Abstract
The nanosized Mn3O4 particles were prepared by microwave-assisted reflux synthesis method. The prepared sample was characterized using various techniques such as X-ray diffraction (XRD), Fourier transform-infrared spectroscopy (FT-IR), Raman analysis, and transmission electron microscopy (TEM). Electrochemical properties of Mn3O4 nanoparticles were investigated using cyclic voltammogram (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge–discharge analysis in different electrolytes such as 1 M KCl, 1 M Na2SO4, 1 M NaNO3, and 6 M KOH electrolytes. XRD pattern reveals the formation of single-phase Mn3O4 nanoparticles. The FT-IR and Raman analysis also assert the formation of Mn3O4 nanoparticles. The TEM image shows the spherical shape particles with less than 50 nm sizes. Among all the electrolytes, the Mn3O4 nanoparticles possess maximum specific capacitance of 94 F g−1 in 6 M KOH electrolyte calculated from CV. The order of capacitance obtained by various electrolytes is 6 M KOH > 1 M KCl > 1 M NaNO3 > 1 M Na2SO4. The EIS and galvanostatic charge–discharge results further substantiate with the CV results. The cycling stability of Mn3O4 electrode reveals that the prepared Mn3O4 nanoparticles are a suitable electrode material for supercapacitor application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, an enormous effort has been focused on the fabrication of electrochemical capacitors or supercapacitors for energy storage due to their long cycle life and higher power and energy density than batteries and conventional capacitors [1]. It is well known that the electrochemical capacitors can be classified into two types such as pseudocapacitor and electric double layer capacitors (EDLC) based on their energy storage mechanism. In pseudocapacitors, the energy is stored through oxidation/reduction or Faradaic reaction occurred at the electrode surface. The transition metal oxides and conducting polymer materials are widely used as electrode material for pseudocapacitors. Similarly in EDLC, the energy is stored at electrode/electrolyte interfaces via double layer formation. Carbon-based materials are widely used as EDLC electrodes due to their higher surface area [2]. There are various metal oxides synthesized for supercapacitor application such as RuO2 [3], NiO [4], Co3O4 [5], Bi2O3 [6], SnO2 [7], and manganese-based oxides [8–10]. Among all the transition metal oxides, RuO2 possesses higher specific capacitance of 720 F g−1 [11]. However, it has been less considered due to their high cost, need of strong acidic electrolyte, and toxicity of the material [12, 13]. Hence, it is believed that the manganese oxide-based electrode materials can replace the RuO2 for supercapacitor applications because of their low cost and environmental friendly nature [14] as well as its different crystallographic forms of MnO, Mn3O4, Mn2O3, and MnO2 due to the existence of various oxidation states [15, 16].
Generally, the specific capacitance of the Mn3O4 mainly depends upon various parameters such as specific surface area [9], preparation conditions [17], synthesis method [16–29], active materials loading [30], morphology [31], conductive additives (carbon, CNT, Graphene) [32–34], electrolyte concentration [22], and potential window [30, 35]. Conventionally, the oxides (MnO2, Mn2O3), carbonate (MnCO3), nitrate (Mn(NO3)2), and sulfate (MnSO4) salts of manganese are heated at above 1000 °C to form a Mn3O4 tetragonal structure [25, 36]. Subsequently, to improve the specific capacitance of Mn3O4, the nanosized particles have been prepared by various methods such as successive ionic layer adsorption and reaction (SILAR) [18], hydrothermal [17, 20], solution combustion [21], chemical bath deposition [22], oxidative precipitation [23], sonochemical [24], solvothermal [25], and microwave [26–29].
In this line, the main aim of this present work is to prepare Mn3O4 nanoparticles by microwave-assisted reflux synthesis with a very short reaction time (5 min) using low power of 20 W without further high-temperature calcinations and explored its application as electrodes for supercapacitors. To the best of our knowledge, there is no previous literature available on the synthesis of Mn3O4 nanoparticles using this procedure and optimization of electrolyte. In addition, here we have used ethylene glycol as a reactive medium for the microwave synthesis due to its high-dissipation factor (1.35) than other solvents such as water (0.157), formic acid (0.722) and so on. Further the prepared sample was characterized by X-ray diffraction (XRD), Fourier transform-infrared spectroscopy (FT-IR), laser Raman spectra, scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The electrochemical performance of Mn3O4 was investigated using cyclic voltammetry, electrochemical impedance, spectroscopic, and galvanostatic charge–discharge analysis in various aqueous electrolytes such as 1 M Na2SO4, 1 M NaNO3, 1 M KCl, and 6 M KOH for optimizing the suitable electrolyte for Mn3O4 electrode in supercapacitor applications. The results indicate that the obtained nanosphere Mn3O4 is a good electrode material for supercapacitor application.
2 Experimental methods and materials
All the chemicals used were of analytical grade and used without any further purification. Manganese chloride tetrahydrate (MnCl2·4H2O) and sodium hydroxide (NaOH) were purchased from Himedia. Ethylene glycol was purchased from Merck. The stoichiometric amounts of MnCl2·4H2O were dissolved in distilled water with EG (50 ml), and NaOH solution was added drop by drop under vigorous stirring. The color of the solution changed in to brown color precipitate. This precipitate was placed in microwave irradiation (Domestic microwave oven, LG) for refluxing with power of 20 W, the ON/OFF cycle duration as 15 s/15 s, with the total reaction time of 5 min [37, 38]. The ON/OFF cycle was used to control the overheating. Finally, the as-prepared sample was centrifuged several times in double distilled water, ethanol, and dried at 100 °C overnight.
The phase purity and compound formation were characterized by an X-ray diffractometer, Bruker D8 Advance with Cu Kα radiation. The morphology of the as-prepared samples was found using TEM analysis (JEOL model JEM 2011 at an accelerating voltage of 200 kV). The functional groups were identified using FT-IR Perkin Elmer make model RXI instrument. The Raman analysis of our samples was carried out in the instrument of laser Raman confocal microprobe (Lab Ram HR 800). He–Ne laser (λ = 633 nm) was used as the excitation source with output power of 17 mW which was focused on to a spot of 1 μm. The cyclic voltammetrics analysis was carried out in CHI 1102A electrochemical workstation. The galvanostatic charge–discharge analysis was carried in Biologic SP-150 electrochemical workstation.
The Mn3O4 active material, carbon black, and PVdF were taken in the weight ratio of 80:15:5. All these were mixed together using NMP (N-methylpyrrolidone) as a solvent. The detailed electrode preparation is given elsewhere [39]. The electrochemical analysis was carried out in three-electrode configuration with Mn3O4-coated graphite sheet, Pt and SCE, Ag/AgCl as working, counter, and reference electrodes, respectively. Here we used different electrolytes such as 1 M Na2SO4, 6 M KOH, 1 M NaNO3, and 1 M KCl to optimize the suitable electrolyte for Mn3O4 electrode material for supercapacitor application.
3 Results and discussion
3.1 Structural and morphological properties
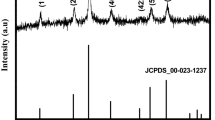
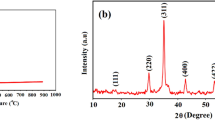
The XRD pattern of Mn3O4 nanoparticles is given in Fig. 1. All the diffraction peaks were indexed to the tetragonal structure (space group I41/amd). No other impurity peaks were found, which reveals the phase purity of the prepared sample. However, the observed broadened and low-intensity peaks indicate the less crystallinity of the as-prepared Mn3O4 nanoparticles. The lattice parameters (a = b = 5.767 Å and c = 9.485 Å), lattice density (4.816 g cm−3), and cell volume (315.5 Å3) of the sample were calculated which are in good consistence with JCPDS data (card no. 89-4837). The crystallite size was calculated using Debye–Scherrer formula, and the average crystallite size is 18 nm, which is less than the reported value [40]. The surface area of the as-prepared Mn3O4 nanoparticles was theoretically calculated [41], and the value is 65 m2 g−1.
The FT-IR spectrum of Mn3O4 (Fig. 2) shows two significant peaks at 635 and 510 cm−1, which corresponds to the coupling between the Mn–O stretching modes of tetrahedral and octahedral sites, respectively. In addition, a broad peak was observed around 3395 cm−1 indicating the presence of –OH group. Further, the small band was observed around 1580 cm−1 corresponding to the adsorption of moisture on the surface of the sample, and also a small peak was observed around 1376 cm−1 corresponding to the bending vibration of O–H bonds connected with Mn atoms [35]. The presence of Mn–O stretching modes and water content was identified through the FT-IR study. Similarly, the characteristic Raman peak of Mn3O4 spinel (Fig. 2 inset) was obtained at 639 cm−1, which is comparable with the reported values of 658.4 and 658 cm−1 [25, 42]. The observed peak broadening reveals the smaller crystallite size as well as the low crystallinity of Mn3O4 nanoparticles [43]. Because of their smaller crystallite size, the material has high uncertainty of momentum which leads to the broadening of the Raman peak [43].
The TEM image of Mn3O4 (Fig. 3) shows the formation of individual spherical nanoparticles with uniform size of less than 50 nm. It can also be seen that some partial aggregation of nanospheres. This aggregation of nanoparticles may be due to the effect of microwave heating. That is, it creates “hot surface” on the initially produced nanoparticles which leads to the particle aggregation. Because of the smaller particle size, it may provide higher surface area which may enhance the electrochemical reaction.
3.2 Electrochemical properties
3.2.1 Cyclic voltammogram analysis
Figure 4a–d shows the cyclic voltammogram (CV) curves of Mn3O4 in different electrolytes (1 M Na2SO4, 6 M KOH, 1 M NaNO3, and 1 M KCl) at different scan rates of 5, 10, 20, 30, 40, 50, and 100 mV s−1. The more or less rectangular shape of the voltammogram reveals the capacitive behavior of Mn3O4. It can be seen that the current under curve increases with increase in scan rate and in turn results in decrease in capacitance (Fig. 4e). It is well known that the voltammetric current is always directly proportional to the scan rate [7]. At low-scan rate, the ions from the electrolyte can utilize all the available sites in the active electrode material, because the ions have enough time to diffuse into all the sites which leads to the higher capacitance. On the other hand, at high-scan rate, the ions from electrolyte confront the difficulty to access all the available sites in the active electrode due to their partial rate of movement in the electrolyte [4]. Comparatively, 6 M KOH and 1 M KCl provide higher specific capacitance when compared with 1 M NaNO3 and 1 M Na2SO4. The calculated maximum specific capacitance of 94 F g−1 was obtained in 6 M KOH. This observed high capacitance may be explained on the basis of ionic conductivity and hydration sphere radii of cations (Na+, K+). Generally, the ionic conductivity and mobility of Na+ ions are lower than the K+ ions in aqueous solvent which reduces the charge propagation [34]. Similarly, the K+ (3.31 Å) ions have low radius of hydration sphere than Na+ ions (3.58 Å) due to the strong interaction of Naδ+–H2Oδ−. Therefore, the Na+ ions have the difficult to move through the electrode when compared with K+ ions [11]. In addition, there may be a large number of K+-free ions near the electrode surface in the KOH electrolyte than KCl, hence more number of ions contributes to charge storage process. Consequently, the size of the Cl− (190 pm) anion is greater than the OH− (133 pm) ion which further enhances the specific capacitance in KOH electrolyte [11]. In contrast, the size of the sulfate (258 pm) anion is bigger than the NO3 − (179 pm) anion, which may lead to the reduction of Na+ ions mobility and finally reduces the specific capacitance of Mn3O4 electrode. Overall, the charge storage mechanism of this manganese-based electrodes are mainly due to the intercalation and deintercalation of cation (Na+, K+) during reduction and oxidation reaction and the adsorption of cations in electrolyte on the surface of the electrode [7, 44]. The observed specific capacitance values are comparatively high when compared with the literature values especially, 14 F g−1 at 5 mV s−1 in 0.5 M K2SO4 reported by Ghodbane et al. [45]. Especially, Komaba et al. reported that the specific capacitance of Mn3O4 electrode depends on the ball milling treatment (specific capacitance of Mn3O4 before and after milling as approximately less than 8 and 35 F g−1, respectively) and the potential window (the maximum specific capacitance (<70 F g−1) obtained when the potential window is in the range of −0.1 to 0.9 V and also the minimum specific capacitance (<20 F g−1) obtained when the potential window is −0.3 to 0.7 V at a scan rate of 10 mV s−1 in 1 M Na2SO4 electrolyte [30]). However, the obtained specific capacitance value is lower than the reported Mn3O4 composites such as Mn3O4/CNT [32], MCMB/Mn3O4 [33], Mn3O4/worm-like mesoporous carbon [16], and Mn3O4/graphene [34] composites which give a higher capacitance value of 143, 178, 266, and 256 F g−1 in 0.5 M Na2SO4 at a scan rate of 50 mV s−1, 1 M LiPF6 (EC + DMC) at a current rate of 330 mA g−1, 6 M KOH at a scan rate of 1 mV s−1, and 6 M KOH at a scan rate of 5 mV s−1, respectively. This observed low capacitance may be due to the less crystallinity of the Mn3O4 nanoparticles which inferred from XRD and Raman spectrum. It is well known that the specific capacitance of the materials is very high when the materials having high crystallinity, because it enhances the ionic mobility of the charge carriers [7, 44].
3.2.2 Electrochemical impedance spectral analysis
In order to substantiate the CV results, the electrochemical impedance spectroscopy (EIS) analysis was carried out for Mn3O4 at different electrolytes of 1 M NaNO3, 1 M Na2SO4, 6 M KOH, and 1 M KCl at open circuit potential in the frequency range of 0.01 Hz to 105 Hz. The typical Nyquist plot of Mn3O4 electrodes are shown in Fig. 5. At high-frequency region, the intersection made on the horizontal axis of the Nyquist plot reveals the solution resistance (R s). At high-to-medium frequency region, one depressed semicircle was observed, which is related with surface property of the Mn3O4 electrode, corresponding to the charge transfer resistance at electrode/electrolyte interface. In addition, at low-frequency region the spike was found, which indicates the characteristic behavior of supercapacitors. The spike represents the Warburg impedance (W) of the electrode, i.e., the diffusive resistance of ions SO4 2−, NO3 −, Cl−, and OH− into electrode [4, 14]. The phase angle of spike greater than 45° for all electrolytes indicates the electrochemical behavior of Mn3O4. Comparing all the electrolytes, the electrolyte 6 M KOH (1.197 Ω) has lower solution resistance than other electrolytes such as 1 M KCl (2.046 Ω), 1 M NaNO3 (2.694 Ω), and 1 M Na2SO4 (2.839 Ω) electrolytes.
3.2.3 Galvanostatic charge–discharge analysis
The galvanostatic charge–discharge analyses of Mn3O4 carried out in all the electrolytes at different current densities such as 0.5, 1, 3, and 5 mA cm−2 and are shown in Fig. 6. It can be seen that a potential or IR drop was found at early discharging time which may be due to the internal resistance of electrode material. It can be related to the contact resistance between the electrode and electrolyte, solution resistance of electrolyte, and charge transfer resistance. The internal resistance of the electrode material increases with increase in current density [46]. The observed symmetric manners of the charge–discharge curves indicate the electrochemical reversibility of Mn3O4 electrode. The maximum discharge capacitance is obtained (74 F g−1) at current density of 0.5 mA cm−2 in 6 M KOH electrolyte which is comparable to the specific capacitance calculated from CV curve. Figure 7a–d shows the variation of discharge capacitance and active sites with different current density in all the electrolytes. It evidences that the electrode has higher specific capacitance and higher number of active sites at low-current density and vice versa, i.e., lower specific capacitance and active sites at higher current density in all electrolytes. It may be due to the fact that ions completely diffuse into the electrode and utilize all the active sites in electrode at low-current density. Therefore, the specific capacitance and active sites are high. Similarly, at higher current density, the ions have time constraint to utilize all the active sites in electrode. Therefore, the specific capacitance and active sites are low [47].
The cyclic stability of the electrode material is important for supercapacitor applications. In order to find the cyclic stability of the Mn3O4 electrode material, the galvanostatic charge–discharge cycle was carried out in all electrolytes at a current density of 5 mA cm−2 up to 500 cycles. Figure 8a–d shows the variation of charge and discharge capacitance, and coulombic efficiency with cycle number of Mn3O4 electrode in all the electrolytes. The first 10 charge–discharge cycles is given as an inset of Fig. 8. It can be seen that during cycling, the discharge and charging capacitance of the electrode material was increased significantly. According to the earlier reports, this may be due to the occurrence of morphological or structural changes while extended cycling [18, 22, 45]. The coulombic efficiency of the material was maintained approximately greater than 100 %. Overall, it shows that the Mn3O4 is a suitable electrode material for supercapacitor application and its suitable electrolyte is 6 M KOH.
4 Conclusions
Mn3O4 nanoparticles were successfully synthesized by microwave-assisted reflux synthesis method within 5 min without any further heat treatment. The as-prepared Mn3O4 samples possess tetragonal structure, and its lattice parameters and grain size were calculated from XRD pattern. FT-IR and Raman analyses confirmed the compound formation and the presence of the functional groups in as-prepared Mn3O4 samples. The TEM result reveals the presence of smaller particle size (50 nm). The electrochemical properties of Mn3O4 electrode were investigated in various aqueous electrolytes. The CV results reveal that the Mn3O4 electrode possesses higher specific capacitance (94 F g−1) in 6 M KOH electrolyte. Therefore, 6 M KOH electrolyte may be adopted as electrolyte for better capacitance performances. The electrochemical impedance analysis and galvanostatic charge–discharge analysis further confirm that the Mn3O4 electrode has lower internal resistance and higher capacitance in 6 M KOH electrolyte than other electrolytes. The long-term cycle stability Mn3O4 electrode reveals that the prepared sample is a suitable electrode material for supercapacitor applications.
References
Zhao X, Sanchez BM, Dobson PJ, Grant PS (2011) Nanoscale 3:839
Conway BE (1999) Electrochemical supercapacitors. Kluwer-Plenum, New York
Devadas A, Baranton S, Napporn TW, Coutanceau C (2011) J Power Sources 196:4044
Meher SK, Justin P, Rao GR (2011) Appl Mater Interfaces 3:2063
Yuan YF, Xia XH, Wu JB, Huang XH, Pei YB, Yang JL, Guo SY (2011) Electrochem Commun 13:1123
Zheng FL, Li GR, Ou YN, Wang ZL, Su CY, Tong YX (2010) Chem Commun 46:5021
Kalai Selvan R, Perelshtein I, Perkas N, Gedanken A (2008) J Phys Chem C 112:1825
Zhang Y, Li GY, Lv Y, Wang LZ, Zhang AQ, Song YH, Huang BL (2011) Int J Hydrogen Energy 36:11760
Xing S, Zhou Z, Ma Z, Wu Y (2011) Mater Lett 65:517
Hu CC, Hung CY, Chang KH, Yang YL (2011) J Power Sources 196:847
Reddy RN, Reddy RG (2003) J Power Sources 124:330
Xu MW, Jia W, Bao SJ, Su Z, Dong B (2010) Electrochim Acta 55:5117
Ding KQ (2008) J Chin Chem Soc 55:543
Xia H, Xiao W, Lai MO, Lu L (2009) Nanoscale Res Lett 4:1035
Hao X, Zhao J, Li Y, Zhao Y, Ma D, Li L (2011) Colloid Surf Physicochem Eng Aspect 374:42
Zhou T, Mo S, Zhou S, Zou W, Liu Y, Yuan D (2011) J Mater Sci 46:3337
Jiang H, Zhao T, Yan C, Ma J, Li C (2010) Nanoscale 2:2195
Dubal DP, Dhawale DS, Salunkhe RR, Lokhande CD (2010) J Electroanal Chem 647:60
Dubal DP, Dhawale DS, Salunkhe RR, Fulari VJ, Lokhande CD (2010) J Alloys Compd 497:166
Li Y, Tan H, Yang XY, Goris B, Verbeeck J, Bals S, Colson P, Cloots R, Tendeloo GV, Su BL (2011) Small 4:475
Gao W, Ye S, Shao M (2011) J Phys Chem Solids 72:1027
Dubal DP, Dhawale DS, Salunkhe RR, Lokhande CD (2010) J Alloys Compd 496:370
Ozkaya T, Baykal A, Kavas H, Kőseoğlu Y, Toprak MS (2008) Phys B 403:3760
Baykal A, Kavas H, Durmuş Z, Demir M, Kazan S, Topkaya R, Toprak MS (2010) Cent Eur J Chem 8(3):633
Zhang W, Yang Z, Liu Y, Tang S, Han X, Chen M (2004) J Cryst Growth 263:394
Bilecka I, Niederberger M (2010) Nanoscale 2:1358
Apte SK, Naik SD, Sonawane RS, Kale BB, Pavaskar Neela, Mandale AB, Das BK (2006) Mater Res Bull 41:647
Berthelin CB, Stuerga D (2005) J Mater Sci 40:253
Malinger KA, Ding YS, Sithambaram S, Espinal L, Gomez S, Suib SL (2006) J Catal 239:290
Komaba S, Tsuchikawa T, Ogata A, Yabuuchi N, Nakagawa D, Tomita M (2012) Electrochim Acta 59:455
Devaraj S, Munichandraiah (2008) J Phys Chem C 112:4406
Cui X, Hu F, Wei W, Chen W (2011) Carbon 49:1225
Wang H, Li Z, Yang J, Li Q, Zhong X (2009) J Power Sources 194:1218
Wang B, Park J, Wang C, Ahn H, Wang G (2010) Electrochim Acta 55:6812
Dubal DP, Dhawale DS, Salunkhe RR, Pawar SM, Lokhande CD (2010) Appl Surf Sci 256:4411
Gibot P, Laffont L (2007) J Solid State Chem 180:695
Patra CR, Gedanken A (2004) New J Chem 28:1060
Wang H, Zhu JJ, Zhu JM, Liao XH, Xu S, Ding T, Chen HY (2002) Phys Chem Chem Phys 4:3794
Senthilkumar B, Thenamirtham P, Kalai Selvan R (2011) Appl Surf Sci 257:9063
Anilkumar M, Ravi V (2005) Mater Res Bull 40:605
Sekar C, Kalai Selvan R, Senthilkumar ST, Senthilkumar B, Sanjeeviraja C (2012) Powder Technol 98:215
Yang LX, Zhu YJ, Tong H, Wang WW, Cheng GF (2006) J Solid State Chem 179:1225
Zuo J, Xu C, Liu Y, Qian Y (1998) Nanostruct Mater 10:1331
Sharma RK, Oh HS, Shul YG, Kim H (2008) Phys B 403:1763
Ghodbane O, Pascal JL, Fraisse B, Favier F (2010) Appl Mater Interfaces 2:3493
Zhang J, Jiang J, Zhao XS (2011) J Phys Chem C 115:6448
Xu J, Gao L, Cao J, Wang W, Chen Z (2010) Electrochim Acta 56:732
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sankar, K.V., Kalpana, D. & Selvan, R.K. Electrochemical properties of microwave-assisted reflux-synthesized Mn3O4 nanoparticles in different electrolytes for supercapacitor applications. J Appl Electrochem 42, 463–470 (2012). https://doi.org/10.1007/s10800-012-0424-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-012-0424-2