this sense, the development of a rapid, sensitive, inexpensive, reliable, on-site detection method for BAP residues in vegetables and fruits is important for concern about food safety. Many methods have been developed for the detection of BAP from food samples [4–10], which generally require high-quality organic solvents, complicated pre-treatments and trained personnel. This has led to the development of faster and more efficient methods that are both inexpensive and environmentally friendly. In contrast, electrochemical sensors [11–14] have received remarkable attention particularly because of their rapid response, high sensitivity, convenient use, time saving, stability, and low cost, which offer good prospects for meeting the growing needs of food safety detection. Furthermore, electrochemical sensors have received successful application in the field of environmental toxins detection [15–17]. Nonetheless, the sensitivity and selectivity of these electrochemical sensors need further improvement.
During the last two decades, molecular imprinting emerged as a valuable technique and has led to the tremendous research interest from scientists devoting to electrochemical sensor development, owing to its unique advantages including ease of preparation, rapidness, low cost, reusability, long-term stability, and predetermined selectivity [18, 19]. Ye et al. [20], Si and colleagues [21] and Sales and colleagues [22] have testified that the highly selective recognition characteristics of the molecularly imprinted polymers (MIPs) on electrode surface are better than those of natural biological species such as receptors and antibodies. Despite the excellent selectivity of molecularly imprinted electrochemical sensors, the main challenge that still remains to be addressed is their poor sensitivity to template molecules resulted from the poor conductivity of MIPs. Then the researchers have made great efforts to find novel nanomaterials to enhance the sensitivity of molecularly imprinted electrochemical sensors [23].
Compared with other potential nanomaterials, multiwall carbon nanotube (MWNT) with one-dimensional tubular structure is acknowledged to be promising material in the field of electrode modification and electrochemical sensor, owing to a number of fascinating properties such as large surface area, high electrocatalytic activity, great mechanical strength, excellent chemical and physical stabilities, and electronic conductivity [24]. These electrochemical sensors fabricated qualified platforms for the acceleration of electron transfer between target molecules and the electrodes, thus improving the possibility of sensitivity enhancement [25]. However, the large length-to-diameter ratio of MWNT causes it to become easily entangled, which finally influences its properties. One effective strategy to overcome this problem points to the fabrication of core/shell structure with a MWNT core and a hierarchical shell, which can extremely amplify the signal transduction efficiency and yield a dramatic improvement of the sensitivity [26]. SnS2 is a crucial IV–VI semiconductor material with a layered CdI2-type structure, whose appealing catalytic activity and high electrical conductivity had been demonstrated in lithium batteries [27]. Moreover, because of its environment friendliness and high carrier mobility, the thin layer of SnS2 is particularly desirable for nanoelectronics applications [28, 29].
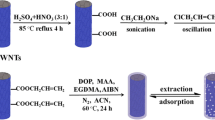
As far as we know, there is no report available in the literature regarding the molecularly imprinted electrochemical sensing of BAP. Herein, a novel electrochemical sensor for the determination of BAP in vegetable and fruit samples was fabricated through the modification of a glassy carbon electrode (GCE) with composite of core/shell-structured MWNT@SnS2 and molecularly imprinted chitosan (CHIT). The schematic representation of the possible mechanism of molecular imprinting at the surface of MWNT@SnS2 is illustrated in Scheme 1. The MWNT@SnS2 as electrode material was used to significantly increase the electron transfer efficiency and surface area of the sensor, which resulted in an improvement of the sensitivity. Simultaneously, the polymeric film with imprinted sites showed ability to selectively bind BAP target molecules. Finally, MIP-MWNT@SnS2 was successfully applied for convenient, rapid, sensitive, and selective detection of trace BAP in vegetable and fruit samples.
2 Experimental
2.1 Chemicals and reagents
MWNTs were purchased from Shenzhen Nanotech Port Co., Ltd. (Shenzhen, China) (www.nanotubes.com.cn). Tin(IV) chloride pentahydrate (SnCl4·5H2O), thioacetamide (TAA), CHIT, BAP, indole-3-acetic acid (IAA), 6-benzyloxypurine (BOP), 6-benzylguanine (BG), kinetin (KIN), m-topolin (MT), roscovitine (ROSC), olomoucine (OLO), and bohemine (BOH) were obtained from Sigma-Aldrich (China) (www.sigmaaldrich.com/china). All reagents were of analytical grade and used without further purification. Stock solution of 0.01 M BAP was prepared with absolute ethanol and diluted before the measurements.
2.2 Instrumentation
Electrochemical measurements were performed using an CHI 660D electrochemical workstation (Shanghai, China), and the data were recorded using a conventional three-electrode system with a MIP-MWNT@SnS2-modified GCE as working electrode, Pt wire as counter electrode, and saturated calomel electrode as reference electrode. X-ray diffraction (XRD) analysis was carried out using a Rigaku TTR-III X-ray diffractometer, with the Cu Kα radiation (λ = 1.5406 Å) as the X-ray source and a monochromator, positioned in front of the detector. Scanning was performed over a 2θ angle ranging from 10° to 70° with a speed of 2° min−1 and step rise of 0.05. Raman spectrum (Renishaw inVia Raman microscope) was recorded on the condition of 514 nm Ar ion laser excitation at 6 mW for 50 s. Energy-dispersive X-ray (EDX) spectrum and field emission scanning electron micrographs (SEMs) were obtained using a Hitachi S-4800 microscope. Field emission transmission electron microscopy (TEM) characterizations were performed on a Tecnai G2 20 S-twin transmission electron microscope operating at 200 kV, for which each sample was prepared by dispersing it in ethanol and drop-casting onto a 200 mesh copper grid coated with holey carbon.
Liquid chromatography-tandem mass spectrometry (LC–MS) method was employed for comparison purpose [4]. A chromatography apparatus, Agilent 1200 (Agilent Technologies, Santa Clara, CA, USA), consists of an autosampler module, a degasser, a binary pump, a column heater/selector and a MS detector from the same provider was used. The column was an Agilent XDB-C18 (1.8 μm, 50 mm × 4.6 mm). The mass detection was achieved with an LTQ XL ion trap (Thermo Fischer Scientific, San Jose, CA, USA), equipped with heated electrospray ionization (HESI-II) probe.
2.3 Preparation of MWNT@SnS2
First, the MWNT was acid-treated according to previous report [30]. 1.0 g of MWNT was refluxed in 30 mL HNO3 (65 wt%) at 140 °C for 6 h. After being rinsed with large amount of water to a neutral pH values, the MWNT was collected by filtration and dried at 60 °C for further use. In a typical procedure of MWNT@SnS2 [31], 0.05 g acid-treated MWNTs were added to 10 mL absolute ethanol and the MWNTs were well dispersed by completely volatilizing the ethanol through violent ultrasonication. Then, 40 mL distilled water, 1.05 g SnCl4·5H2O, and 2.25 g TAA were added to the MWNTs successively at room temperature. The resultant mixture was continually stirred at 25 °C for 1 h and then transferred to a Teflon-lined stainless-steel autoclave (capacity of 80 mL). The autoclave was sealed and maintained at 100 °C for 4 h. The system was then cooled to ambient temperature naturally. The final product was collected and washed with ethanol and distilled water for six times, followed by vacuum drying. The SnS2 was also prepared according to the above procedures without the addition of MWNTs. A suspension containing 20 mg MWNT, SnS2, or MWNT@SnS2 and 10 mL water was prepared by ultrasonication for 2 h.
2.4 Sensor fabrication
A bare GCE with diameter of 3 mm was sequentially polished on micro-cloth pads using 0.1 and 0.05 μm alumina slurries until a mirror-like surface was achieved. Then, the GCE was thoroughly rinsed with water and consecutively sonicated for 1 min in ethanol/water (1:1, v/v) and water, the electrode was dried at room temperature.
CHIT solution was prepared by dissolving 100 mg CHIT in 10 mL aqueous acetic acid (1 wt%) at room temperature. Afterward, 1.0 mL of 15 mM BAP was mixed with 1 mL CHIT solution and 1 mL MWNT@SnS2 suspension by ultrasonic agitation for 30 min, followed by magnetic stirring for 1 h. A black and homogeneous solution of BAP–CHIT-MWNT@SnS2 was obtained. 4 μL of the solution was spread evenly on the surface of a GCE with a syringe and allowed to evaporate water in air to form BAP–CHIT-MWNT@SnS2/GCE. Finally, the MIP-MWNT@SnS2/GCE was obtained by immersing BAP–CHIT-MWNT@SnS2/GCE in absolute ethanol for 40 min to completely remove the BAP molecules, creating the specific-binding cavities. As a control, non-imprinted polymer sensor (NIP-MWNT@SnS2/GCE) was also fabricated using the same procedure mentioned above, but excluding BAP from the CHIT solution. In order to exhibit the unique properties of MWNT@SnS2, the imprinted CHIT film modified GCE (MIP/GCE) was also prepared. These electrodes were stored at room temperature before use.
2.5 Preparation and determination of vegetable and fruit samples
Vegetable samples (soybean sprout, mung bean sprout, potato, and tomato) and fruit samples (pear and apple) were obtained from the local markets. Five gram of each sample was homogenized and extracted with 25 mL ethyl alcohol with ultrasonic treatment for 30 min [32]. Then, the supernatants were taken, filtered through a 0.45 μm membrane, and finally concentrated to 5.0 mL. The obtained sample extracts were stored at 4 °C before being determined by this sensor and LC–MS method directly. The concentration of BAP in these samples could be obtained from standard addition method.
2.6 Electrochemical measurements
The cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were carried out in 2.0 mM K3Fe(CN)6 as a probe in 0.2 M PBS (pH 6.5). The cyclic voltammograms were recorded between −0.2 and 0.6 V at a scan rate of 100 mV s−1. The impedance measurements were carried out at a potential of 0.2 V over the frequency ranging from 0.1 Hz to 100 kHz, using an alternating voltage of 2 mV. The differential pulse voltammetry (DPV) measurements were performed in 0.2 M PBS (pH 6.5) containing 0.5 mM K3Fe(CN)6 in a potential range of −0.2 to −0.5 V, a amplitude of 50 mV, a pulse width of 0.2 s, and a pulse period of 0.5 s. All experiments were conducted at room temperature. All experiments were performed in sextuplicate, and curves were fitted by using the average values of all replicates.
3 Results and discussion
3.1 Characterization of MWNT@SnS2
The surface composition of the MWNT@SnS2 was analyzed by EDX. The result shown in Fig. 1a reveals that C, Sn, and S coexist in the product. According to the integral area of Sn–S, the atomic ratio of Sn/S is about 1:2.1, which is close to stoichiometric ratio of pure SnS2.
The crystalline phases of the as-prepared MWNT@SnS2 were confirmed with XRD (Fig. 1b). The strong peak locates at 2θ = 26.05° and a weak peak locates at 2θ = 42.77° are observed and, respectively, correspond to the (002) and (100) diffraction patterns of typical graphite [33], which indicate that the MWNT keeps well graphitization degree after the coverage of SnS2. Moreover, seven diffraction peaks are also observed at 2θ of 14.81°, 28.55°, 31.89°, 50.29°, 52.32°, 56.03°, and 58.79° corresponding to the (001), (100), (101), (110), (111), (103), and (201) reflections of the hexagonal crystalline SnS2 with lattice constants a = 3.649, c = 5.899 Å [34], which are in good agreement with the values in the standard card (JCPDS no. 23-0677). This product has a high purity because no impurity peaks are observed, which is in accordance with the EDX analysis.
Raman measurement is a simple and powerful technique for confirmation of the formation of MWNT@SnS2 structure. In the typical Raman spectrum of Fig. 1c, the peaks near 1350 and 1581 cm−1 are the D and G bands, respectively. The D band is related to disordered sp2-hybridized carbon atoms containing vacancies, or other symmetry-breaking defects. And the G band is related to graphite E 2g symmetry of the interlayer mode reflecting structural integrity of sp2-hybridized carbon atoms. Furthermore, the extent of carbon-containing defects can be evaluated by the intensity ratio of G band–D band (I G/I D) [35]. Therefore, HNO3 treatment results in high-purity MWNT by removing amorphous carbon and metallic impurities from the purchased MWNT. This is further testified by the presence of strong 2D band at about 2697 cm−1 in the MWNT@SnS2, because the 2D band is originated from the two-photon elastic scattering process, which is always used as an indication of the purity of carbon nanotube. In addition, a strong peak at 311 cm−1 corresponds to A1g mode that appears in the MWNT@SnS2 spectrum [36], indicating the formation of SnS2 on MWNT surface.
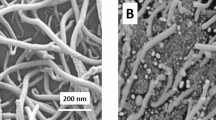
The morphology of the MWNT@SnS2 was observed using SEM. Figure 2a certainly indicates that the high-structured MWNT@SnS2 nanotubes form a dense network. The encapsulation of MWNT with SnS2 shell is clearly shown in Fig. 2b, which displays the good distribution of MWNT in the SnS2 matrix without any agglomeration. The SnS2 coating appears to be quite uniform, which may help to separate the MWNT in the final compound due to their large scale.
TEM micrographs of the acid-treated MWNT and MWNT@SnS2 are presented in Fig. 2c–f. Consistent with the manufacture provided data, the acid-treated MWNT (Fig. 2c) consist of long individual tubes with diameters ranging from 18 to 25 nm, up to micrometer lengths and smooth surface. Almost no trace of other carbon impurities is detected. After surface modification of MWNT with SnS2, the diameters of the MWNT increase to 54–61 nm (Fig. 2d), providing evidence that SnS2 is successfully loaded on the surface of MWNT. Moreover, the MWNT do not suffer any structure destruction after being modified with SnS2. Therefore, outstanding physicochemical properties of MWNT can be maintained after surface modification. The TEM image in Fig. 2e shows the SnS2 nanoflakes with about 14 nm are dispersed uniformly on the outer MWNT walls. The close interaction between SnS2 nanoflakes and MWNT in the contact interface can be found, which will be favorable to enhance the electron transfer rate of the sensor. Figure 2f displays the high-resolution TEM image of the top view of SnS2 nanoflakes coated on the MWNT surface. It can be seen that the interplanar distance of 0.32 nm is in agreement with the d-spacing of (100) planes of the hexagonal SnS2.
3.2 Electrochemical characterization of the MIP sensor
Cyclic voltammetric behaviors at bare GCE and different modified GCE were recorded in PBS solution (pH 6.5) containing 2.0 mM K3Fe(CN)6 to confirm the stepwise fabrication process of the MIP sensor, the results are shown in Fig. 3A. As observed in curve a, a couple of typical redox peaks attributing to K3Fe(CN)6 appear on the bare GCE, and the change of redox peak currents is used to investigate the change in electrochemical resistance to characterize the electrochemical behavior of MIP sensor. On a MWNT film modified GCE (curve b) and SnS2 film modified GCE (curve c), the redox peak currents of K3Fe(CN)6 have evident increase. The obvious increase in the redox peak currents demonstrates that an efficient catalytic reaction occurs in the MWNT and SnS2 film. After the surface of GCE is functionalized with core/shell-structured MWNT@SnS2, an increment of redox peak current is seen in curve d when compared with the values at the bare GCE, MWNT/GCE, and SnS2/GCE, which can be ascribed to the good electrical conductivity and high surface-to-volume ratio of MWNT@SnS2 film. Furthermore, the core/shell architecture maximizes the SnS2-support interaction through the three-dimensional contact between the SnS2 nanoflakes and the MWNT, thereby facilitating the electron transfer and thus increasing its rate. Curve e shows the cyclic voltammogram of BAP–CHIT-MWNT@SnS2/GCE in the same solution. The result indicates that the presence of BAP hinders K3Fe(CN)6 from getting access to the surface of GCE, so this modified electrode before template removal is almost non-conductive. After the removal of BAP molecules from BAP–CHIT-MWNT@SnS2 film through washing the modified electrode with ethanol, the MIP-MWNT@SnS2/GCE is obtained and its electrochemical behavior is studied and recorded as curve f. When compared with curve e, the redox peak currents shown in curve f enhance evidently and almost equal to the peak heights in curve d. The embedded BAP molecules that hinder the electron transfer of MIP-MWNT@SnS2/GCE are removed, which implies that lots of imprinted cavities are produced in the imprinted membrane. These cavities can be used as channels for the diffusion of [Fe(CN)6]3−/4− and enhance the redox reaction activity of [Fe(CN)6]3−/4− ion pair on the MIP sensor surface. In order to investigate the determination of BAP, the process of rebinding was conducted. The curve g in Fig. 3A shows the CV response of the MIP sensor after incubation for 12 min in the presence of BAP at 10 μM. The lower peak currents can be found through a comparison with curve f, attributing to the occupation of imprinted cavities by BAP molecules after rebinding step.
Cyclic voltammograms (A) and electrochemical impedance spectra (B) of the bare GCE (a), MWNT/GCE (b), SnS2/GCE (c), MWNT@SnS2/GCE (d), BAP–CHIT-MWNT@SnS2/GCE (e), MIP-MWNT@SnS2/GCE (f), and MIP-MWNT@SnS2/GCE after incubation in 10 μM BAP (g) in PBS (pH 6.5) containing 2.0 mM K3Fe(CN)6. Scan rate is 100 mV s−1
EIS was further used as an efficient tool to investigate the interface properties of modified electrodes. The Nyquist diagrams of various modified electrodes in the presence of 2.0 mM K3Fe(CN)6 in pH 6.5 PBS solution are shown in Fig. 3B. In electrochemical impedance measurements, the electron transfer resistance (R ct) during the modification process can be reflected by the semicircle diameter at high frequencies, and a linear part at low frequencies is relative to the diffusion control process. The simple electrical equivalent circuits in the inset of Fig. 3B are used to fit the impedance diagrams. As shown in curve a of Fig. 3B, the bare GCE displays a small semicircle (R ct = 327.6 Ω). In an attempt to find out the effect of core/shell structure on the catalytic activity of MWNT@SnS2, the EIS data obtained for MWNT/GCE (curve b), SnS2/GCE (curve c), and MWNT@SnS2/GCE (curve d) were compared. The results exhibit that R ct for MWNT/GCE and SnS2/GCE are 229.1 and 121.5 Ω, respectively, much lower than that for bare GCE. After modification of the GCE with MWNT@SnS2, an almost straight line in the Nyquist pot is displayed, which corresponds to the characteristic diffusion-limiting process known as Warburg element. The value of R ct for the MWNT@SnS2/GCE is calculated as 81.40 Ω, which means that the MWNT@SnS2 core/shell-structured nanocomposite has excellent electric conducting property. Subsequently, when the BAP-imprinted CHIT-MWNT@SnS2 film is coated on the GCE surface, as shown in curve e, the semicircle of the BAP–CHIT-MWNT@SnS2/GCE increases dramatically, and the value of R ct increases to 1776 Ω, indicating that the MIP film before the removal of BAP molecules forms a barrier on the surface of GCE which can block the electron transfer between [Fe(CN)6]3−/4− ion pair and the GCE. The electron transfer resistance of the MIP sensor reduces contrarily (R ct = 191.9 Ω) when the template BAP molecules are removed from the imprinted film (curve f), suggesting that the reserved specific recognition cavities after the removal of template molecules enhance the electron transfer significantly. When BAP molecules are selective rebound on the MIP membrane, as shown in curve g, the R ct (i.e., 568.9 Ω) is higher than that of the MIP-MWNT@SnS2/GCE. This can be attributed to the reason that the re-adsorbed BAP molecules on the surface of electrode hinder the electron transfer once again. The results are in accord with that obtained from the CV scanning in Fig. 3A.
3.3 Confirmation of BAP molecules in imprinted film
In order to confirm the existence of BAP molecules in imprinted films, DPV of BAP–CHIT/GCE, BAP–CHIT-MWNT@SnS2/GCE, MIP-MWNT@SnS2/GCE, and NIP-MWNT@SnS2/GCE were recorded in the PBS (pH 6.5) solution as a supporting electrolyte, respectively, as shown in Fig. 4. An oxidation peak at 0.98 V, which reveals the typical peak of BAP, can be seen clearly both in curves a and b for BAP–CHIT/GCE and BAP–CHIT-MWNT@SnS2/GCE, indicating that BAP molecules have been embedded into the imprinted membrane. As expected, the oxidation peak current in curve b increases significantly compared with curve a, attributing to the core/shell structure of MWNT@SnS2 nanomaterial, which maximizes the interaction through three-dimensional contact between the MWNT core and the SnS2 shell, therefore exhibiting excellent electrocatalytic effect and good conductivity for the electron transportation. Furthermore, the SnS2 shell offers protection to the MWNT core from coagulation and undoubtedly increases the effective electrode surface area for BAP binding. After immersing into absolute ethanol for 40 min, there is no peak observed for the MIP-MWNT@SnS2/GCE (curve c), suggesting that the BAP template molecules embedded in the MIP film can be totally removed by washing with absolute ethanol. In the case of NIP-MWNT@SnS2/GCE, no oxidation peak is observed (curve d). The results are ascribed to large amount of recognition cavities for BAP in the MIP-coated MWNT@SnS2, while NIP-coated MWNT@SnS2 has no recognition cavities for binding BAP. The non-printed films were expected to be less porous than the imprinted film, since the extracted template left more cavities and revealed the pores in the film. The porosity enhanced the diffusion of template into the film and could promote the binding within the film.
3.4 Optimization of the experimental parameters
The electrochemical activity of BAP templates is poor, so the “gate-controlled” method with K3Fe(CN)6 as the probe is used here to improve the determination sensitivity. After the extraction of BAP template molecules, the electron transportation of K3Fe(CN)6 can be realized easily using the imprinted cavities as channels. However, some cavities will be blocked by the adsorbed BAP molecules after the rebinding process. Consequently, the electrochemical redox signal derived from the K3Fe(CN)6 system can be controlled by using the extraction and rebinding of BAP template molecules as a switch. By the aid of the “gate-controlled” method, the redox current of K3Fe(CN)6 is indirectly related with BAP template concentration, and the magnified response is achieved. Herein, ΔI = I 0 − I c , I 0 and I c are the currents when the concentrations of BAP are 0 and c μM in MIP-MWNT@SnS2 film.
The influence of K3Fe(CN)6 solution with different pH values of 5.8–8.0 on the electrochemical behavior of MIP-MWNT@SnS2/GCE toward BAP (10 μM) was studied with DPV technique. As shown in Fig. 5a, a maximum electrochemical response current is observed at pH 6.5. Thus, pH 6.5 PBS was adopted in subsequent experiments.
The effect of MIP-MWNT@SnS2 amount was studied in the range of 2–6 μL (Fig. 5b). The electrochemical response current increases when the amount of MIP-MWNT@SnS2 changes from 2 to 4 μL. Further increase of MIP-MWNT@SnS2 on electrode surface leads to a gradual decrease of peak current. The imprinted amount of BAP molecules increases with the amount of MIP-MWNT@SnS2 increasing, and therefore more cavities are produced. But overmuch MIP-MWNT@SnS2 on electrode surface results in the increase of resistance and the reduction of electrochemical response current. Furthermore, the template molecules are difficult to be removed completely from the excessively thick MIP membrane. Therefore, 4 μL MIP-MWNT@SnS2 was a balanced point and was modified on the GCE surface.
Only if the template molecules are removed completely from the imprinted film, high sensitivity, selectivity, and reproducibility of the sensor can be obtained. Various eluents including methanol, ethanol, acetic acid, and their mixture were optimized for removing BAP from the imprinted film because H-bond is the main binding force that combines the imprinted film and BAP template molecules. The results reveal that the BAP molecules are prone to remove completely and the imprinted cavities can maintain integrity and stability when the ethanol is used as eluent. Therefore, absolute ethanol was chosen as the optimum eluent for BAP template removal. Furthermore, the extraction time was investigated (Fig. 5c). The value for I c gradually increases and reaches a maximum at about 40 min and then remains stable over 10 min, which means that the BAP template molecules are washed out at about 40 min in the absolute ethanol. As a result, an extraction time of 40 min was selected for all subsequent analysis.
The influence of incubation time on the electrochemical response current was also considered in the range of 2–16 min (Fig. 5d). It is found that the electrochemical response current increases gradually when extending incubation time from 2 to 12 min. Then it keeps almost unchanged, which indicates the readsorption of BAP is saturated. Thus, 12 min was chosen as incubation time so that the BAP solutions had enough time to reach saturated adsorption.
3.5 Selectivity, repeatability, reproducibility, and stability of the MIP-based electrochemical sensor
The selectivity of MIP-MWNT@SnS2/GCE was checked with some analogs, such as IAA, BOP, BG, KIN, MT, ROSC, OLO, and BOH were chosen as objective molecules to study their influence on this MIP-based electrochemical sensor. As shown in Fig. 6, 10-fold concentration of IAA, BOP, BG, KIN, MT, ROSC, OLO, and BOH hardly cause any evident change of peak current for the MIP-MWNT@SnS2/GCE (signal change <5 %), indicating a good selectivity of this MIP film for BAP.
The repeatability of this MIP sensor was investigated at a BAP concentration of 10 μM with the same MIP-MWNT@SnS2/GCE. The relative standard deviation (RSD) of 1.14 % for 10 DPV measurements is calculated, which indicates the satisfactory repeatability of the sensor. Additionally, 10 repeated MIP-MWNT@SnS2/GCEs were prepared under the same conditions, and the reproducibility was also studied after incubation of the MIP-MWNT@SnS2/GCE in 10 μM BAP. The RSD was calculated to be 2.42 %, implying acceptable interelectrode reproducibility. In order to evaluate the stability of this MIP sensor, it was stored at room temperature over 20 days, and no significant change of peak current was observed in the same experimental condition. It retained 96.8 % of its original response. Moreover, the response of MIP film for BAP hardly changed after 5 min of ultrasonication. All measurements show the excellent stability of this MIP sensor.
3.6 Electrochemical detection of BAP
Under the optimized conditions for the proposed MIP sensor, differential pulse voltammograms of BAP with different concentrations were recorded at the MIP-MWNT@SnS2/GCE. Figure 7 shows the dependence of the electrochemical response on the concentrations of BAP. Owing to the occupation of imprinted cavities by BAP template molecules, the I c decreases with the increasing BAP concentrations, and inclines to be stable at high concentration of BAP. The inset of Fig. 7 displays that the ΔI is linear with logarithm of BAP concentration over a range from 0.1 nM to 10 mM with the regression equation: ΔI (μA) = 8.007 + 1.351 logc (unit of c, μM) (r 2 = 0.996). The detection limit is 50 pM based on the 3S b/S rules [37], where S b represents the standard deviation of the peak currents of the bland solution (n = 6) and S represents the slope of the calibration curve for BAP. The electrochemical methods for the determination of BAP have been summarized in Table 1. Compared with the other electrochemical sensors for the assay of BAP, the excellent sensitivity and wide linear range are observed for this proposed sensor.
Differential pulse voltammograms of 0.5 mM K3Fe(CN)6 in pH 6.5 PBS at the MIP-MWNT@SnS2/GCE after interaction with different concentrations of BAP solution. Concentration of BAP (from a to n): 0, 0.1 nM, 1.0 nM, 5.0 nM, 0.1 μM, 1.0 μM, 5.0 μM, 10 μM, 50 μM, 0.1 mM, 0.5 mM, 1.0 mM, 5.0 mM, and 10 mM. Other conditions are as in Fig. 4
3.7 Determination of BAP in real samples
In order to study the practical applicability of the proposed MIP sensor, the content of BAP in vegetable and fruit samples including soybean sprout, mung bean sprout, potato, tomato, pear, and apple was analyzed, and the results are shown in Table 2. BAP in different samples was detected with the MIP-MWNT@SnS2/GCE based on standard addition method. Each sample was determined for six times and the RSD was not higher than 3 %, revealing good precision. The results were also compared using LC–MS and are also tabulated in Table 2. The results show that the two methods are in good agreement and the MIP-MWNT@SnS2/GCE can be used for accurate determination of BAP in these samples. Otherwise, the average recovery of the MIP-MWNT@SnS2/GCE toward BAP in these samples ranges from 91.0 to 103 %, validating the good recovery and practicability of the developed MIP sensor.
4 Conclusions
In conclusion, the development of a simple molecular imprinting procedure to prepare a BAP-imprinted voltammetric sensor based on a GCE modified with molecularly imprinted CHIT-MWNT@SnS2 composite film was described here. Due to the excellent electrocatalytic activity and high conductivity of MWNT@SnS2 and the selective recognition of CHIT with BAP, the prepared sensor exhibited enhanced response for the electrochemical oxidation of BAP. This sensing platform not only showed significant improved sensitivity by the strategy of gate-controlled electrocatalytic effect, but also offered the advantages of convenience, long-term stability, and efficient assay in target detection, which were essential for the BAP detection in food safety monitoring. Moreover, this strategy was employed to detect BAP in vegetable and fruit samples successfully, which could further be expected to be used in fabrication of various MIP-based electrochemical sensors by replacing BAP with other template molecules for chemical/biological detections.
References
Bui MPN, Seo SS (2015) Electrochemical analysis of parathion-ethyl using zirconium oxide–laponite nanocomposites-modified glassy carbon electrode. J Appl Electrochem 45:365–373. doi:10.1007/s10800-015-0789-0
Barclay GF, McDavid CR (1998) Effect of benzylaminopurine on fruit set and seed development in pigeonpea. Sci Hortic Amst 72:81–86. doi:10.1016/S0304-4238(97)00110-6
Li X, Chen X, Yao X, Jin M, Jiang J (2005) Determination of 6-benzylaminopurine in bean-sprout by high performance liquid chromatography. Chin J Health Lab Technol 15:149–151. doi:10.3969/j.issn.1004-8685.2005.02.010
Zhang YL, Liu H, Wang SL, Ding T, Xu NS, Yu KY, Shen WJ, Zhao ZY, Liu Y, Wu B, Zhang R, Shen CY (2015) Simultaneous determination of eight kinds of drug residues in sprouts by high performance liquid chromatography–tandem mass spectrometry. J Instrum Anal 34:164–170. doi:10.3969/j.ssn.1004-4957.2015.02.007
Qiao M, Liu SP, Liu ZF, Yang JD, Zhu JH, Hu XL (2014) Overlapping of second order scattering and frequency double scattering spectra method and resonance Rayleigh scattering method for the determination of 6-benzyladenine in bean sprout. Food Anal Methods 7:1737–1744. doi:10.1007/s12161-014-9812-z
Wu PG, Tan Y, Zhang J, Wang LY, Tang J, Jiang W, Pan XD, Ma BJ, Ni ZN, Wang TJ (2014) Determination of 10 plant growth regulators in bean sprouts by sequential cleaning-gas chromatography–mass spectrometry. Chin J Anal Chem 42:866–871. doi:10.3724/SP.J.1096.2014.40105
Zhao XH, Feng XM, Zhu QH, Yang Q, Li XY, Zhang J (2014) Determination of kinetin and 6-benzylaminopurine in fruits and vegetables based on l-Phe fluorescence quenching. Jiangsu J Agric Sci 30:880–884. doi:10.969/j.ssn.1000-4440.2014.04.030
Lee S, Kim GY, Moon JH (2013) Detection of 6-benzylaminopurine plant growth regulator in bean sprouts using OFRR biosensor and QuEChERS method. Anal Methods 5:961–966. doi:10.1039/c2ay26136g
Zhang P, Zheng DW, Liu J, Zhong RG, Liu CW, Guo X, Wang H (2012) Rapid detection of 6-benzylaminopurine residues in sprout beans by surface-enhanced Raman spectroscopy. Spectrosc Spectr Anal 32:1266–1269. doi:10.3964/j.issn.1000-0593(2012)05-1266-04
Assuncao NA, Arruda SCC, Martinelli AP, Carrilho E (2009) Direct determination of plant-growth related metabolites by capillary electrophoresis with spectrophotometric UV detection. J Braz Chem Soc 20:183–187. doi:10.1590/S0103-50532009000100027
Zhao G, Liu KZ, Lin S, Liang J, Guo XY, Zhang ZJ (2003) Application of a carbon nanotube modified electrode in anodic stripping voltammetry for determination of trace amounts of 6-benzylaminopurine. Microchim Acta 143:255–260. doi:10.1007/s00604-003-0078-1
Li CY (2006) Electrochemical determination of 6-benzylaminopurine (6-BAP) using a single-wall carbon nanotube-dicetyl phosphate film coated glassy carbon electrode. Bull Korean Chem Soc 27:991–994. doi:10.5012/bkcs.2006.27.7.991
Sun D, Zhang HJ (2006) Voltammetric determination of 6-benzylaminopurine (6-BAP) using an acetylene black–dihexadecyl hydrogen phosphate composite film coated glassy carbon electrode. Anal Chim Acta 557:64–69. doi:10.1016/j.aca.2005.10.002
Zhang YF, Bo XJ, Guo LP (2012) Electrochemical behavior of 6-benzylaminopurine and its detection based on Pt/ordered mesoporous carbons modified electrode. Anal Methods 4:736–741. doi:10.1039/C2AY05712C
Rahman LU, Shah A, Khan SB, Asiri AM, Hussain H, Han C, Qureshi R, Ashiq MN, Zia MA, Ishaq M, Kraatz HB (2015) Synthesis, characterization, and application of Au–Ag alloy nanoparticles for the sensing of an environmental toxin, pyrene. J Appl Electrochem 45:463–472. doi:10.1007/s10800-015-0807-2
Rahman LU, Shah A, Qureshi R, Khan SB, Asiri AM, Shah AUHA, Ishaq M, Khan MS, Lunsford SK, Zia MA (2015) Spectroscopic analysis of Au–Cu alloy nanoparticles of various compositions synthesized by a chemical reduction method. Adv Mater Sci Eng. doi:10.1155/2015/638629
Rahman LU, Shah A, Lunsford SK, Han C, Nadagouda MN, Sahle-Demessie E, Qureshi R, Khan MS, Kraatz HB, Dionysiou DD (2015) Monitoring of 2-butanone using a Ag–Cu bimetallic alloy nanoscale electrochemical sensor. RSC Adv 5:44427–44434. doi:10.1039/c5ra03633j
Huang JD, Zhang XM, Liu S, Lin Q, He XR, Xing XR, Lian WJ (2011) Electrochemical sensor for bisphenol A detection based on molecularly imprinted polymers and gold nanoparticles. J Appl Electrochem 41:1323–1328. doi:10.1007/s10800-011-0350-8
Wang YY, Ya Y, Tan XH, Huang KJ, Li CY (2010) Investigation on electrochemical preparation of methyl parathion imprinted film modified electrode and its application. J Xinyang Norm Univ (Nat Sci Ed) 23:566–570. doi:10.3969/j.issn.1003-0972.2014.01.018
Ye J, Chen Y, Liu Z (2014) A boronate affinity sandwich assay: an appealing alternative to immunoassays for the determination of glycoproteins. Angew Chem Int Ed 53:10386–10389. doi:10.1002/anie.201405525
Zhang CJ, Si SH, Yang ZP (2015) Design of molecularly imprinted TiO2/carbon aerogel electrode for the photoelectrochemical determination of atrazine. Sens Actuators B 211:206–212. doi:10.1016/j.snb.2015.01.079
Moreira FTC, Sharma S, Dutra RAF, Noronha JPC, Cass AEG, Sales MGF (2015) Detection of cardiac biomarker proteins using a disposable based on a molecularly imprinted polymer grafted onto graphite. Microchim Acta 182:975–983. doi:10.1007/s00604-014-1409-0
Justino CIL, Freitas AC, Pereira R, Duarte AC, Santos TAPR (2015) Recent developments in recognition elements for chemical sensors and biosensors. Trends Anal Chem 68:2–17. doi:10.1016/j.trac.2015.03.006
Liu YX, Wang WW, Wei H, Li JW, Lu XQ, Liu XH (2014) Simultaneous determination of dihydroxybenzene isomers based on thionine functionalized multiwall carbon nanotubes modified electrode. J Appl Electrochem 44:667–674. doi:10.1007/s10800-014-0674-2
Inam R, Bilgin C (2013) Square wave voltammetric determination of methiocarb insecticide based on multiwall carbon nanotube paste electrode. J Appl Electrochem 43:425–432. doi:10.1007/s10800-013-0526-5
Xu SJ, Zhang SM, Zhang JX, Tan T, Liu Y (2014) A maize-like FePO4@MCNT nanowire composite for sodium-ion batteries via a microemulsion technique. J Mater Chem A 2:7221–7228. doi:10.1039/c4ta00239c
Liu ZX, Deng HQ, Mukherjee PP (2015) Evaluating pristine and modified SnS2 as a lithium-ion battery anode: a first-principles study. ACS Appl Mater Interfaces 7:4000–4009. doi:10.1021/am5068707
Zhong HX, Zhou P, Yue L, Tang DP, Zhang LZ (2014) Micro/nano-structured SnS2 negative electrodes using chitosan derivatives as water-soluble binders for Li-ion batteries. J Appl Electrochem 44:45–51. doi:10.1007/s10800-013-0590-x
Li J, Yang ZJ, Tang Y, Zhang YC, Hu XY (2013) Carbon nanotubes–nanoflake-like SnS2 nanocomposite for direct electrochemistry of glucose oxidase and glucose sensing. Biosens Bioelectron 41:698–703. doi:10.1016/j.bios.2012.09.059
Wang Z, Luan D, Madhavi S, Hu Y, Lou XW (2012) Assembling carbon-coated α-Fe2O3 hollow nanohorns on the CNT backbone for superior lithium storage capability. Energy Environ Sci 5:5252–5256. doi:10.1039/C1EE02831F
Sun HY, Ahmad M, Luo J, Shi YY, Shen WC, Zhu J (2014) SnS2 nanoflakes decorated multiwalled carbon nanotubes as high performance anode materials for lithium-ion batteries. Mater Res Bull 49:319–324. doi:10.1016/j.materresbull.2013.09.005
Jin MC, Ren YP, Chen XH (2007) Determination of 6-benzyladenine in bean sprout by LC–ESI-IT–MS-MS. Chromatographia 66:407–410. doi:10.1365/s10337-007-0311-x
Yang L, Yan XP, Wang QW, Wang Q, Xia H (2015) One-pot catalytic conversion of cellulose into polyols with Pt/CNTs catalysts. Carbohydr Res 404:87–92. doi:10.1016/j.carres.2014.12.001
Liu H, Su Y, Chen P, Wang Y (2013) Microwave-assisted solvothermal synthesis of 3D carnation-like SnS2 nanostructures with high visible light photocatalytic activity. J Mol Catal A 378:285–292. doi:10.1016/j.molcata.2013.06.021
Gong J, Liu J, Jiang ZW, Wen X, Mijowska E, Tang T, Chen XC (2015) A facile approach to prepare porous cup-stacked carbon nanotube with high performance in adsorption of methylene blue. J Colloid Interface Sci 445:195–204. doi:10.1016/j.jcis.2014.12.078
Hai B, Tang KB, Wang CR, An CH, Yang Q, Shen GZ, Qian YT (2001) Synthesis of SnS2 nanocrystals via a solvothermal process. J Cryst Growth 225:92–95. doi:10.1016/S0022-0248(01)01030-2
Wang Q, Paim LL, Zhang XH, Wang SF, Stradiotto NR (2014) An electrochemical sensor for reducing sugars based on a glassy carbon electrode modified with electropolymerized molecularly imprinted poly-ophenylenediamine film. Electroanalysis 26:1612–1622. doi:10.1002/elan.201400114
Acknowledgments
The authors gratefully acknowledge the financial support from the National Science Foundation of China (No. 61201091), the Program for University Key Scientific Research of Henan (No. 15A150025), the Program for University Innovative Research Team of Henan (No. 15IRTSTHN001), and the Key Scientific and Technological Project of Henan (No. 152102210341). The Center of Analysis and Testing of Xinyang Normal University was also acknowledged for its help in the EDX, SEM, and TEM observation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gan, T., Lv, Z., Sun, Y. et al. Highly sensitive and molecular selective electrochemical sensing of 6-benzylaminopurine with multiwall carbon nanotube@SnS2-assisted signal amplification. J Appl Electrochem 46, 389–401 (2016). https://doi.org/10.1007/s10800-016-0923-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-0923-7