Abstract
Cyclic voltammogram of methiocarb in 0.1 M H2SO4 exhibited an irreversible anodic peak at about +1285 mV versus Ag/AgCl. Electro-oxidation and determination of methiocarb in spiked soil, river water and agrochemical formulation were realized on a newly prepared carbon-nanotube paste electrode by applying square wave voltammetry (SWV). The dE p /dpH value indicated that the oxidation mechanism involved the coupling of H+ with the oxidation process. The peak signals were linearly related to methiocarb concentration in the range of 1.5–59.1 mgL−1 with a detection limit of 0.45 mgL−1. The accuracy and selectivity of the proposed method were shown by calculating the recoveries of methiocarb from soil, river water and pesticide formulation Mesurol®. The calculated percent recoveries for soil and river water samples spiked with 30.0 μg g−1 and 40.0 μg mL−1 levels were 99.3 ± 1.2 and 98.5 ± 0.3 at 95 % confidence limit, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There are a number of carbamate insecticides formed from carbamic acid, and their poisonous effects and actions on pest are different. Those types of insecticides are applied as either sprays or allurements to kill insects by damaging their nervous systems and brains. Due to their broad biological activities, these insecticides are used massively worldwide [1]. Carbamate insecticides are used on crops or to kill ants, fleas, crickets, scale, whitefly, lace bugs, cockroaches, aphids, and mealy bugs. Carbaryl was introduced in 1956 as a first successful carbamate. A cockroach has developed resistance to organophosphates, but as an alternative, Propoxur is highly effective. Bendiocarb has generally been used as a household, turf, and foliage plant insecticide. As an insecticide, acaricide, and molluscicide, methiocarb is a highly important N-methylcarbamate pesticide and used worldwide in agriculture and health programs [2]. Methomyl is usually applied as adult fly bait and used to fight slugs, snails, spider mites, and insects on lawns, turf, and foliage plants around building gardens. As with other carbamate pesticides, methiocarb exterminates insects by affecting the activity of acetylcholinesterase enzyme in the nervous system. Methiocarb has been proved to be highly toxic (the highest of four levels) [3] to birds by the acute oral route of exposure, with most LD50 s in the 5–15 mg/kg range. In plants and animals, the metabolism occurs by carbamate ester cleavage and oxidation to sulfoxides and sulfones. At neutral pH, the half-life is nearly a month, extending to approximately a year under acidic action (pH 4–5) but going to hours in basic solution (pH 9) [4].
Chromatographic methods have been usually used for methiocarb detection. A variety of chromatographic detection methods, including liquid chromatography/electrospray ionization mass spectrometry (LC/ESI–MS) [ 5–7], high-performance liquid chromatography (HPLC) with UV-diode array (DAD) [8, 9], chemiluminescence (HPLC-CL) [10] or fluorescence(HPLC/FL) detection [1, 11, 12], and gas chromatography–mass spectrometry (GC–MS) [13] are the preferred methods of choice for carbamate insecticide analysis. Despite the use of electroanalytical methods for the determination of pesticides in general [14–18], only a few studies are available for the electrochemical investigation of carbamate pesticides [19–21], and, as far as we know, no work dealing with electro-oxidation behavior of methiocarb and its detection using carbon-nanotube electrode appeared so far. However, cholinesterase sensors modified with processed polyaniline have been developed for methiocarb detection, but it depends on potentiometric measurement rather than voltammetric investigation. The detection limit for cholinesterase sensor was found to be 0.08 mg L−1, but linearity range was limited with 0.2–5.7 mg L−1[22]. In another study, differential pulse adsorptive stripping voltammetric method was proposed for quantification of methiocarb with a detection limit of 1.0 × 10−8 M, but it was based on the reduction of the molecule on mercury electrode, and therefore the possibility of interfering effects should also be considered because of the ability of reducible behavior of many trace elements on mercury electrode[23].
Voltammetric methods have some advantages compared with the chromatography, i. e., their low cost and the possibility of analysis without extraction or pre-concentration, as well as the short time required for analysis. In this study, electrochemical oxidation of methiocarb was investigated using newly prepared carbon nanotube paste electrode (CNTPE) by applying square wave voltammetry (SWV). This method was successfully applied for its determination in spiked soil and river water samples. Carbon nanotubes are desirable materials owing to their extraordinary mechanical and electrical properties such as electrical conductivity, mechanical strength, and a wide operational potential range. The aim of the present study is to display oxidation behavior of methiocarb and to build the optimum conditions for its determination in soil, water, and commercial samples.
2 Experimental
2.1 Instrumentation
The voltammograms were recorded with a Bioanalytical Systems- Epsilon potentiostat/galvanostat (BAS, West Lafayette, IN, USA) analyzer connected with a BAS-C3 cell stand. A three-electrode system was used including a platinum counter electrode, Ag/AgCl (3 M NaCl) reference electrode, and multiwall carbon nanotube paste electrode (MWCNTPE). All experiments were carried out at room temperature. A Hanna HI 8521 model (Hanna Instruments, Singapore) pH meter with combined glass electrode was used to measure pH of all the solutions. Agilent 1100 HPLC system (Agilent Technologies, USA) coupled with a quaternary pump, a Rheodyne injector equipped with a 20-mL sample loop, 150 mm Zorbax Eclipse XDB C18 (150 mm × 4.6 mm, id, 5 mm) column, a model of L-7455 diode array, and multiple wavelength UV–vis detector (200 nm) controlled by Agilent Chem workstation was used for chromatographic studies. To prepare carbon nanotube paste electrode, multiwall carbon nano tube powder (Sigma Aldrich, Inc) was mixed with mineral oil (0.15–0.85 mass ratios). The homogenized mixture was then mixed, and the paste was inserted in a plastic syringe needle using a 3-mm diameter copper wire that was connected to the system.
2.2 Reagents
Methiocarb (99 % purity) was obtained from Bayer crop science. Agrochemical formulation Mesurol® (equivalent to 50.0 % m/m of methiocarb) was supplied by Bayer crop science in Turkey. Methiocarb stock solutions (500 mg/L) were daily prepared by dissolving 0.0050 g methiocarb in 5.0 mL acetonitrile and diluting up to 10.0 mL with water. This solution was kept in the dark refrigerator when not in use. A series of Britton–Robinson buffer solutions were prepared from the mixed acids of 0.04 mol L−1 acetic, orthophosphoric, and boric acids. Dilute solutions were freshly prepared in volumetric flasks from stock solutions upon completion of final volume of with double-distilled water. The chemicals used in this study were of analytical reagent grade and used without further purification.
2.3 Procedure
An accurate volume of 10.0 mL of the 0.1 M H2SO4 was transferred to the voltammetric cell. Afterward, the electrodes were put in the solutions through which pure nitrogen gas was passed for 15 min before obtaining the voltammograms. After recording the voltammogram of the blank solution, an accurate concentration of the methiocarb solution was added. The accumulation potentials from −0.00 mV to +400 mV were applied during the accumulation periods from 0.0 to 90 s under stirring at 400 rpm. The stirring is stopped, and after waiting for 5 s equilibrium period, the square wave voltammogram of methiocarb was obtained by making a positive potential scan. The peak intensities quietly depend on the square wave voltammetric parameters. To achieve the maximum amount of square wave anodic peak, frequency (f), amplitude (∆E), and staircase step potential (∆E s) parameters have been optimized. The oxidation peak currents were then obtained under different pHs, pulse amplitude, frequency, step potential, accumulation potentials, and times. Peak currents were increased with pulse amplitude up to 100 mV, but the base-line current also increased. The peak intensity initially increased with frequency from 25 to 100 Hz, and then became distorted and ill-defined. The peak response increased linearly with the step potential up to 5 mV. The maximum peak intensity was obtained with an accumulation potential of +400 mV and gradually decreased with further applications. The influence of accumulation time (t acc) was maximum at 60 s and did not change with further increasing due to the saturation of electrode surface. The optimum conditions selected for the SW voltammetric determination of methiocarb were: 0.1 M H2SO4 as supporting electrolyte, ΔE s = 5 mV, f = 100 Hz, ΔE = −50 mV, t acc = 60 s, and E acc = +400 mV owing to the best current responses.
For the determination of methiocarb in soil, 5.0 g ground and dried soil samples were spiked with 4–8 mL of stock methiocarb (500 mL−1) solution and subsequently diluted to 20.0 mL. After homogenizing the samples, they were put into a shaker consisting of a temperature-controlled water-bath, shaken for 12 h at 20 °C, and centrifuged for 10 min at 3,000 rpm, and then extracted with 20 mL of 1:1 acetonitrile–water solutions. One milliliter of aliquots from the supernatant was added to 9.0 mL of 0.10 M H2SO4 in the voltammetric cell. The pesticide determination was performed successfully from the peak current generated at +1,265 mV with successively standard methiocarb additions. For the application of water samples, an aliquot of river water sample was fortified with methiocarb to achieve a final concentration of 20–40 μg mL−1. Toward this goal, 1.0 mL of river water samples was spiked with 5 mL of 500.0 μg mL−1 stock methiocarb solution. The sample solutions in the voltammetric cell were deoxygenated with a high purity of nitrogen (99.999 %) for 300 s before saving voltammograms. The optimized accumulation potential of +400 mV was applied for the electro-deposition process during the accumulation period (t acc = 60 s) and under the nitrogen atmosphere. Five seconds of equilibrating time was applied just after stopping the stirring process. A potential scan, hereafter, from +400 to +2,000 mV, was performed by using square wave voltammetry (Osteryoung version). For the determination of commercial insecticide, Mesurol® WP 50 solution equivalent to 500 μg mL−1 methiocarb was precisely prepared in a 10.0 mL of acetonitryl (50 %) and sonicated 5 min. Peak responses were recorded at +1265 mV after 0.2 mL of 500 μg mL−1 of an aliquot of this clear supernatant liquor was put into 10.0 mL 0.1 M H2SO4 in the voltammetric cell. The methiocarb in insecticide formulation was quantified by the standard addition procedure.
3 Results and discussion
3.1 Cyclic voltammetry
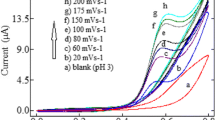
Voltammetric techniques (especially cyclic voltammetry) are the most convenient ones for clarifying the oxidation–reduction behavior of the organic compounds. Therefore, cyclic voltammetric records resulting from the oxidation–reduction properties of electrochemically active compounds might have marker effects on the comprehension of the redox mechanism. In order to expose the oxidation process of methiocarb on the carbon nanotube paste electrode (CNTPE), cyclic voltammetric behavior of 25 μg mL−1 methiocarb was evaluated with several scan rates (Fig. 1). As shown in Fig. 1, cyclic voltammograms obtained in 0.1 M H2SO4 exhibit an anodic peak at about +1285 mV(Ag/AgCl, 3 M NaCl), whereas no peak on the cathodic way. This voltammetric process proves that the particular oxidation peak of methiocarb is irreversible.
Potential scan rate studies are also decisive to understand whether the electrode process is diffusion or adsorption controlled. The logarithm of oxidation peak currents versus scan rates (from 25 to 1,600 mVs−1) showed linear relationship with a slope of 0.34. This value is very close to the theoretical one of 0.5 which has been accepted for an ideal diffusion-controlled electrode process
On the other hand, the peak potentials were shifted to more positive ways (vs Ag/AgCl, 3 M NaCl) with the increments of scan rate from 25 to 1,600 mV s−1, which approved the irreversibility of the oxidation reaction (Fig. 1, inset).
3.2 Effect of pH
The effect of pH was investigated in detail, because the pH of the electrolysis medium affects not only the appearance of voltammograms, peak potentials, and currents, but also gives useful information about the electrode reaction mechanisms. The SW voltammograms of 10 μg mL−1 methiocarb at the CNTPE over a wide range of pH (1.0–10) had a single well-defined oxidation peak (Fig. 2). The pH effect on this peak showed two linear segments. The oxidation peak was first significantly shifted to more positive potentials with a slope of 35.0 mV/pH in the pH range of 1.0–3.0, and it was then shifted to less positive potentials with a slope of 13.0 mV/pH in the pH range of 3.0–10.0. The two linear segments depending on pH can be stated by the following linear equations:
The pH effect revealed that the oxidation peak was most probably due to the oxidation of the protonated form of the thiomethyl group in the insecticide. The effects of pH on the methiocarb peak currents were also appraised to achieve the optimum pH for highest sensitivity. Accordingly, 0.1 M H2SO4 was selected as an optimum pH for analytical determination of methiocarb.
The number of electrons included in the electrode reaction could be determined from the semi-differentiation of a voltammogram [24]. For the diffusion-controlled reaction, the peak half width is W 1/2 = 3.52 RT/nF, where R, T, n, and F are commonly accepted designations. The peak half width for 10 μg mL−1 methiocarb at 25 °C was measured as 53.6 mV. By using this equation, the number of electrons transferred per mole of methiocarb was nearly two. Square wave frequency (f) determines the intensity of the peak current and therefore affects the sensitivity of the technique. Furthermore, the peak potential should vary linearly with the logarithm of the frequency [25]
where α and n are the transfer coefficient and number of electrons involved in the above equation, respectively. Figure 3 displays the dependence of E p with log f. When the slope of the straight line was equated to 2.303 RT/nα, αn and α values were obtained as 1.08 and 0.54, respectively. This behavior also explains the irreversibility of the electrode process.
The δE p/δpH value (35 mV/pH) over the pH range studied also indicates that the oxidation process involves the coupling of H+. In other words, rate determining step includes protonation, and proton transfer precedes the electron transfer [26]. The electro oxidation was catalyzed with protons by a proposed mechanism illustrated in Scheme 1. The electroactivity of methiocarb in sulfuric acid was attributed to anodic oxidation of the thiomethyl group, resulting in methiocarb sulfoxide.
3.3 Analytical applications
Quantitative evaluation was carried out from the linear relationship between the peak current and concentration. The best peak response for the oxidation process was achieved using the previously optimized experimental conditions of pH ≈ 1.0 (H2SO4), accumulation potential 400 mV, accumulation time 60 s, frequency 60 Hz, pulse amplitude 50 mV, and step potential 5 mV. Square wave voltammograms of methiocarb showed that the peak current increased linearly on increasing the insecticide concentration in the range of 1.5 and 59.1 mgL−1 (Fig. 4) with an analytical equation given by:
SWS voltammograms for the calibration graph of methiocarb at CNTPE. (pH ≈ 1.0 H2SO4; E s = 5 mV; f = 100 Hz; ∆E = 50 mV; t acc. = 60 s; E acc = + 400.0 mV) (a) 0.1 M H2SO4 and (b) 1.50 μg mL−1, (c) 2.98 μg mL−1, (d) 4.46 μg mL−1, (e) 9.80 μg mL−1, (f) 15.50 μg mL−1, (g) 21.99 μg mL−1, (h) 30.08 μg mL−1, (i) 38.75 μg mL−1, (j) 51.17 μg mL−1, and (k) 59.08 μg mL−1 of methiocarb
The detection limit (DL) and quantification limit (QL) were calculated using the following equations from IUPAC [27].
where S b is the standard deviation of the current measured for the blank solution, and m is the slope of the calibration curve. The calculated values of DL and QL were 0.45 and 1.5 mg L−1, respectively. The reproducibility was acquired from five different measurements of the 1.50 mg L−1 methiocarb with a relative standard deviation of 2.07 %. Analytical performance data were summarized in Table 1. These values show quite good precision, accuracy, and repeatability.
3.4 Selectivity
Some interfering effects from the species present in the samples such as soil or environmental water could be taken into account before the determination of target molecule. The interfering species were selected on the basis of criteria such as their usage in agriculture or presence in environmental samples. The co-existing ions such as Ni2+, Co2+, Cu2+, Mn2+, Zn2+, Ca2+, Pb2+, Mg2+, K+, Cr2O7 2−, and Br− were taken as one or twofold mass excess of methiocarb (10.0 μg mL−1), and they did not show serious interfering effects on the determination of methiocarb. The influence of some other pesticides [e.g., phenyl methyl carbamate insecticides (propoxur and diaxacarb), anilide fungicides(carboxin), and pyramidinylsulfonylurea herbicides(halosulfuron)] falling to the same classification with methiocarb were also examined (Table 2). Propoxur, diaxacarb, and halosulfuron did not generate an oxidation signal, but carboxin gave an oxidation peak at about +1,000 mV. Hence, with the exception of relatively excess amount of carboxin, the other pesticides did not significantly interfere with the methiocarb signal. The recovery of methiocarb in the presence of co-existing species indicated that the degree of the peak current did not deviate by more than ±5 %, confirming that the developed SWV method is free from serious interferences and can therefore be regarded as a selective method.
3.5 Determination of methiocarb in soil and river water
Accuracy of the proposed method was shown by the recoveries of methiocarb from soil and river water as well as agrochemical pesticide formulation Mesurol®. Therefore, following the procedure given in the experimental sections, pesticide determination was performed successfully from the peak current generated at +1,265 mV with successively standard methiocab additions (Fig. 5).
Determination of methiocarb in soil. (a) 9.0 mL 0.1 M H2SO4 + 1.0 mL soil aliquot and (b) 1.50 μg mL−1, (c) 2.98 μg mL−1, (d) 4.46 μg mL−1 (e) 9.80 μg mL−1, (f) 19.23 μg mL−1 (g) 28.30 μg mL−1, (h) 37.04 μg mL−1 (i) 53.57 μg mL−1 methiocarb. (pH ≈ 1.0 H2SO4; E s = 5 mV; f = 100 Hz; ∆E = 50 mV; t acc = 60 s; E acc = + 400.0 mV)
The recovery values for the spiked soil and river water samples with some selected concentrations were introduced in Table 3. The values obtained from soil and river water samples spiked with 30.0 μg g−1 and 40.0 μg mL−1 levels were 29.8 ± 0.9 μg g−1 and 39.4 ± 0.3 μg mL−1 at 95 % confidence level, respectively. The percent recoveries were calculated as 99.3 ± 1.2 and 98.5 ± 0.3 % with relative standard deviations of 1.2 and 0.3 %, respectively. Very high recoveries and low relative standard deviations reflect reasonable accuracy and precision.
3.6 Determination of methiocarb in pesticide formulation
The adequacy of the recommended methods was estimated by quantifying methiocarb in commercial insecticide Mesurol® WP formulation containing 50 % methiocarb by mass. For this purpose, Mesurol® WP 50 solution equivalent to 500 μg mL−1 methiocarb was precisely prepared in 10.0 mL of acetonitryl (50 %) and sonicated 5 min. Peak responses were recorded at +1,265 mV after 0.2 mL of 500 μg mL−1 of an aliquot of this clear supernatant liquor was put into 10.0 mL 0.1 M H2SO4 in the voltammetric cell. The methiocarb in insecticide formulation was quantified by the standard addition procedure. The recommended SWV method can be confidently used for the direct determination of methiocarb either in commercial formulation or natural samples using the correspondent calibration equation, without extraction or filtration step, but just dissolution and a suitable dilution of the analyte compound present in the solution of Mesurol® WP 50. As shown in Table 4, the recommended method was agreeably applied for the evaluation of methiocarb in its commercial dosage form. The accuracy of the method has been evaluated by recovering consciously added methiocarb from the previously labeled insecticide formulation Mesurol® WP using the stated experimental procedure.
The developed method was validated, and results are given in Table 4. These data introduce an average methiocarb content of 50.51 ± 3.52 (n = 3) % for SWSV, rather close to 50 % value specified by the manufacturer (Table 4). After statistical calculations, the results were compared with those obtained by HPLC [(50.64 ± 0.43) %; (n = 5)] using Student’s t distribution and variance ratio F test. Regarding the accuracy and precision, statistical results of both methods indicated no significant difference. The proof of this is that , at 95 % confidence level, the experimentally obtained values of t and F do not exceed the theoretical ones (t = 0.87 < t theoretical = 2.31, and F = 16.6 < F theoretical = 19.3, respectively). Thus, in terms of accuracy and precision, the results obtained with both techniques were found to be compatible.
4 Conclusions
A novel voltammetric method involving SWV at newly prepared multiwall carbon nanotube electrode was recommended to determine methiocarb content in water, soil, and insecticide formulation. The proposed square wave voltammetric method enables simple, less influence of the matrix effect, rapid, selective, and accurate analysis of methiocarb in insecticide formulation and natural samples. The most important advantage of this method is the possibility to determine the active ingredient of the pesticide from the commercial and natural samples without the need for any time-consuming and polluting pre-processing steps such as extraction, cleanup, derivatization, or pre-concentration.
References
Li HP, Li JH, Li GC, Jen JF (2004) Talanta 635:47–553
Ozden S, Catalgol B, Gezginci-Oktayoglu S, Arda-Pirincci P, Bolkent S, Alpertunga B (2009) Food Chem Toxicol 47:1676–1684
United States Environmental Protection Agency, Prevention, Pesticides and Toxic Substances (7508W) February (1994), EPA-738-F-94-002
The Reconsideration of Methiocarb, Registrations of Products containing Methiocarb and their Associated Labels. Australian Pesticides & Veterinary Medicines Authority (2005) Australia
Kruve A, Herodes K, Leito I (2011) Rapid Commun Mass Spectrom 25:1159–1168
Wang JA, Cheung W (2006) J AOAC Int 89:214–224
Wang J, Cheung W, Grant D (2005) J Agric Food Chem 53:528–537
Moreno-Gonzalez D, Huertas-Perez JF, Gamiz-Gracia L, Garcia-Campana AM (2011) Anal Chem 91:1329–1340
Topuz S, Alpertunga B (2003) Int J Environ Anal Chem 83:787–795
Huertas-Perez JF, Garcia-Campana AM (2008) Anal Chim Acta 630:194–204
Koc F, Yigit Y, Das YK, Gurel Y, Yaral C (2008) J Food Drug Anal 16:39–45
Sanchez-Brunete C, Albero B, Tadeo JL (2004) J Food Prot 67:2565–2569
Carabias-Martinez R, Garcia-Hermida U, Rodriguez-Gonzalo E, Ruano-Miguel L (2005) J Sep Sci 28:2130–2138
İnam R, Tekalp T (2012) Int J Environ Anal Chem 92:85–95
Pelit FO, Ertaş H, Ertaş FN (2011) J Appl Electrochem 41:1279–1285
Mai NN, Liu XY, Wei WZ, Luo SL, Liu W (2011) Microchim Acta 174:89–95
Galli A, De Souza D, Machado SAS (2011) Microchem J 98:135–143
Mercan H, İnam R, Aboul-Enein HY (2011) Anal Lett 44:1392–1404
Radovan C, Manea F (2007) Electroanalytical 19:91–95
Ni YN, Qiu P, Kokot S (2005) Anal Chim Acta 537:321–330
İnam R, Cicek E (2009) Clean Soil Air Water 37:75–79
Ivanov AN, Lukachova LV, Evtugyn GA, Karyakina EE, Kiseleva SG, Budnikov HC, Orlov AV, Karpacheva GP, Karyakin AA (2002) Bioelectrochemistry 55:75–77
Rajasekhar RS, Chandramohan K, Ravindranath BT, Subrahmanyam K (2011) Int J Environ Sci 2:1119–1126
Bobrowski A, Kasprzyk G, Mocak J (2000) Collect Czech Chem C 65:979–994
Lovric M, Komorsky-Lovric S (1988) J Electroanal Chem 248:239–253
Grimshaw J (2000) Electrochemical reactions and mechanisms in organic chemistry. Elsevier, Amsterdam, p 187
Currie LA (1999) Anal Chim Acta 391:103
Acknowledgments
We would like to thank “Gazi University” for supporting this project (Grant No. BAP-05/2011-51) financially.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
İnam, R., Bilgin, C. Square wave voltammetric determination of methiocarb insecticide based on multiwall carbon nanotube paste electrode. J Appl Electrochem 43, 425–432 (2013). https://doi.org/10.1007/s10800-013-0526-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-013-0526-5